


Trepostome bryozoans encrusting Silurian gastropods: A taphonomic window and its implications for biodiversity
CAROLINE J. BUTTLER, LESLEY CHERNS, and LUCY M.E. MCCOBB
Buttler, C.J., Cherns, L., and McCobb, L.M.E. 2022. Trepostome bryozoans encrusting Silurian gastropods: A taphonomic window and its implications for biodiversity. Acta Palaeontologica Polonica 67 (3): 569–577.
Silurian turreted gastropods from the Upper Leintwardine Formation, Ludlow Series, collected in Delbury Quarry, Shropshire, UK, are all encrusted by the trepostome bryozoan Homotrypa cochlea sp. nov. Bryozoans were not found to encrust any other component of the shelly fauna and thus seemed preferentially to choose the gastropod shells. The relationship between these two organisms was examined to consider whether the bryozoans were using the dead, empty mollusc shells as a substrate, if they were living symbiotically with live gastropods, or if the shells were inhabited by a non-gastropod host. There is evidence that the bryozoans encrusted the shells of living gastropods but continued growing after the death of the mollusc, potentially with the shell then occupied by a conchicole. Bryozoans encased the gastropod shells and, after death of the mollusc, the internal cavity became a “closed” microenvironment where the shell form and sometimes the recrystallised shell became preserved. The aragonitic shells of these gastropods were prone to dissolution early in diagenesis, and no gastropods are found without encrusting bryozoans. Bryoimmuration resulted in a local taphonomic window for the molluscs, which are notably sparse in most early Palaeozoic shelly faunas—the so-called “missing molluscs” phenomenon.
Key words: Gastropoda, Bryozoa, symbiosis, overgrowth, aragonite, taphonomy, Silurian, Shropshire, UK.
Caroline J. Buttler [Caroline.Buttler@museumwales.ac.uk] and Lucy M.E. McCobb [Lucy.McCobb@museumwales.ac.uk], Department of Natural Sciences, Amgueddfa Cymru National Museum Wales, Cathays Park, Cardiff CF10 3NP, Wales, UK.
Lesley Cherns [cherns@cardiff.ac.uk], School of Earth and Environmental Sciences, Cardiff University, Cardiff CF10 3AT, Wales, UK.
Received 3 December 2021, accepted 2 March 2022, available online 21 July 2022.
Copyright © 2022 C.J. Buttler et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Bryozoans have a long geological history of using mollusc shells as a substrate, and symbiotic associations between the two phyla have been recognised (Buttler and Taylor 2020). However, it can be difficult to identify from fossils what the relationship between the organisms was in life.
At Delbury Quarry, Silurian bryozoans are found encrusting all turreted gastropods, that are identified as species of Murchisonia, among a brachiopod dominated shelly fauna. An originally aragonitic shell for this genus is evident from its typical fossil preservation as sediment moulds and casts (e.g., Mazaev 2011). A general sparsity of fossils of organisms with original aragonitic shells in lower Palaeozoic rocks can be attributed to early diagenetic dissolution, the “missing molluscs” problem of Cherns and Wright (2000). We examine the relationship between the trepostome bryozoans and gastropods, and discuss the taphonomic history. The bryozoan is a new species, described below.
Institutional abbreviation.—NMW, Amgueddfa Cymru –National Museum Wales, Cardiff, Wales, UK.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:act:D1C4A37E-3C72-4AE5-B6E5-A444A3623B09
Geological setting
The bryozoan-encrusted gastropods described here come from the Upper Leintwardine Formation (Ludfordian, upper Ludlow) in the Wenlock Edge area at Delbury Quarry (SO 494 862, Lat/Long 52.47140087-2.74484452), ca. 10 km north of the Ludlow Series type area of Shropshire, United Kingdom (Fig. 1). Extraction of building stone from the overlying Lower Whitcliffe Formation in the working quarry has left a platform (ca. 4 m) of fossiliferous nodular calcareous siltstone flags of the Upper Leintwardine Formation (Fig. 1; both mapped by the British Geological Survey as “Upper Ludlow Shales”). These comprise shallow marine siliciclastic shelf sediments deposited in the Welsh Basin near the edge of the Midland Platform (Cherns et al. 2006).
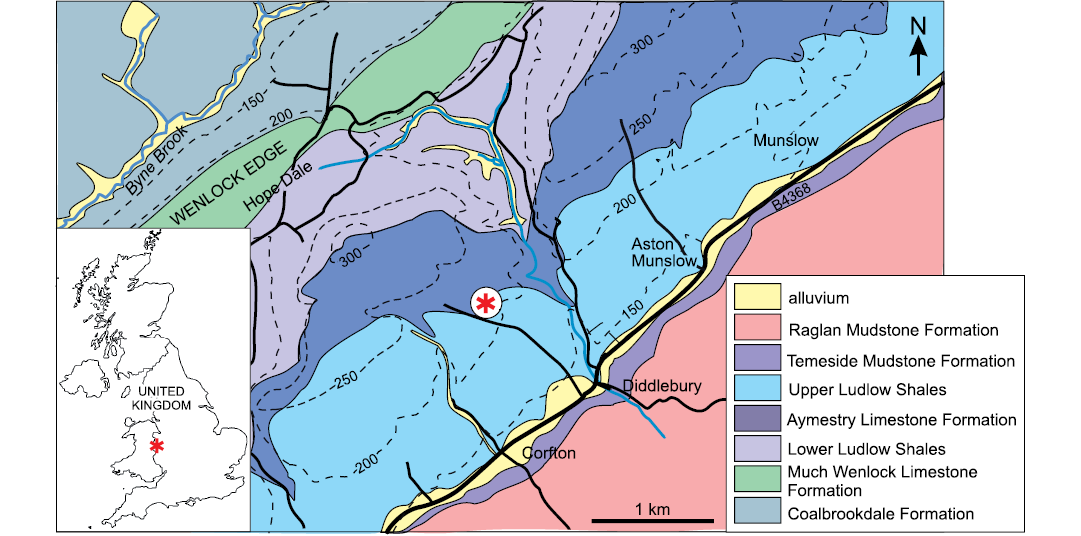
Fig. 1. Map showing the location and geological setting (asterisk) of Delbury Quarry in Shropshire, UK (amended from https://digimap.edina.ac.uk/roam/map/geology and https://digimap.edina.ac.uk/roam/map/os; accessed 05/08/21).
The Upper Leintwardine Formation is a thin unit recognised throughout the shelf area by its distinctive shelly assemblage, most notably the brachiopod Shaleria ornatella, trilobites Calymene puellaris and Encrinurus stubblefieldi and in more distal shelf environments by the brachiopod–ostracode Aegiria grayi–Neobeyrichia lauensis assemblage (Holland et al. 1963; Cherns 1999). The graptolite Saetograptus leintwardinensis occurs rarely in this and the underlying Lower Leintwardine Formation, placing both within the Saetograptus leintwardinensis Biozone (Holland et al. 1963). At Delbury, other brachiopods are also common, e.g., Atrypa reticularis, Leptaena depressa, Isorthis orbicularis, Microsphaeridiorhynchus nucula, Protochonetes ludloviensis, Dayia navicula, and Salopina lunata. Some bedding planes are highly fossiliferous and dominated by large numbers of brachiopods. Less common fossils include bryozoans, molluscs (bivalves, gastropods, nautiloid cephalopods), ostracods, “Serpulites longissimus” and Cornulites serpularius. Bryozoan-encrusted gastropods are locally numerous.
Relationship of bryozoans and gastropods from Delbury Quarry
All gastropods from Delbury Quarry are found with the trepostome bryozoan Homotrypa cochlea sp. nov. covering the surface of the shells (Fig. 2), but the colonies do not grow across the apertures (Fig. 3A3, A4, C). They are encrusting rather than massive and did not grow beyond the surface of the shell onto the sea floor, unlike those illustrated by Snell (2004: pl. 3: 2, 3).
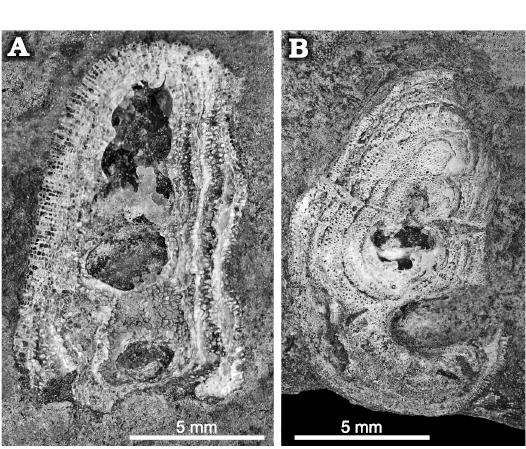
Fig. 2. Trepostome bryozoan Homotrypa cochlea sp. nov. from Upper Leintwardine Formation; Ludfordian, upper Ludlow, Silurian; Delbury Quarry, Shropshire, UK. A. NMW 2019.21G.7.1, bryozoan encrusting the surface of a gastropod shell. B. NMW 2019.21G.33, bryozoan encrusting the surface of a gastropod shell but not growing across aperture.
Borings can be recognised in the bryozoan colonies (Fig. 3B); they are straight with a cylindrical cross section and identified as belonging to Trypanites Mägdefrau, 1932. This trace fossil is commonly found in early Palaeozoic trepostome bryozoans and was a significant bioeroder of hard substrates (Wilson and Palmer 2006). The borings are not seen penetrating into the gastropod shells.
The bryozoan colonies are comprised of a single layer or multiple layers caused by self-overgrowths (Fig. 3A1, A2). Thin sections reveal that the colonies have not grown evenly across the surface of the shell. On the apertural side the colony is often thinner than on the abapertural side. The thinner side has no zooecia apertures towards the surface but is composed of extremely thick calcite wall (Fig. 3B); this indicates that there were no feeding zooids present in this area of the colony. This is a similar pattern of encrusting to that described by Morris et al. (1991), who suggested that the shell was occupied either by the mollusc or a conchicole.
Further evidence for the occupation of the shell is that the bryozoan colonies are not seen growing across the gastropod apertures. In one specimen bryozoan calcite appears to be present between two layers of gastropod shell, indicating that the bryozoan and gastropod were alive at the same time (Fig. 3A4). In some specimens (Figs. 3A3, 4) the bryozoan colony can be observed growing just inside the aperture, implying that the shell was no longer occupied by the mollusc but not precluding a conchicole being present.
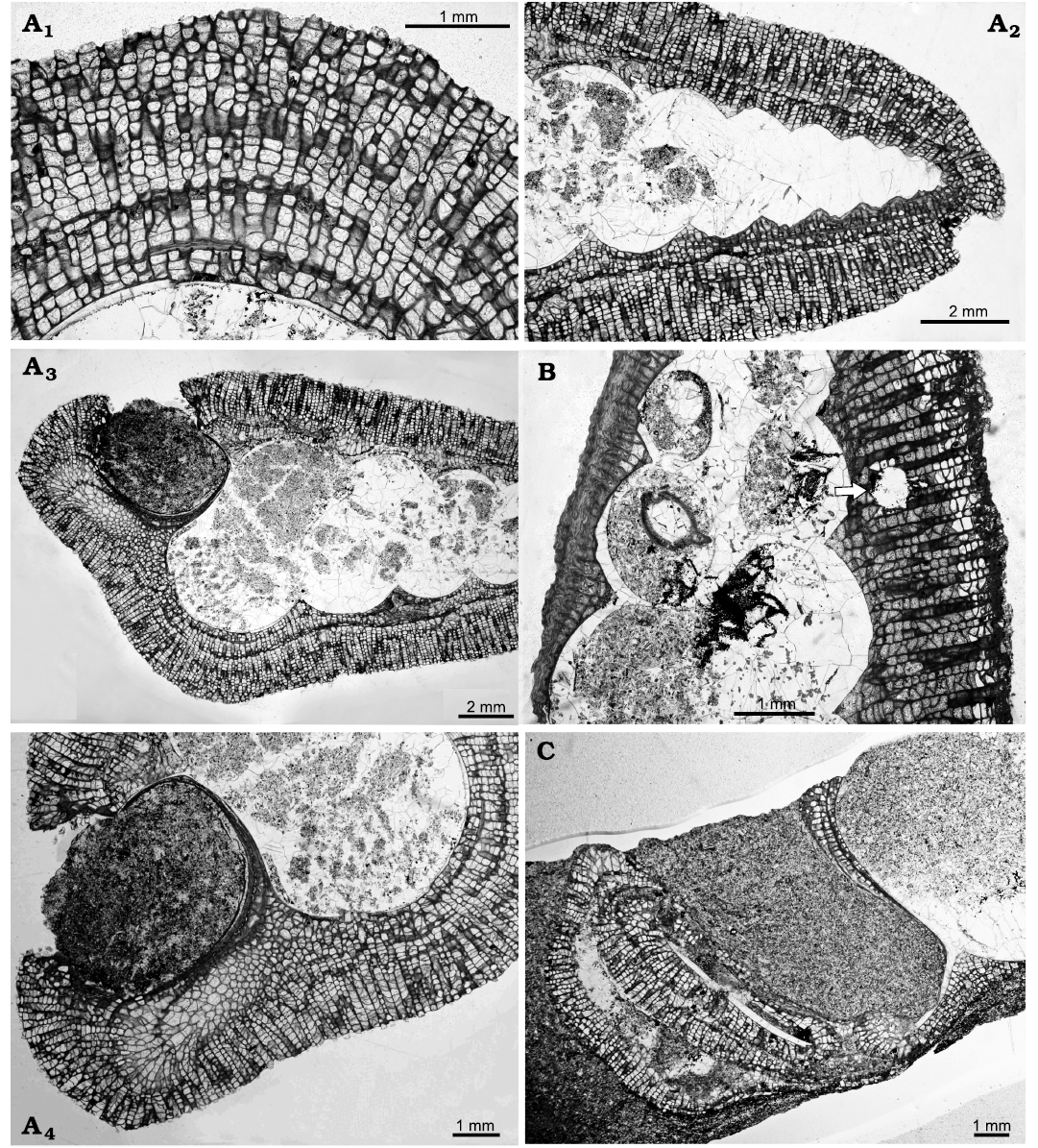
Fig. 3. Thin sections of trepostome bryozoan Homotrypa cochlea sp. nov. from Upper Leintwardine Formation; Ludfordian, upper Ludlow, Silurian; Delbury Quarry, Shropshire, UK. A. NMW 2019.21G.5.2, encrusting colonies comprising multiple layers caused by self-overgrowths (A1), apex of gastropod shell encrusted with bryozoan colony (A2), colony does not grow over the gastropod shell aperture (A3, A4). B. NMW 2019.21G.6.2, colony encrusting apertural side of shell thinner than on the abapertural side, arrow indicates Trypanites boring in bryozoan colony. C. NMW 2019.21G.7.3, colony can be seen inside the aperture of gastropod shell.
All bryozoan colonies identified on the gastropod shells are encrusting. One colony (Fig. 4) shows an erect branch that has grown from an encrusting form, located at the anterior end of the shell adjacent to where part of the colony is growing inside the aperture. It is suggested that the bryozoan settled on the gastropod whilst it was alive and continued to grow after death in a ramose form. Potentially at this time the empty shell may have been occupied by a conchicole that lifted the shell off the surface and enabled the bryozoan to grow erect. This has similarities to the growth pattern which is observed today in bryozoan colonies encrusting shells occupied by hermit crabs (Taylor 1994: fig. 2).
Systematic palaeontology
Phylum Bryozoa Ehrenberg, 1831
Class Stenolaemata Borg, 1926
Superorder Palaeostomata Ma, Buttler, and Taylor, 2014
Order Trepostomata Ulrich, 1882
Family Monticuliporidae Nicholson, 1881
Genus Homotrypa Ulrich, 1882
Type species: Homotrypa curvata Ulrich, 1882; Cincinnatian (Upper Ordovician), North America.
Emended diagnosis.—Colonies generally frondose, less commonly dendroid, may be encrusting or massive. Maculae typically formed of clusters of mesozooecia with thick diaphragms or solid stereom surrounded irregularly with large macrozooecia. Endozones uniform in cross-sectional size and autozooecial pattern, autozooecia in disordered pattern throughout, polygonal in cross-section. Diaphragms moderate in spacing and evenly spaced to few and scattered. Autozooecial walls in endozones typically irregularly undulating, some crenulated, polygonal in cross-section. In exozones autozooecial boundaries polygonal in cross-section, serrated in longitudinal sections. Some colonies with thin continuous cingulum throughout, or with cingulum irregularly distributed. Cystiphragms located in exozone. Diaphragms in exozone primary attached to cortex or secondary attached to cystiphragms or cingulum. Mesozooecia rare to abundant. Zooecial and endozonal styles may be present, some oblique to autozooecia. Small exozonal styles common.
Stratigraphic and geographic range.—Middle Ordovician–Silurian; North America, Europe, Australia, Siberia, China.
Homotrypa cochlea sp. nov.
Figs. 3, 4, 5; Table 1.
Zoobank LSID: urn:lsid:zoobank.org:act:D1C4A37E-3C72-4AE5-B6 E5-A444A3623B09
Etymology: From Latin cochlea, snail; named because all specimens are found encrusting gastropods.
Type material: Holotype: NMW 2019.21G.2, whole specimen cut into sections and including prepared thin sections. Paratypes: NMW 2019.21G.1–10, 12, 19, 35, 36, whole specimens cut into sections and including prepared thin sections, all from the type locality.
Type locality: Delbury Quarry, Shropshire (SO 494 862, Lat/Long 52.47140087–2.74484452)
Type horizon: Upper Leintwardine Formation, Ludlow, Silurian.
Diagnosis.—Colonies typically encrusting but can develop ramose form. Endozonal autozooecia recumbent, exozonal budding irregular. Autozooecial apertures irregularly oval to circular, basal diaphragms closely spaced and localised cystiphragms. Mesozooecia common, fairly large with thick walls, develop as closed cysts in early exozone, with abundant diaphragms, in cross-section chambers circular to ovoid. Exozonal styles present.
Description.—Colonies typically encrusting, single or multi laminar, up to five layers recognised. One colony with single erect branch (Fig. 4: 6.5 mm diameter, 10 mm high) developed after encrusting phase, maculae unknown. In endozones of encrusting form autozooecia restricted to recumbent buds grown from exterior basal colony walls. In ramose form autozooecia with disordered pattern in endozones. Autozooecial walls thin, smoothly curved to irregularly undulating in profile. Diaphragms typically widely spaced in endozone of erect branch. In exozones of the erect form the budding is irregular, some autozooecia budded from mesozooecia and mesozooecia budded from autozooecia, both intrazooidally. Autozooecia and mesozooecia disordered in arrangement. Autozooecial boundaries both merged and serrated in longitudinal sections, cortex walls irregular in thickness with some small patches of cingulum. In tangential sections autozooecial apertures irregularly oval to circular, mean average autozooecial diameter maximum 0.19 mm and minimum 0.16 mm, some irregularly shaped. Autozooecial basal diaphragms primary and closely spaced (0.11 mm mean average), some diaphragms inclined or attached to cystiphragms. Mesozooecia abundant and quite large (0.12 mm mean average in diameter), with thick walls, in cross-section chambers circular to ovoid. Mesozooecia develop as closed cysts in early exozone (Fig. 5A2), thick diaphragms and walls combine longitudinal views to compartmentalize chambers, followed by moderate to extremely thick annular mesozooecia walls connected by diaphragms to continue cystose shape of earlier growth. Some mesozooecial diaphragms extremely thick. Some autozooecia contain abundant cystiphragms in the exozone. Exozonal styles present (Fig. 5B), irregularly arranged, some on autozooecial boundaries and some inflecting autozooecial walls.
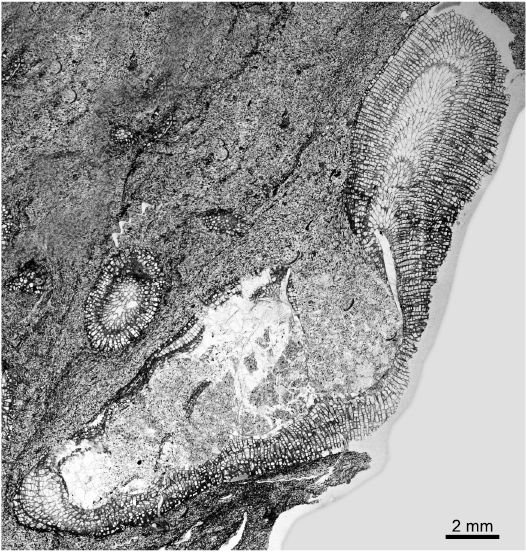
Fig. 4. Thin section of trepostome bryozoan Homotrypa cochlea sp. nov. from Upper Leintwardine Formation, Ludfordian, upper Ludlow; Delbury Quarry, Shropshire, UK. Holotype, NMW 2019.21G.2.3, bryozoan colony encrusting gastropod shell and developing ramose form at shell aperture.
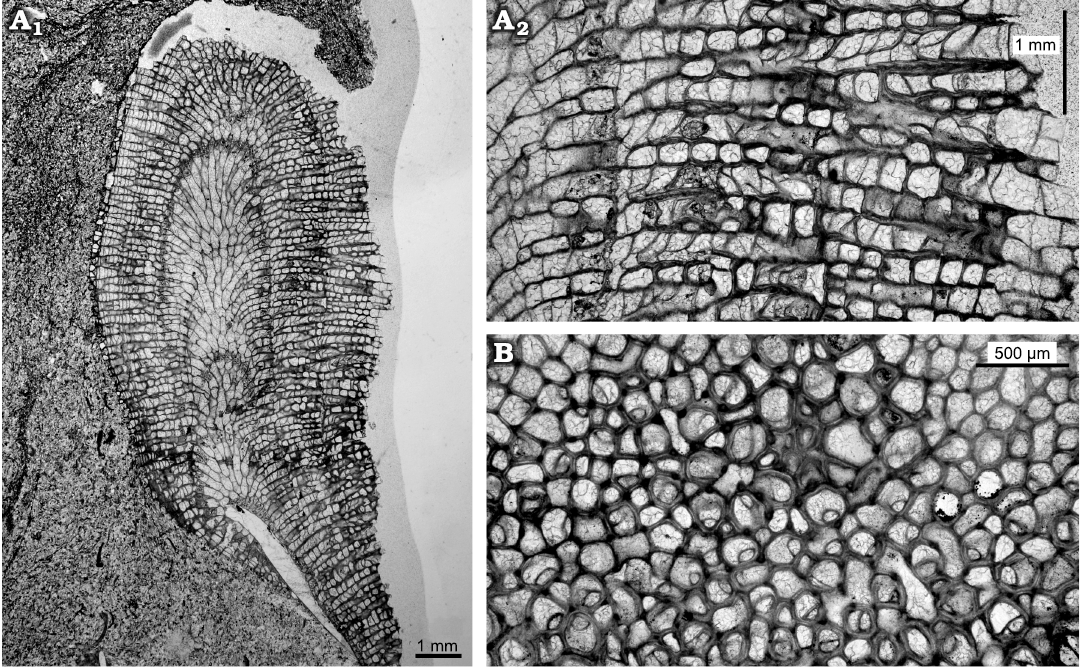
Fig. 5. Thin sections of trepostome bryozoan Homotrypa cochlea sp. nov. from Upper Leintwardine Formation, Ludfordian, upper Ludlow, Silurian; Delbury Quarry, Shropshire, UK. A. Holotype, NMW 2019.21G.2.3, ramose colony form (A1), thick mesozooecia walls connected by diaphragms, continue cystose shape of earlier growth (A2). B. NMW 2019.21G.2.4, autozooecia rounded in cross-section, some irregularly shaped.
Remarks.—Homotrypa cochlea sp. nov. is characterised by the cystose or beaded shape of the mesozooecia which is unusual in species of this genus. It differs from Homotrypa oweni (Ross, 1965) from the Caradoc (upper Ordovician) of Shropshire by having more abundant diaphragms and the nature of the mesozooecia. In morphology it is similar to Asperopora ludlovensis (Owen, 1962), known from the Ludlow (upper Silurian) of England and Sweden, which also forms encrusting colonies with ramose branches, but the latter species has very abundant styles and cystiphragms are absent.
Stratigraphic and geographic range.—Upper Leintwardine Formation, Ludlow Series, Silurian; Shropshire, England.
Table 1. Measurements (in mm) of Homotrypa cochlea sp. nov.
| |
Number of measurements |
Minimum |
Maximum |
Mean/average |
Standard deviation |
|
Maximum autozooecial diameter |
22 |
0.14 |
0.24 |
0.19 |
0.03 |
|
Minimum autozooecial diameter |
22 |
0.12 |
0.23 |
0.16 |
0.03 |
|
Diaphragms spacing in autozooecia |
48 |
0.06 |
0.15 |
0.11 |
0.03 |
|
Mesozooecia diameter |
12 |
0.07 |
0.17 |
0.12 |
0.03 |
|
Diaphragm spacing in mesozooecia |
60 |
0.07 |
0.17 |
0.11 |
0.02 |
|
Zooecial wall thickness |
21 |
0.01 |
0.05 |
0.03 |
0.01 |
Discussion
Bryozoan-gastropod interactions in the early Palaeozoic.—Bryozoan colonies originate when their planktotrophic larvae settle on a surface and undergo metamorphosis. Gastropod shells have been used as substrates by bryozoans since the Ordovician. Three types of associations between bryozoans and gastropod shell substrates can be recognised (Buttler and Taylor 2020): bryozoan colonies can grow on shells of living gastropods, on empty shells of dead gastropods, and on shells of dead gastropods housing secondary occupants or “conchicoles” (Vermeij 1987). It is usually difficult to determine unequivocally the nature of interaction in bryozoans encrusting fossil gastropod shells.
From the lower Palaeozoic, there are Ordovician and Silurian examples of bryozoans encrusting gastropod shells. Bassler (1911: figs. 27, 179) described two types of overgrowth from the Ordovician of Estonia: a thin single layer of the cystoporate Crepipora lunatifera growing on the surface of the gastropod Hormotoma insignis and a massive colony of the trepostome Hemiphragma subspheriricum completely covering a small gastropod shell. Snell (2004: pl. 3: 2b) illustrated similar massive colonies overgrowing shells. In the case of the cystoporate Favositella interpunctata, the colony grew completely over the shell and onto the surrounding substrate, revealing the basal layers of epitheca on the underside of specimens. Examples where the gastropod shell and aperture are completely covered by the bryozoan colony indicate that the mollusc was dead during at least part of the life of the bryozoan. It is however extremely difficult to ascertain if colony growth started while the mollusc was alive or if the larvae settled on an empty shell.
McNamara (1978) described interactions between trepostome bryozoan and gastropod shells from the Upper Ordovician Coniston Limestone Formation of Cumbria and inferred that the bryozoans encrusted both empty shells and the shells of mature living molluscs. The colonies interpreted as encrusting living molluscs were located at the apex of the shell and on the abapertural side.
The Silurian trepostome Orbignyella fibrosa was described by Owen (1961: pl. 14: E, F) encrusting small gastropod shells. In this example the gastropods are encrusted by a multilaminar bryozoan colony that did not grow across the shell aperture.
Morris et al. (1991) suggested that a Middle Devonian Palaeozygopleura gastropod shell, encrusted by the trepostome bryozoan Leptotrypella from the Hamilton Group of New York State, USA, was occupied by a conchicole. They recognised that the encrustation was on all sides of the shell, including the parietal lip. However, the colony was thicker on the abapertural side, suggesting a secondary occupant who dragged the shells aperture-down. They explained the thin bryozoan layer on the apertural side could be due to the secondary occupant lifting the shell from the substrate and allowing the colony to grow. A similar pattern on other Palaeozygopleura shells was recognised by Brett and Cottrell (1982) but here they were encrusted by the tabulate coral Pleurodictyum. Morris et al. (1991) suggested sipunculan worms as an example of a modern analogue for the secondary occupant.
The evidence from the specimens from Delbury Quarry indicates that the bryozoan larvae settled on the gastropods’ shells whilst the mollusc was alive. The bryozoans would have benefited from the mobile host, giving them protection from being buried and possibly elevating the zooids above turbid layers to more nutrient rich water currents (Buttler and Taylor 2020). The bryozoans continued to grow after the death of the gastropod, growing inside the aperture and developing ramose forms potentially with a secondary occupant in the shell.
Taphonomy and sedimentary history.—The typical sparsity of originally aragonitic shelly organisms in the early Palaeozoic marine fossil record, which is dominated by brachiopods and other originally calcitic shells, including the shell-encrusting bryozoans described here, has been attributed to taphonomic bias arising from early diagenetic dissolution (e.g., Cherns and Wright 2000, 2009). At Delbury, non-encrusted gastropods are lacking and other molluscs are sparse among a rich shelly fauna. While the encrusting bryozoan skeletons at Delbury are well preserved, none of the originally aragonitic gastropod shell has survived although the shell form in some is retained after inversion to calcite. Wilson et al. (2019) reported in vivo encrustation by bryozoans, bryoimmuration, leading commonly to preservation of moulds of encrusted aragonitic mollusc shells, including nautiloid cephalopods, bivalves and monoplacophorans, in the Upper Ordovician of the USA; they ascribed this to protection of vulnerable shells from seafloor dissolution in a “calcite sea” before final burial.
The high turreted gastropods from Delbury can be referred to species of the long-ranging genus Murchisonia (Ordovician–Triassic), which is characterised by a fairly central labral slit and selenizone. The selenizone profile is more prominent in the apical part of the shell than in lower, larger whorls (Fig. 6A1, B). Fairly rounded whorls may indicate species of Murchisonia (Hormatoma). Allbutt et al. (2002) recorded the encrusted gastropods at Delbury as Cyclonema corallii, which Lawson (1975: text-fig. 2) had indicated as fairly common only in the Upper Leintwardine Formation. However, this platycerid genus is ruled out not only by its squat turbiform shell without a slit but also by its bimineralic shell composition with a thin outer calcitic layer, which preserves more commonly as shell when most other gastropods are found only as moulds (e.g., Mazaev 2011). There is no indication of a calcitic outer shell layer in the material here.
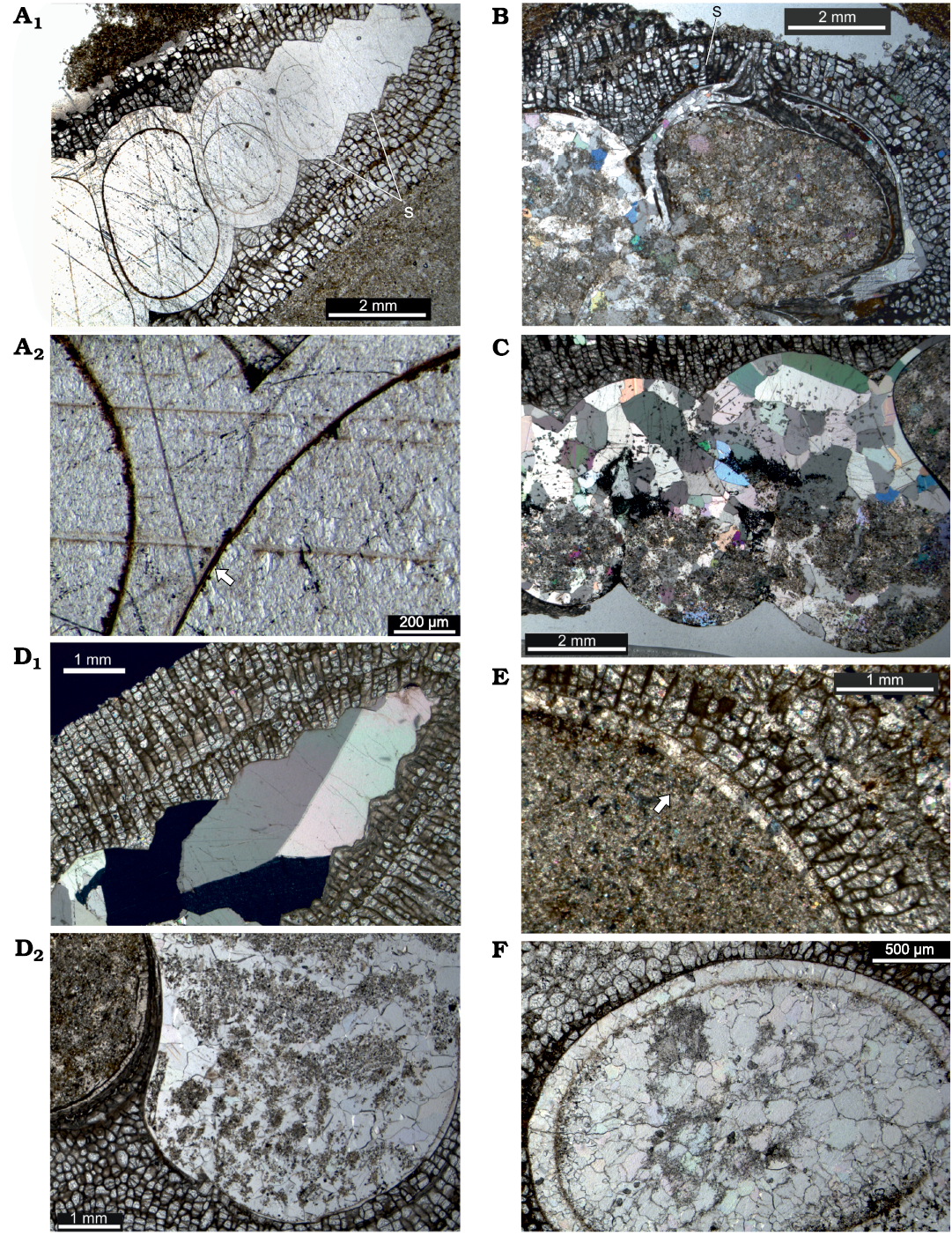
Fig. 6. Taphonomy of encrusted gastropods in longitudinal thin section from Upper Leintwardine Formation, Ludfordian, Upper Ludlow, Silurian; Delbury Quarry, Shropshire, UK. A. NMW 2019.21G.8.2, spar-filled gastropod shell, shell wall distinct only in lower whorls, with sharp outline against encrusting bryozoan, note selenizone profile more marked in upper whorls (A1), shell wall and fill of sparite continuous across inner shell wall marked by dark patchy layer but locally preserved as thin innermost shell layer (arrowed), possibly originally a nacreous lining (A2). B. NMW 2019.21G.36.2, apertural whorl of gastropod showing encrusting bryozoan entering through selenizone, shell wall recrystallized to microspar, shell fill of sediment and microspar. C. NMW 2019.21G.6.2, gastropod whorls with geopetal fill, shell recrystallized to spar and microspar, sparite crystals continuous across inner wall into sparite shell fill. D. NMW 2019.21G.5.2, direct sharp contact of encrusting bryozoan with sparite shell fill (D1), remnant shell wall reduced to very thin outer layer directly adjacent to bryozoan in parts, inner layer recrystallized to irregular microspar (D2). E. NMW 2019.21G.12.2, two layers in remnant shell wall (arrowed) adjacent to the bryozoan, shell fill of sediment. F. NMW 2019.21G.9.2, recrystallized shell wall retaining distinct inner outline marked by dark patchy organic layer, shell fill of microspar and sparite. Abbreviation: s, selenizone.
Murchisonia shells from Delbury are partially to completely encrusted externally and, although in some the bryozoan curls around the shell lip, the aperture remains clear. This supports interpretation of a symbiotic relationship in life, either with the gastropod or a subsequent shell inhabitant. The lower whorls of most shells are partially to completely infilled with sediment and microsparitic to sparitic cement. Coarse sparite is more common filling apical shell regions, although it may occupy most of the shell cavity. Geopetal fills illustrate post-depositional orientations on or in the sediment (Fig. 6C). There is no significant compaction of the gastropod shells (cf. Morris et al. 1991).
The shell exterior of gastropods from Delbury is closely moulded by the attached bryozoan. In some gastropod specimens, the bryozoans show a sharp direct contact with coarse sparry calcite crystals where the original shell outline is apparently lost (Fig. 6D1); in others irregular microspar rims a sediment-microspar shell fill (Fig. 6A1, D1). More commonly the shell has undergone inversion to calcite and there are at least remnant areas where the original inner shell outline remains distinct from the cavity fill. Completely spar-filled upper shells preserve a thin dark layer along the inner shell wall except at the apical end, even though all original shell material is lost and large sparite crystals are continuous across walls (Fig. 6A1, A2, C). The shell appears to have two layers and, from the thin remnant shell adjacent to the bryozoan in some specimens, it appears that the inner layer was more readily lost through dissolution (Fig. 6D2, E). In addition a very thin innermost shell layer was presumably a nacreous shell lining (Fig. 6A2). A thin patchy layer of organic material preserves the inner shell outline even where the shell is replaced (Fig. 6A1, A2, F). Where bryozoan growth rounds the aperture lip or enters the interior through the selenizone the enclosed shell is recrystallized to spar (also Morris et al. 1991; Fig. 6B). No specimens retain a mouldic gap between the fill and the bryozoan (McNamara 1978); equally, encrustation was directly onto the shell and not onto internal moulds following dissolution (Palmer et al. 1988).
Conclusions
The presence of gastropods in the Silurian rocks at Delbury Quarry, Shropshire, is only recorded due to their entombment by encrusting bryozoans. No aragonite is preserved in the gastropods but their recrystallised shells or the shell form are retained within the encrusting epizoan colonies. Indications are that the bryozoan larvae settled on the gastropod shells whilst the molluscs were alive and that there may have been a symbiotic relationship. After the death of the mollusc the empty shells may have been inhabited by conchicoles.
Acknowledgements
We are grateful to David Loydell (University of Portsmouth, UK) for providing us with the specimens, Brittany Evans (Nuffield Foundation placement, Aberdare Community School, Rhondda Cynon Taff, UK) for assisting with photography, Amanda Valentine-Baars (NMW) for preparing the thin sections and Mark Wilson (The College of Wooster, USA) and Andrej Ernst (University of Hamburg, Germany) for providing helpful reviews of the manuscript.
References
Allbutt, M., Moseley, J., Rayner, C., and Toghill, P. 2002. The Geology of South Shropshire. Geologists’ Association Guide 27. 116 pp. Geologists’ Association, London.
Bassler, R.S. 1906. The bryozoan fauna of the Rochester Shale. United States Geological Survey Bulletin 292: 1–137.
Bassler, R.S. 1911. The early Palaeozoic Bryozoa of the Baltic Provinces. United States National Museum Bulletin 77: 1–382.
Borg, F. 1926. Studies on Recent cyclostomatous Bryozoa. Zoologiska Bidrag från Uppsala 10: 181–507.
Brett, C.E. and Cottrell, J.F. 1982. Substrate specificity in the Devonian tabulate coral Pleurodictyum. Lethaia 15: 247–262. Crossref
Buttler, C.J. and Taylor, P.D. 2020. Review of symbioses between bryozoans and primary and secondary occupants of gastropod shells in the fossil record. In: P.J.N. Wyse Jackson and K. Zágoršek (eds.), Bryozoan Studies 2019, 11–22. Czech Geological Survey, Prague.
Cherns, L. 1999. Faunal associations of the Lower Leintwardine Formation of the Anglo-Welsh Basin. In: A.J. Boucot and J.D. Lawson (eds.), Paleocommunities: A Case Study from the Silurian and Lower Devonian, 373–379. Cambridge University Press, Cambridge.
Cherns, L. and Wright, V.P. 2000. Missing molluscs as evidence of large scale, early skeletal aragonite dissolution in a Silurian sea. Geology 28: 791–794. Crossref
Cherns, L. and Wright, V.P. 2009. Quantifying the impacts of early diagenetic aragonite dissolution on the fossil record. Palaios 24: 756–771. Crossref
Cherns, L., Cocks, L.R.M., Davies, J.R., Hillier, R.D., Waters, R.A., and Williams, M. 2006. Silurian: the influence of extensional tectonics and sea-level changes on sedimentation in the Welsh Basin and on the Midland Platform. In: P.J. Brenchley and P.F. Rawson (eds.), The Geology of England and Wales, 2nd edition, 75–102. Geological Society, London. Crossref
Ehrenberg, C.G. 1831. Symbolae Physicae, seu Icones et descptiones Corporum Naturalium novorum aut minus cognitorum, quae ex itineribus per Libyam, Aegiptum, Nubiam, Dongalaam, Syriam, Arabiam et Habessiniam, studia annis 1820–1825, redirent. Pars Zoologica, 4, Animalia Evertebrata exclusis Insectis. 10 pls. Officina Academica Berolini, Berolini.
Holland, C.H., Lawson, J.D., and Walmsley, V.G. 1963. The Silurian rocks of the Ludlow district, Shropshire. Bulletin of the British Museum, Natural History (Geology) 8: 93–171.
Lawson, J.D. 1975. Ludlow benthonic assemblages. Palaeontology 18: 509–525.
McNamara, K.J. 1978. Symbiosis between gastropods and bryozoans in the late Ordovician of Cumbria, England. Lethaia 11: 25–40. Crossref
Ma, J.-Y., Buttler, C.J., and Taylor, P.D. 2014, Cladistic analysis of the “trepostome” Suborder Esthonioporina and the systematics of Palaeozoic bryozoans. Studi Trentini di Scienze Naturali 94: 153–161.
Mägdefrau, K. 1932. Über einige Bohrgänge aus dem Unteren Muschelkalk von Jena: Paläontologische Zeitschrift14: 150–160. Crossref
Mazaev, A.V. 2011. Pennsylvanian gastropods of the suborders Murchisoniina Cox et Knight, 1960 and Sinuspirina Mazaev subordo nov. from the central regions of the Russian Platform: Morphology, taxonomy, and phylogeny. Paleontological Journal 45: 1533–1599. Crossref
Morris, P.J., Linsley, R.M., and Cottrell, J.F. 1991. A Middle Devonian symbiotic relationship involving a gastropod, a trepostomatous bryozoan, and an inferred secondary occupant. Lethaia 24: 55–67. Crossref
Nicholson, H.A. 1881. On the structure and affinities of the genus Monticulipora and its subgenera with critical descriptions of illustrative species. 235 pp. William Blackwood and Sons, Edinburgh. Crossref
Owen, D.E. 1961. On the species Orbignyella fibrosa (Lonsdale). Geological Magazine 98: 230–234. Crossref
Owen, D.E. 1962. Ludlovian Bryozoa from the Ludlow district. Palaeontology 5: 195–212.
Palmer, T.J., Hudson, J.D., and Wilson, M.A. 1988. Palaeoecological evidence for early aragonite dissolution in ancient calcite seas. Nature 335: 809–810. Crossref
Ross, J.R.P. 1965. Homotrypa and Amplexopora? from the Caradoc series, Shropshire. Palaeontology 8: 5–10.
Snell, J. 2004. Bryozoa from the Much Wenlock Limestone (Silurian) Formation of the West Midlands and Welsh Borderland. Monograph of the Palaeontographical Society 157: 1–136. Crossref
Taylor, P.D. 1994. Evolutionary palaeoecology of the symbioses between bryozoans and hermit crabs. Historical Biology 9: 157–205. Crossref
Ulrich, E.O. 1882. American Palaeozoic Bryozoa. The Journal of the Cincinnati Society of Natural History 5: 121–175, 233–257.
Vermeij, G.J. 1987. Evolution and Escalation. 527 pp. Princeton University Press, Princeton.
Wilson, M.A. and Palmer, T.J. 2006. Patterns and processes in the Ordovician bioerosion revolution. Ichnos 13: 109–112. Crossre
Wilson, M.A., Buttler, C.J., and Taylor, P.D. 2019. Bryozoans as taphonomic engineers, with examples from the Upper Ordovician (Katian) of Midwestern North America. Lethaia 52: 403–409. Crossref
Acta Palaeontol. Pol. 67 (3): 569–577, 2022
https://doi.org/10.4202/app.00964.2021