

A glimpse into ancient food storage: Sequestrichnia and associated nucleocave Chondrites from Eocene deep-sea deposits
JAROSLAV ŠAMÁNEK, LOTHAR H. VALLON, RADEK MIKULÁŠ, and MICHAL VACHEK
Šamánek, J., Vallon, L.H., Mikuláš, R., and Vachek, M. 2022. A glimpse into ancient food storage: Sequestrichnia and associated nucleocave Chondrites from Eocene deep-sea deposits. Acta Palaeontologica Polonica 67 (3): 767–779.
The old brickyard Velká nad Veličkou in the Czech Republic offers a unique insight into an Eocene deep-sea ichnoassemblage that is dominated by sequestrichnia. Secondary use of such food-stowing was made by the tracemaker of Chondrites intricatus as evidenced by its frequent penetration of the trace fossil Zoophycos brianteus and its occurrence preferentially on the surface of Zoophycos spreiten. Here, the Chondrites tracemaker presumably exploited the nutrient-enriched micro-environment (nucleocave). This close interrelations between Chondrites and Zoophycos support the hypothesis of a sequestrichnial behaviour of the Zoophycos producer. The studied locality also yields Helminthopsis tenuis, Tubulichnium mediterranensis, Scolicia strozzii, ?Dactyloidites isp., Megagrapton irregulare, and Planolites isp.
Key words: Zoophycos, trace fossils, deep-marine, flysch, Bílé Karpaty Unit, Outer Western Carpathians, Czech Republic.
Jaroslav Šamánek [samanek.j@mail.muni.cz], Department of Geological Sciences, Faculty of Science, Masaryk University, Kotlářská 267/2, 611 37 Brno, Czech Republic.
Lothar H. Vallon [kv@oesm.dk], Geomuseum Faxe, Østsjællands Museum, Rådhusvej 2, DK-4640 Faxe, Denmark.
Radek Mikuláš [mikulas@gli.cas.cz], Institute of Geology of the Czech Academy of Sciences, Rozvojová 269, 165 02 Praha 6, Czech Republic.
Michal Vachek [michal.vachek66@gmail.com], State Land Office, Bratislavská 1/6, 695 01 Hodonín, Czech Republic.
Received 16 December 2021, accepted 11 April 2022, available online 8 September 2022.
Copyright © 2022 J. Šamánek et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
At first glance, deep-tier trace fossils such as various ichnospecies of Zoophycos, Chondrites, Spirophyton, Polykampton, and Pilichnus reflect a feeding behaviour. On closer inspection, however, the ethology of these trace fossil makers becomes more complicated as frequently discussed in recent years. A thorough investigation led Uchman and Wetzel (2016) to introduce a new ethological group—sequestrichnia or food-storage or stowing traces. Stowing behaviour is predominantly applied by tracemakers in deep-sea settings, where food availability fluctuates seasonally or episodically. When highly nutritious material is deposited, the tracemakers collect it on the sediment surface and stow it in deep-tiers where shallow deposit feeders cannot reach it and where little- or not oxygenated porewater favours the preservation of organic matter (Uchman and Wetzel 2016; for shallower environments see also Bromley 1996: 100–101, fig. 4.25; Seilacher 2007: 54, pl. 18). Sequestration was further analysed in the ichnogenera Polykampton (Uchman and Rattazzi 2017; Uchman et al. 2019, 2020) and Tubulichnium (Uchman and Wetzel 2017). Additionally, Jurkowska et al. (2018) discussed food storage in Lepidenteron mantelli and Rodríguez-Tovar et al. (2019) in the ichnogenus Halimedides.
The trace fossil Chondrites records a wide variety of behaviour of its producers, living in anoxic (Bromley and Ekdale 1984) or oxic settings (Wetzel and Uchman 2012), showing either a deposit feeding, farming, or chemosymbiotic behaviour (e.g., Fu 1991, Baucon et al. 2020). In particular, deep-tier Chondrites ispp. appear to follow a chemosymbiotic strategy that can be utilised in order to identify trace fossils applying stowing behaviour (sequestrichnia), which became reworked by the Chondrites-producer. In this context, the Velká nad Veličkou locality (Eocene of the Carpathian Flysch Belt, Czech Republic) where Chondrites and supposed sequestrichnial ichnogenera occur in close relationship within a specific bed is of particular interest. The aim of the present paper is to describe this distinct ichnoassemblage in which both food-storage and chemosymbiotic behaviours were applied in dependence upon each other.
Institutional abbreviations.—ICN, Natural History Museum of Verona, Italy; MZM, Moravian Museum, Brno, Czech Republic; Z, City Museum in Strážnice, Czech Republic.
Geological setting
The old brickyard Velká nad Veličkou (formerly known as the Javorník brickyard; 48°51’41.067”N, 17°31’4.278”E) is located near the Javorník nad Veličkou railway station in the south-eastern part of Moravia (Czech Republic), close to the border with the Slovak Republic (Fig. 1). The quarrying ceased in 1993 and since 2013 the locality is protected by law as a natural monument.
The outcrop is the stratotype of the Kuželov Formation of the Hluk Facies of the Bílé Karpaty Unit (Stráník et al. 1995). The Kuželov Formation is dominated by greenish, brownish, and reddish foliated claystone with intercalated mainly thin-bedded, laminated sandstone beds. Strongly lithified marl beds (in Czech “pelokarbonát”) are a diagnostic feature of the Kuželov Formation. The formation is about 250 m thick and represents sediments deposited in a distal part of a deep-sea turbidity fan from the late Paleocene to the early Eocene (Stráník et al. 1995). From the studied locality, Bubík (1995) described an assemblage of agglutinated foraminifera that is dominated by tubular forms indicative for depths of the lower slope. Index fossil for the Planktonic Foraminifera Zone P6 and the Nannoplankton Zone NP10, i.e., Rheophax elongatus provided an early Eocene age (see Bubík 1995; Stráník et al. 1995). The Kuželov Formation is underlain by the Nivnice Formation or Paleocene–Eocene variegated beds (Stráník et al. 1995). There are neither indications of shallowing during the sedimentation of the Kuželov Formation nor of discontinuous sedimentation. The overlying sediments have been eroded (Bubík 1995).
So far, the only ichnological study of the outcrop was a brief mention in an unpublished Ph.D. thesis by Němcová (1973) written in Czech; she described Rhabdoglyphus grossheimi and a new, but invalid ichnospecies, Spirophycus ornatus. Although she did not mention the exact collection point, the specimens must derive from a more sandy part of the quarry considering the lithology of the depicted samples.
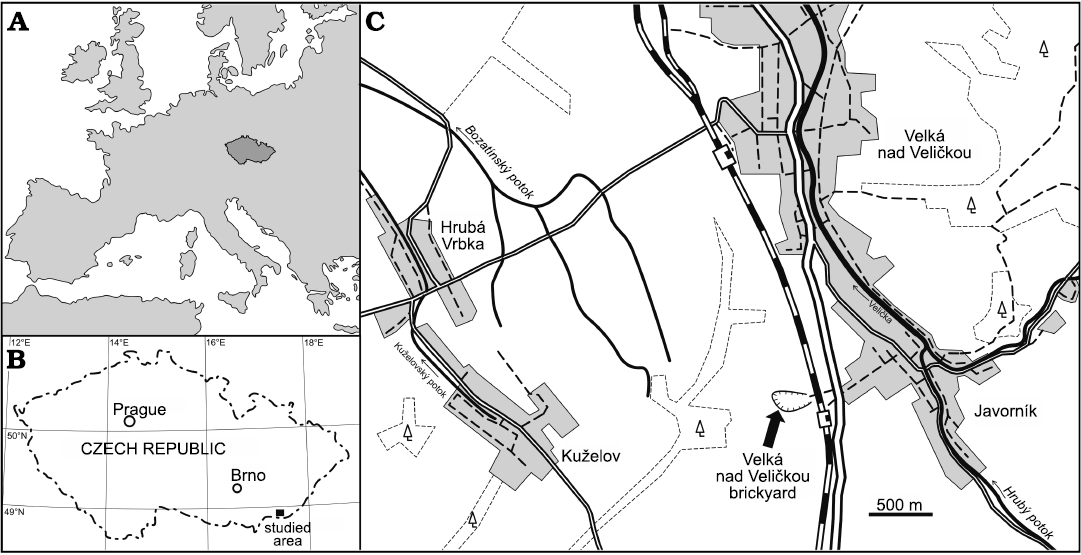
Fig. 1. Location of the studied area in Europe (A) and the Czech Republic (B). Schematic map of the studied locality (C).
Material and methods
The studied material was collected exclusively at the old brickyard Velká nad Veličkou. Most of the trace fossils come from a 170 mm thick, moderately bioturbated bed (further referred to as Zoophycos Bed) in the southern part of the outcrop (Fig. 2). The strata are tectonically tilted and inclined at an angle of 45°. The Zoophycos Bed yields trace fossils mostly preserved in full relief within the strongly lithified marl. Their infill derives from an overlying 50 mm thick siltstone layer. Thus, we may presume that this siltstone bed was deposited before (or during) the production of the observed trace fossils.
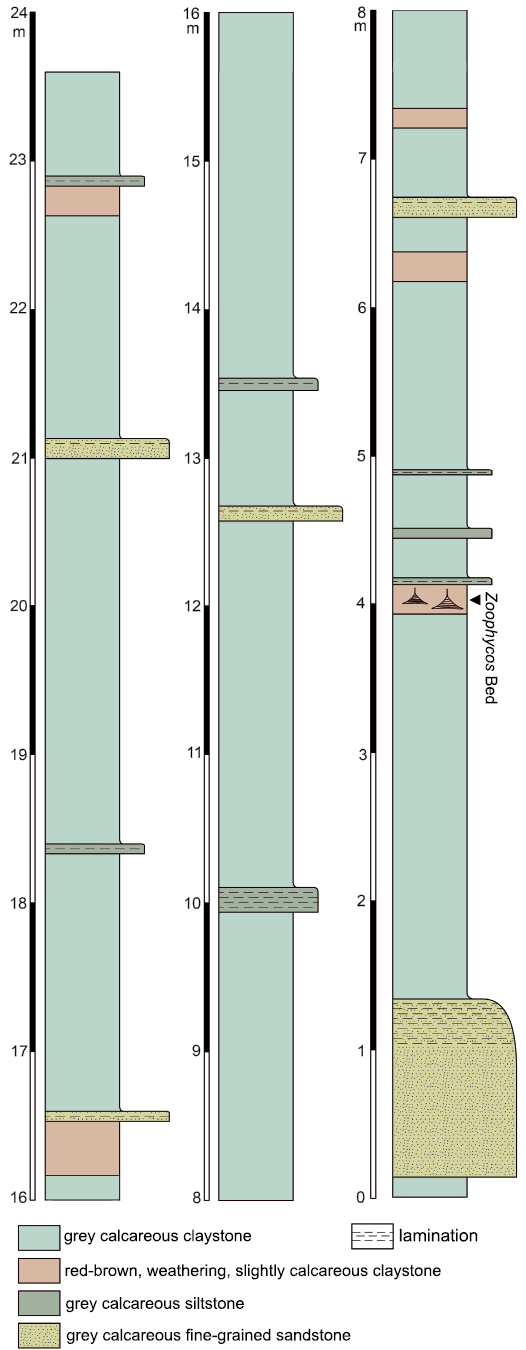
Fig. 2. Stratigraphic position of the Zoophycos Bed in the southern part of the studied locality.
Additional trace fossil material was collected from the talus. These trace fossils do not display the unique preservational features (see below) as the specimens in the Zoophycos Bed and are derived from other strata.
Measurements introduced by Wetzel and Bromley (1996) were used for Helminthopsis tenuis.
The herein described specimens are housed in the collection of the Moravian Museum, Brno, Czech Republic (MZM) under the inventory numbers Ge 32946-32961 and the City Museum in Strážnice, Czech Republic under the inventory number Z 69.
Systematic palaeoichnology
The ichnogenera are arranged according to the morphological system by Buatois et al. (2017).
Simple horizontal trails
Ichnogenus Helminthopsis Wetzel and Bromley, 1996
Type ichnospecies: Helminthopsis hieroglyphica Wetzel and Bromley, 1996, Ganei, Switzerland, Paleocene–lower Eocene.
Helminthopsis tenuis Książkiewicz, 1968
Fig. 3.
Material.—Single large specimen on a sandstone slab, collected from talus (MZM Ge 32946) from Velká nad Veličkou, Czech Republic, Eocene.
Description.—Incomplete specimen of an unbranched, horizontal burrow with a fairly constant diameter of about 10 mm and a somewhat rectangular cross-section. Preserved as convex hyporelief with a passive, structureless fill (no pellets were observed). Burrow segments are rarely straight and often slightly wiggly. Two meanders, one wide and one narrow, are preserved. The broad meander is 120 mm wide and 86.6 mmm high, the narrow one measures 61.3 mm in width and 80 mm in height. The burrow possesses a partially preserved median furrow.

Fig. 3. Simple horizontal trail Helminthopsis tenuis Książkiewicz, 1968, MZM Ge 32946, from Velká nad Veličkou, Czech Republic, Eocene.
Remarks.—Wetzel and Bromley (1996) interpreted Helminthopsis as feeding burrows constructed in shallow depths of sediments probably rich in benthic food. Although collected from talus, the trace fossil might therefore have been constructed within a layer that was deposited in times of nutrition-rich sedimentation. Thus, it might have been constructed by a shallow tier tracemaker that the producers of sequestrichnia aimed to avoid (cf. Uchman and Wetzel 2016).
Stratigraphic and geographic range.—Ediacaran to Recent (Buatois et al. 2014; Kundal et al. 2022); worldwide.
Simple actively filled (massive) horizontal to oblique structures
Ichnogenus Planolites Nicholson, 1873
Type ichnospecies: Planolites beverleyensis (Billings, 1862), near Beverley in the township of Bastard, Ontario, Canada, Potsdam Sandstone, Cambrian.
Planolites isp.
Material.—Six specimens on 2 samples collected from the siltstone layer overlying the Zoophycos Bed (MZM Ge 32948) from Velká nad Veličkou, Czech Republic, Eocene.
Description.—Near-surface, unbranched, cylindrical burrows with a smooth wall and no lining, preserved as positive hyporelief. Course of burrow generally horizontal but undulating vertically. Occurring in two different size ranges; the smaller one is about 1 mm wide and preserved over a length of about 30 mm, the larger type has a constant width of about 5 mm and is preserved for lengths between 30 to 80 mm. The latter size variant is more common.
Simple, actively filled (pelletoidal) horizontal to oblique structures
Ichnogenus Tubulichnium Książkiewicz, 1977
Type ichnospecies: Tubulichnium incertum Książkiewicz, 1977, Gurnigel Nappe in the Fribourgian Alps, Switzerland, Maastrichtian–lower Palaeogene.
Remarks.—Originally described as Tubotomaculum García-Ramos, Mángano, Buatois, and Rodríguez-Tovar, 2014, the ichnogenus was considered a junior synonym of Tubulichnium Książkiewicz, 1977, by Uchman and Wetzel (2017) and its only ichnospecies Tubotomaculum mediterranensis García-Ramos, Mángano, Buatois, and Rodríguez-Tovar, 2014, was transferred to Tubulichnium by Uchman and Wetzel (2017).
Tubulichnium mediterranensis (García-Ramos, Mángano, Buatois, and Rodríguez-Tovar, 2014)
Fig. 4.
Material.—In the field, about 200 observed, usually partially preserved specimens, derived from the Zoophycos Bed; 113 collected (owing to the large number of specimens, inventorying was done in lots: MZM Ge 32958 (figured herein), MZM Ge 32959 (not figured) from Velká nad Veličkou, Czech Republic, Eocene.
Description.—All Tubulichnium mediterranensis specimens were observed in mid-tier position within the Zoophycos Bed as horizontal, unbranched burrows with no obvious connection to the surface. The burrows have a fairly straight to slightly winding course and blind endings. The diameter of the burrows is fairly constant in the range of 4.4 to 13.5 mm, but irregular swellings occur along the burrow. The burrows are lined with elongated, small, 1 mm long and 0.4 mm wide, silty pellets consisting of material derived from the overlying siltstone layer. The maximal preserved length is 60 mm. Larger burrows of probably higher ichnogenic stages display a spindle-like morphology and often retrusive, vertical spreiten with oblique lamellae. Burrows are often colour-enhanced by manganese- or/and iron-rich oxides.
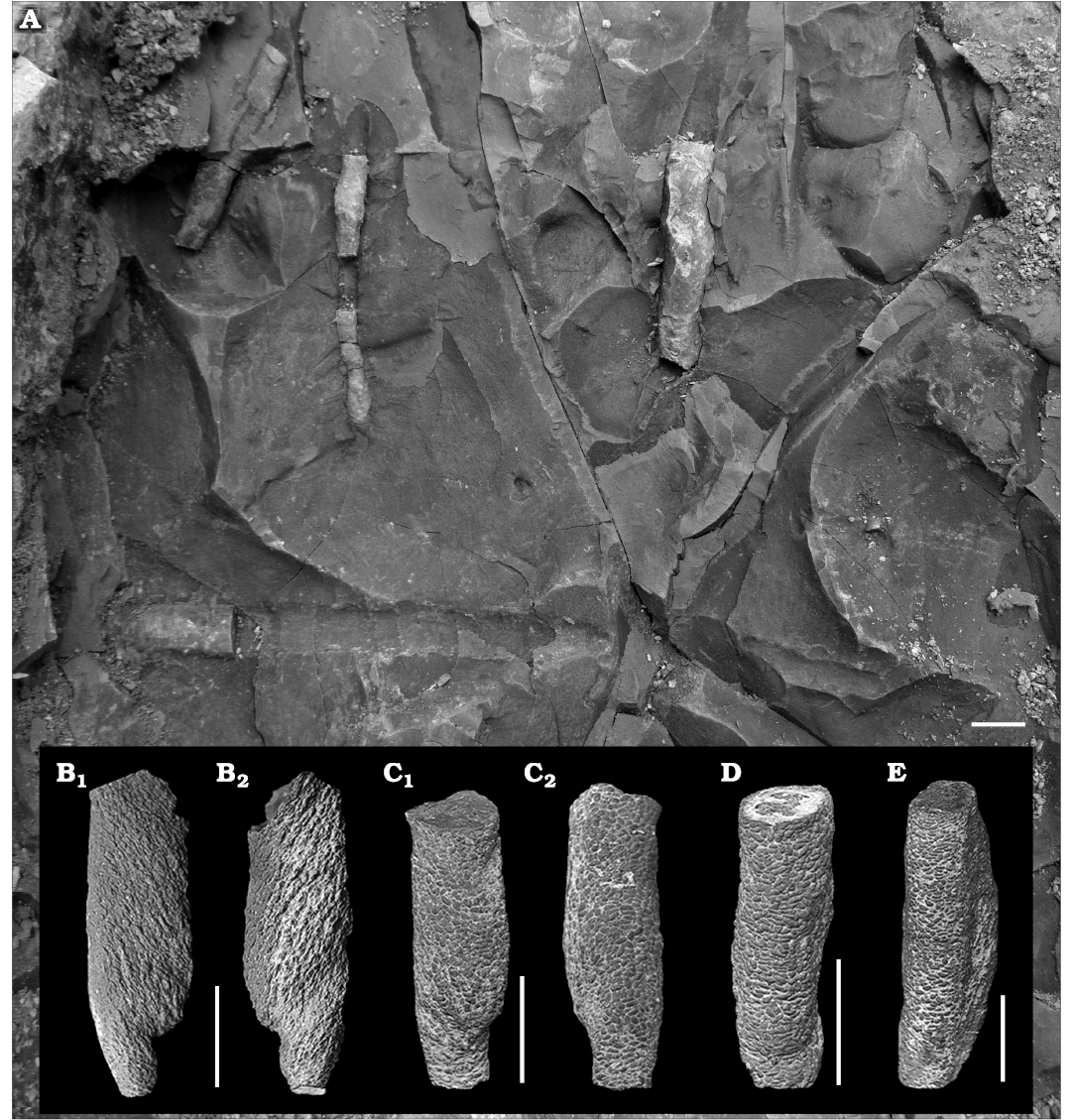
Fig. 4. Simple, actively filled (pelletoidal) horizontal to oblique structure Tubulichnium mediterranensis (García-Ramos, Mángano, Buatois, and Rodríguez-Tovar, 2014), from Velká nad Veličkou, Czech Republic, Eocene. A. Field photo of in situ specimens. B–E. MZM Ge 32958, partially preserved burrows with clearly visible spindle-like shape and pelletal infill. Same specimens from different angles (B1 and B2; C1 and C2). Scale bars 10 mm.
Remarks.—Tubulichnium mediterranensis is relatively common in Cretaceous to Eocene strata in the Mediterranean and occurs according to García-Ramos et al. (2014) often in association with Rotundusichnium zumayensis Plička, 1989, Zoophycos isp., Chondrites isp., Gyrophyllites isp., and Stelloglyphus llicoensis Le Roux, Nielsen, and Henríquez, 2008. Other occurrences are restricted to the Miocene of Borneo (Seilacher 2007; García-Ramos et al. 2014). Tubulichnium was interpreted by Uchman and Wetzel (2017) to represent a sequestrichnion.
Stratigraphic and geographic range.—Upper Cretaceous to Miocene (García-Ramos et al. 2014); probably worldwide.
Complex actively filled horizontal structures
Ichnogenus Scolicia de Quatrefages, 1849
Type ichnospecies: Scolicia prisca Quatrefages, 1849, Donostia-San-Sebastián, Guipuscoa, Spain, Paleogene.
Scolicia strozzii (Savi and Meneghini, 1850)
Fig. 5.
Material.—Single specimen collected in the talus (MZM Ge 32949) from Velká nad Veličkou, Czech Republic, Eocene.
Description.—Only a small section of a guided meander is preserved as bilobate positive hyporelief with a median groove. The burrow is 40 mm wide and each of the two limbs has a length of about 95 mm. Most of the original internal structures have been destroyed by small indetermined burrows.

Fig. 5. Complex actively filled horizontal structure Scolicia strozzii (Savi and Meneghini, 1850) MZM Ge 32949, from Velká nad Veličkou, Czech Republic, Eocene.
Stratigraphic and geographic range.—Tithonian to Recent (Tchoumatchenco and Uchman 2001); probably worldwide.
Radial to rosette structures
Ichnogenus Dactyloidites Hall, 1866
Type ichnospecies: Dactyloidites asteroides (Fitch, 1850), New York, USA, Granville Formation, Cambrian.
Remarks.—Dactyloidites is an ichnogenus that deserves further investigations. It was synonymized with Haentzschelinia Vialov, 1964, and with Brooksella Walcott, 1896 (Fürsich and Bromley 1985; Pickerill et al. 1993; Wilmsen and Niebuhr 2014; Boyd and McIlroy 2016). Brooksella was later re-described as a body fossil by Ciampaglio et al. (2006). Wilmsen and Niebuhr (2014) revisited the type material of Dactyloidites ottoi and discussed the synonymisation of Haentzschelinia and Dactyloidites. They concluded (in accordance with Fürsich and Bromley 1985) that there is no valid argument to use Haentzschelinia as separate ichnogenus. However, Haentzschelinia is still retained as separate ichnogenus by some authors and since its defence by Vialov (1989), Haentzschelinia was mainly used for more radial forms (e.g., Belaústegui et al. 2015; Muñoz et al. 2019) and for post-Palaeozoic forms, although a certain time range is not regarded as ichnotaxobase. Recently, Boyd and McIlroy (2016) described a new ichnospecies of Dactyloidites and emended the diagnosis of this ichnogenus.
?Dactyloidites isp.
Fig. 6.
Material.—Eight incomplete traces (MZM Ge 32950–32957) from the Zoophycos Bed, about 30 more observed in the field, from Velká nad Veličkou, Czech Republic, Eocene. In average, 10–15 specimens occur on one square metre, usually arranged in groups with individual burrows about 50–100 mm apart.
Description.—Burrow system with numerous cylindrical galleries radiating from a centre creating radial or fan-like structures up to 70 mm in diameter. Individual tunnels have a constant diameter of about 1 mm. They are commonly unbranched and rarely bifurcating at acute angles near their blind tapering endings. The individual J-shaped tunnels usually form multiple layers and are not lined or coated with pellets. The burrows are circular in cross-section and do not overlap. Unlike the specimens that are usually referred to Dactyloidites, the central axis of the observed specimens consists of multiple, steeply inclined to subvertical shafts; the parts of shafts connecting the galleries with the surface were not observed.

Fig. 6. Radial to rosette structures ?Dactyloidites isp., from Velká nad Veličkou, Czech Republic, Eocene. A–C. Field photos of in situ specimen (indicated by arrows, A), close ups (B, C).
Remarks.—Species of at least four different ichnogenera are similar to the studied material: Asterosoma Otto, 1854, Phycodes Richter, 1850, Phymatoderma Brongniart, 1849, and Dactyloidites Hall, 1886. However, none of the first three fits perfectly. Species of Asterosoma have a concentrically layered wall around a passively filled lumen. Some of our more fan-like specimens resemble Phycodes auduni Dam, 1990, or Phycodes bromleyi Dam, 1990. However, the ichnospecies of Phycodes are best restricted to burrows having a (sub-)quadrate cross-section (Seilacher 2000). Ichnospecies of Phymatoderma are irregularly branched, and pellets frequently occur (e.g., Izumi and Yoshizawa 2016). Multiple shafts are not found in any ichnospecies of Dactyloidites. Nevertheless, the defined ichnotaxobases for Dactyloidites matches the studied material best. The ichnogenus Dactyloidites comprises five valid ichnospecies: Dactyloidites ottoi (Geinitz, 1849), Dactyloidites asteroides Fitch, 1850, and Dactyloidites cabanasi (Meléndez in Cabanás, 1966), exhibit simple, less radiating morphotypes, whereas Dactyloidites peniculus D’Alessandro and Bromley, 1986, and Dactyloidites jordii Boyd and McIlroy, 2016, represent more complex radiating burrows with multiple layers of tunnels that sometimes are overlapping.
The studied material of ?Dactyloidites isp. from the Velká nad Veličkou shares similarities with more densely radiating ichnospecies. Dactyloidites peniculus D’Alessandro and Bromley, 1986, occurs as a radial pile or brush-like structure. The studied material is distinguishable from D. peniculus by presence of dichotomous branching tunnels running from the central shaft without lining or pelleted structure. Dactyloidites jordii Boyd and McIlroy, 2016, has a bilateral symmetry, exhibits multiple orders of branching, and the burrows display an n-shaped cross-section. In contrast, the studied material has radial or bilateral symmetry (in the case of fan-like morphotypes), exhibits only first order branching, and the cross-section of tunnels is circular. Furthermore, it also shares similarities with D. ottoi (Haentzschelinia ottoi sensu Seilacher 2008) such as dichotomous branching of tunnels and a fan to radial outline.
Burrows with helicoidal spreiten
Ichnogenus Zoophycos Massalongo, 1855
Type ichnospecies: Zoophycos brianteus Massalongo, 1855, Livorno, Tuscany (Italy), Upper Cretaceous.
Zoophycos brianteus Massalongo, 1855
Fig. 7A1.
Material.—Three nearly complete specimens from the Zoophycos Bed (MZM Ge 32960, MZM Ge 32961; Z 69) from Velká nad Veličkou, Czech Republic, Eocene.
Description.—The three studied, almost complete specimens exhibit the typical spreite of Zoophycos helically coiled around a vertical axis. Both sinistral and dextral helices were observed, and the structures were constructed from top to bottom. The spirals’ diameters increase downward. The first has a nearly circular outline, the second is rather oval, and the last one is slightly lobate. The largest diameters of the three specimens measure 139 mm, 167 mm, and 225 mm respectively. They penetrate from the top of the Zoophycos Bed for about 150 mm with a total height of the Zoophycos spreite whorls of about 40 mm each. In vertical sections, each spreite-whorl is bent concave upwards with both limits (marginal tunnel and contact at the centre) being roughly at the same level. The thickness of the spreite increases from 2.8 mm at the top whorl to up to 6.4 mm as a maximum. This maximal thickness is already reached about halfway through the burrow’s vertical extension. From there on, the spreite thickness does not increase further. An inner, not bioturbated area with a subcircular cross-section is present having a diameter 7.6–8.5 mm. A central shaft has not been observed. In all specimens, the marginal (unlined) tunnel is well developed as well as primary and secondary lamellae of the spreite. The maximal distance between two subsequent primary lamellae increases from the top to the bottom of the helix. On the surfaces of the whorls, faecal pellets have been observed. They have an elongate ellipsoidal to slightly ovoid or capsule-like shape. Their size is fairly constant in all specimens; ranging 0.8–1.4 mm in length and 0.4–0.5 mm in width. With depth protrusion, these faecal pellets increase in size.
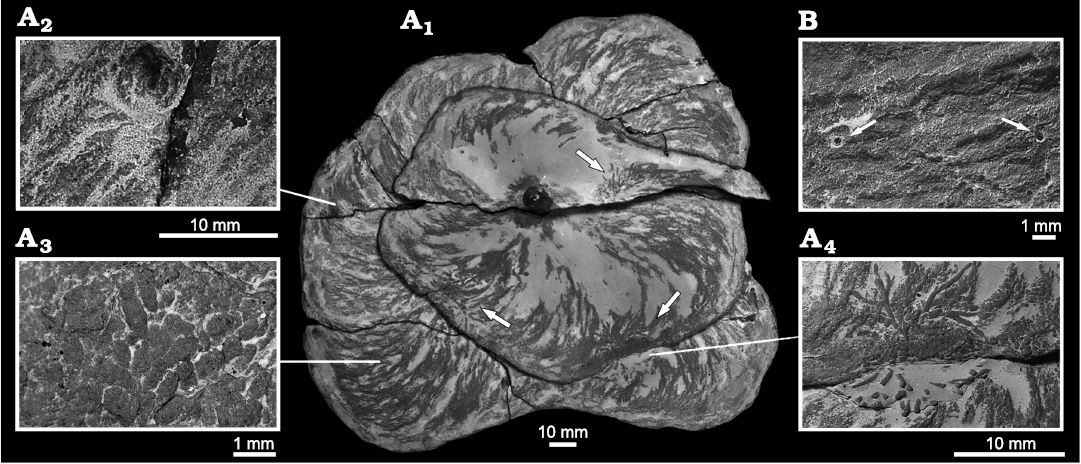
Fig. 7. Zoophycos brianteus Massalongo, 1855, with Chondrites intricatus (Brongniart, 1823). A. MZM Ge 32960, from Velká nad Veličkou, Czech Republic, Eocene. A1, bottom two coils of Z. brianteus, arrows mark specimens of Ch. intricatus; A2, cylindrical structure penetrating a Z. brianteus spreite; A3, pellets on the surface of the spreite; A4, Ch. intricatus on the surface of a spreite. B. MZM Ge 32961, from Velká nad Veličkou, Eocene, minute shafts cross-cutting a Z. brianteus spreite, note the diagenetic halo around the shafts.
Remarks.—Olivero (2007) redescribed the specimens of the three proposed Zoophycos-species by Massalongo, 1855. He selected lectotypes and a new type ichnospecies of Zoophycos since the originally designated type ichnospecies constitutes rather a fossil alga than a trace fossil (Olivero 2007). The studied specimens match with the redescription of Zoophycos brianteus by Olivero (2007).
In general, two ways of construction exist in Zoophycos, from top to bottom or from bottom to top. This can be inferred by the convex side of the lamellae within the spreite (Olivero and Gaillard 1996). According to the findings by Olivero and Gaillard (1996, 2007), the bottom to top mode of building seems to be more common but the (lecto-)type material of Zoophycos brianteus (ICN1, ICN2, ICN3, ICN4) seems to be constructed from top to bottom (see Olivero 2007).
For the studied specimens, ichnogenetic stages (Belaústegui et al. 2016) are transitional but a growth of the tracemaker during construction can be inferred owing to increases of spreite thickness, diameter of the whorls, pellet size, dimensions of the marginal tunnel etc. (cf. Kotake 1989). The tracemaker seems to have reached adult size before the structure was completed.
All specimens exhibit a continuous spreite with mixture of silty material and pellets (Fig. 7A3) indicating an active downward-movement of overlying more nutritious material into the nutrition-poor Zoophycos Bed. The deposition of faecal pellets within the structure points towards a sequestration behaviour (cf. Uchman and Wetzel 2016, 2017). The tracemaker purposely stowed material in order to either re-digest it or to utilise it indirectly by consuming microbes cultivated on it or their processed compounds. The same behaviour by a Recent Zoophycos-tracemaker was observed by Wetzel et al. (2011).
Some individual coils of the largest specimen are cross-cut by younger shafts of an undetermined trace fossils with a diameter up to 10 mm (Fig. 7A2). On the spreite surfaces, several specimens of Chondrites intricatus (Fig. 7A4) and minute circular shafts (diameter less than 1 mm) occur (Fig. 7B).
Stratigraphic and geographic range—Lower Permian to Recent (Hu et al. 2010); worldwide.
Burrows with shaft or bunch with downwards radiating probes
Ichnogenus Chondrites Sternberg, 1833
Type ichnospecies: Chondrites targionii (Brongniart, 1828), locality and age unknown.
Chondrites intricatus (Brongniart, 1823)
Fig. 7A4.
Material.—12 specimens on the surface of the above mentioned Zoophycos brianteus specimens (MZM Ge 32960, MZM Ge 32961; Z 69), 3 isolated specimens observed in sediment (collected in situ from the Zoophycos Bed) from Velká nad Veličkou, Czech Republic, Eocene.
Description.—Regularly branching, small, dendritic burrow systems covering an area of up to 90 mm2, with numerous minute branches, less than 1 mm in diameter, usually preserved in close contact with both upper and lower surfaces of Zoophycos spreite/whorls; isolated occurrence of Chondrites intricatus in the surrounding claystone is rare.
Remarks.—All above described Zoophycos brianteus specimens are pierced by minute vertical shafts filled with pyrite having a diagenetic halo (Fig. 7B). The diameter of these shafts is in the same size-range as the diameter of the individual branches of Chrondrites intricatus (less than 1 mm). Thus, these shafts may represent connections of the branched section of Chondrites with the ancient water-sediment interface.
Stratigraphic and geographic range—Lower Silurian (Rindsberg and Chowns 1986) to Recent; worldwide.
Regular to irregular network graphoglyptids
Ichnogenus Megagrapton Książkiewicz, 1968
Type ichnospecies: Megagrapton irregulare Książkiewicz, 1968, Berest near Grybów, Beloveža Beds, Poland, lower Eocene.
Megagrapton irregulare Książkiewicz, 1968
Fig. 8.
Material.—Single incomplete specimen (MZM Ge 32947) collected from the talus, preserved as positive hyporelief from Velká nad Veličkou, Czech Republic, Eocene.
Description.—Trace fossil commonly preserved as hypichnial irregular nets. Megagrapton with meshes bordered by only slightly winding strings, which commonly branch at approximately right angles. The incomplete specimen is confined by the edges of the slab and has a length of 150 mm and a width of 60 mm with a tunnel diameter of 1 mm. It consists of an irregular network of winding galleries, which branch at approximately 90°. Branching occurs only on the convex sides of the winding galleries.
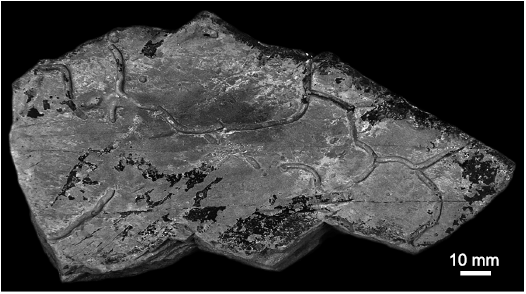
Fig. 8. Regular to irregular network graphoglyptid Megagrapton irregulare Książkiewicz, 1968, MZM Ge 32947, from Velká nad Veličkou, Czech Republic, Eocene.
Remarks.—Megagrapton irregulare occurs from Silurian (Crimes and Crossley 1991) to Miocene (D’Alessandro 1982) in deep-sea, mainly flysch deposits. For more in-depth discussion on Megagrapton, see Uchman (1998).
Stratigraphic and geographic range—Lower–Middle Ordovician to Miocene (Uchman et al. 2005); worldwide.
Discussion
The studied trace fossils from the old brickyard Velká nad Veličkou belong to the Zoophycos ichnofacies and represent a deep-sea flysch ichnoassemblage (see also Ekdale and Bromley 1984) confirming previous bathymetric interpretations of a lower slope setting based on agglutinated foraminifera (Bubík 1995). It is dominated by Zoophycos brianteus, Chondrites intricatus, ?Dactyloidites isp., and Tubulichnium mediterranensis. The studied Zoophycos Bed (Fig. 9) yielded mid- to deep-tier trace fossils and most tracemakers applied food-stowing behaviour (sequestrichnia). Shallow or surface traces are preserved in the overlying siltstone layer and are represented by Planolites isp. The interrelationships of these near-surface burrows and the trace fossils in the Zoophycos Bed are difficult to evaluate because no cross-cuts or avoidance behaviour could be observed. Given the fact that these are near-surface burrows and most of the trace fossils of the Zoophycos Bed are mid- to deep tier burrows, it can be assumed that all these traces were made simultaneously. The upper mid-tier ichnosuite is represented by T. mediterranensis and the lower mid-tier by ?Dactyloidites isp. Finally, the deepest tier is represented by Chondrites intricatus and Zoophycos brianteus, the biggest Zoophycos, however, already has its first whorl in the lower mid-tier. Considering that the Z. brianteus whorls were cross-cut by minute vertical shafts and intricate networks of Chondrites intricatus, the latter were the last constructed ichnotaxa in the ichnoassemblage.
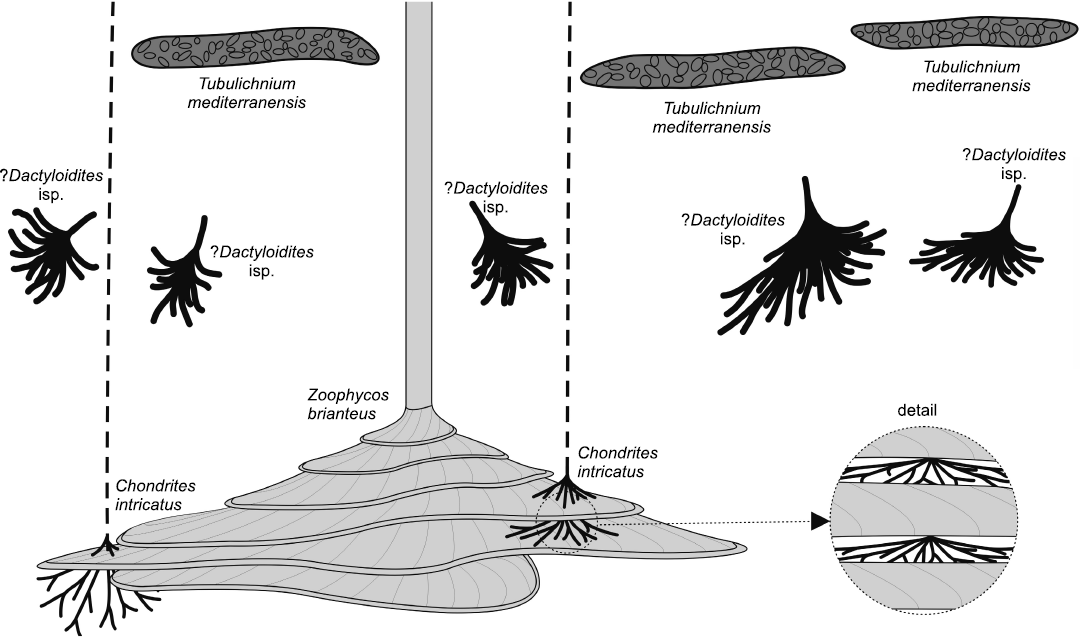
Fig. 9. Tiering of the Zoophycos Bed at Velká nad Veličkou, Czech Republic, Eocene. Upper tier dominated by Tubulichnium mediterranensis, mid-tier dominated by ?Dactyloidites isp., lower tier dominated by Zoophycos brianteus and interacting nucleocave Chondrites intricatus (enlargement).
When proposing the new ethological category “sequestrichnia” for stowing traces, Uchman and Wetzel (2016) listed common morphologies for trace fossils belonging to this ethological category. They also described the strategies applied by the tracemakers in order to stow food items. For the studied Z. brianteus specimens, most of the described strategies can be recognized: (i) The trace fossils are embedded within an organic-poor sediment (the Zoophycos Bed), which is overlain by an organic-rich layer (siltstone); (ii) The traces within the Zoophycos Bed are actively filled with material originating from the organic-rich stratum above and this material was transferred deep below the water sediment-interface keeping it out of reach of the shallower living bioturbators and probably also to preserve it in an anoxic environment. In the studied case, this fill is deposited as pellets and within the spreiten (compare Uchman and Wetzel 2016; see also Bromley 1991). During times of nutritional scarcity, the tracemakers remobilize the food from their storage either indirectly by solutions or ectoenzymes or directly by reworking and re-digestion. This “recycling-diet” was probably enriched in microbes growing within the active fill (cf. Jurkowska et al. 2017).
Similarly to Zoophycos brianteus, Chondrites intricatus is filled with the silty material deriving from the overlying stratum (except the shafts probably belonging to Chondrites). The preferred and close contact suggests that the Zoophycos structures represented a favourable (micro-) environment for the Chondrites tracemakers to exploit (compare Uchman and Wetzel 1999; Mikuláš et al. 2012; Rodríguez-Tovar et al. 2019). Chondrites has repeatedly been interpreted as chemichnion (for summary see Fu 1991; Bromley 1996; Vallon et al. 2016) and the diagenetic halos around the penetrating minute shafts together with their pyritic core, support a chemichnial behaviour. Chemichnia are produced by tracemakers that live in symbiosis with chemoautotrophic bacteria. Hence, their burrows need to serve both the needs of the oxygen-dependent tracemaker and its sulphate-reducing bacteria. The burrows, therefore, commonly exhibit an upper part where the tracemaker lives (usually not preserved in the fossil record) and a lower part reaching down into the anoxic area of the sediment as for instance shown for modern sediments by Wetzel (2008: fig. 5D). Here sulphide- or ammonium-rich porewaters are mined which are essential for respiration of the symbiont-microbes (Seilacher 1990; Kędzierski et al. 2015). Although Chondrites intricatus may also appear isolated within the Zoophycos Bed, their preferred occurrence in-between individual Z. brianteus whorls points to favourable conditions for the Chondrites tracemakers in this nucleocave micro-environment. The faecal pellets within the Z. brianteus spreite probably enriched the already anoxic sediments with additional metabolic products locally, intensifying the sulphide- or ammonium-rich porewater. Because the Z. brianteus whorls were filled with the silty material from above, they might have acted as reservoir of pore water enriched in sulphide owing to their coarser grain size and more open pore-space compared to the surrounding mud. In the studied material from Velká nad Veličkou, the individual Chondrites tracemakers repeatedly exploited the same Z. brianteus specimen in the same manner. Once a reservoir was depleted, the tracemaker burrowed deeper through the coil and exploited a new source. Although the two ichnogenera Zoophycos and Chondrites are very common, are frequently reported together and have a large stratigraphic range (e.g., Vinn et al. 2020 and references herein), we do not know of any similar interaction as reported herein.
The ichnogenus Tubulichnium is present in Triassic to Oligocene deposits and it occurs together with trace fossils typical of the Nereites ichnofacies (Uchman and Wetzel 2017). Tubulichnium rectum occupies a relatively deep tier in depths between 30 and 65 mm (Uchman and Wetzel 2017) although García-Ramos et al. (2014) treated T. mediterranensis as a structure formed in a shallower tier. Proposed cache behaviour of T. mediterranensis (García-Ramos et al. 2014) corresponds with the sequestration interpretation by Uchman and Wetzel (2017). The possible tracemakers are under discussion. For T. rectum a vermiform animal (Uchman and Wetzel 2017) was proposed in contrast to crustacean tracemaker of T. mediterranensis (García-Ramos et al. 2014).
The ichnogenus Dactyloidites usually occurs in shallow, sandy marine to brackish environments (D’Alessandro and Bromley 1986; Agirrezabala and Gibert 2004; Uchman and Pervesler 2007; Boyd and McIlroy 2016) whereas the studied specimens derive from a deep-sea environment. The studied material is therefore in concordance with the type ichnospecies Dactyloidites asteroides (Fitch, 1850) (see Hall 1886; cf. Landing 2012) from the Granville Formation, New York, USA, which was interpreted to derive from a lower slope environment (Donald H. Goldstein, personal communication 2021). Although recent deep-sea radial burrows were reported (Huggett 1987; Gaillard 1991; Bett et al. 1995; Turnewitsch et al. 2000; Bell et al. 2013), all the structures were observed on the sediment surface. The studied material shows distinct infaunal features similarly to burrows of Arenicola marina (Linnaeus, 1758) reported by Rijken (1979). He noticed that the stimulus for this atypical behaviour of A. marina is the presence of nutrient-rich sediment at living depth. Despite A. marina is an inhabitant of shallow seas, this hypothesis can be applied to deep-sea environments as well. Further, the presence of plant remains within or in close contact with Dactyloidites ottoi was repeatedly reported (see Agirrezabala and Gibert 2004; and references therein). This behaviour of incorporating plant material has been interpreted as farming (agrichnia) by Agirrezabala and Gibert (2004). Alternatively, this and our observations of deep-marine Dactyloidites isp. may also point to sequestration (e.g., stacked J-shape burrows combined with pellet deposition). The studied deep-sea material further supports this interpretation as these infaunal burrows were actively filled with silty material from the layer above the Zoophycos Bed.
Conclusions
The Velká nad Veličkou brickyard exemplifies deep-sea flysch sedimentation with uniquely preserved trace fossils and a relatively rich ichnoassemblage. The Zoophycos Bed offers a rare glimpse into the ecology of deep-sea dwellers and their feeding strategies, that mostly applied the recently recognised behaviour of sequestration. Chondrites intricatus burrows occur in close contact with Zoophycos brianteus, in particular to the spreite whorls inferring a highly specialised behaviour of the Chondrites intricatus tracemakers. This is interpreted as an opportunistic exploitation of a nucleocave environment with sulphide- or ammonium-rich pore waters. ?Dactyloidites isp. in the Velká nad Veličkou brickyard outcrop contributes to the broader knowledge about this ichnogenus. The studied material represents a deep-sea environment (like its type ichnospecies) whereas most Dactyloidites ichnospecies are reported from shallow-marine settings.
Acknowledgements
We are greatly thankful to Arden. R. Bashforth (Natural History Museum of Denmark, Copenhagen, Denmark) for access to Dam’s material deposited at the museum. We would also like to thank Michał Stachacz (Kraków, Poland), Donald H. Goldstein (University of Connecticut, Storrs, USA), and Andrew K. Rindsberg (University of West Alabama, Livingston, USA) for discussions and providing further information or hardly accessible literature and improving language. Christian Klug (University of Zürich, Switzerland) translated the French passage. Alfred Uchman (Jagiellonian University, Kraków, Poland), Olev Vinn (University of Tartu, Estonia), and Andreas Wetzel (University of Basel, Switzerland) provided helpful and constructive comments on the manuscript with their reviews. JŠ acknowledges research project MUNI/A/1394/2021. RM was supported by the Institute of Geology of the Czech Academy of Sciences No. RVO 679 858 31 and the Czech Science Foundation (Project No. 18-05935S).
References
Agirrezabala, L.M. and Gibert, J.M. de 2004. Paleodepth and paleoenvironment of Dactyloidites ottoi (Geinitz, 1849) from Lower Cretaceous deltaic deposits (Basque-Cantabrian Basin, West Pyrenees). Palaios 19: 276–291. Crossref
Baucon, A., Bednarz, M., Dufour, S., Felletti, F., Malgesini, G., de Carvalho, C.N., Niklas, K. J., Wehrmann, A., Batstone, R., Bernardini, F., Briguglio, A., Cabella, R., Cavalazzi, B., Ferretti, A., Zanzerl, H., and McIlroy, D. 2020. Ethology of the trace fossil Chondrites: Form, function and environment. Earth-Science Reviews 202: 102989. Crossref
Belaústegui, Z., Doménech, R., and Martinell, J. 2015. Trace fossils of the Middle Miocene of the El Camp de Tarragona Basin (NE Spain). In: D. McIlroy (ed.), Ichnology: Papers from Ichnia III. Geological Association of Canada, Miscellaneous Publication 9: 15–30.
Belaústegui, Z., Muñiz, F., Mángano, M.G., Buatois, L.A., Domènech, R., and Martinell, J. 2016. Lepeichnus giberti igen. nov. isp. nov. from the upper Miocene of Lepe (Huelva, SW Spain): Evidence for its origin and development with proposal of a new concept, ichnogeny. Palaeogeography, Palaeoclimatology, Palaeoecology 452: 80–89. Crossref
Bell, J.B., Jones, D.O.B., and Alt, C.H.S. 2013. Lebensspuren of the bathyal Mid-Atlantic Ridge. Deep-Sea Research Part II: Topical Studies in Oceanography 98: 341–351. Crossref
Bett, B.J., Rice, A.L., and Thurston, M.H. 1995. A quantitative photographic survey of “spoke-burrow” type lebensspuren on the Cape Verde abyssal plain. Internationale Revue der gesamten Hydrobiologie und Hydrographie 80 (2): 153–170. Crossref
Billings, E. 1862. New species of fossils from different parts of the Lower, Middle and Upper Silurian rocks of Canada. In: E. Billings (ed.), Palaeozoic Fossils, Vol. 1 (for 1861–1865), 96–168. Geological Survey Canada, Montreal. Crossref
Boyd, C. and McIlroy, D. 2016. Three-dimensional morphology and palaeobiology of the trace fossil Dactyloidites jordii nov. isp. from the Carboniferous of England. Geobios 49: 257–264. Crossref
Bromley, R.G. 1991. Zoophycos: strip mine, refuse dump, cache or sewage farm? Lethaia 24: 460–462. Crossref
Bromley, R.G. 1996. Trace Fossils: Biology, Taphonomy and Applications. Second edition. 361 pp. Chapman & Hall, London.
Bromley, R.G. and Ekdale, A.A. 1984. Chondrites: a trace fossil indicator of anoxia in sediments. Science 224: 872–874. Crossref
Brongniart, A.T. 1823. Observations sur les Fucoids. Mémoires de la Société d’Histoire Naturelle de Paris1: 301–320.
Brongniart, A.T. 1828. Histoire des végétaux fossiles ou recherches botaniques et géologiques sur les végétauxrenfermés dans les diverses couches du globe. 488 pp. G. Dufour & E. D’Ocagne, Paris. Crossref
Brongniart, A.T. 1849. Tableau des generes de végétaux fossiles considérés sous le point de vue de leur classification botanique et de leur distribution géologique. Dictionnaire universel d’histoire naturelle 13: 1–27 (152–176).
Buatois, L.A., Narbonne, G.M., Mángano, M.G, Carmona, N.B., and Myrow, P. 2014. Ediacaran matground ecology persisted into the earliest Cambrian. Nature Communications 5: 3544. Crossref
Buatois, L.A., Wisshak, M., Wilson, M.A., and Mángano, M.G. 2017. Categories of architectural designs in trace fossils: A measure of ichnodisparity. Earth-Science Reviews 164: 102–181. Crossref
Bubík, M. 1995. Cretaceous to Paleogene agglutinated foraminifera of the Bílé Karpaty unit (West Carpathians, Czech Republic). In: M.A. Kaminski, S. Geroch, and M.A. Gasiński (eds.), Proceedings of the Fourth International Workshop on Agglutinated Foraminifera, Kraków, Poland, September 12–19, 1993. Grzybowski Foundation Special Publication 3: 71–116.
Cabanás, G. 1966. Notas estratigraficas de la provincia de Cordoba. Con una nota sobre un nuevo fosil del Cambriano por Bermudo Meléndez. Notas y Comunicaciones del Instituto Geológico y Minero de España 90: 77–84.
Ciampaglio, C.N., Babcock, L.E., Wellman, C.L., York, A.R., and Brunswick, H.K. 2006. Phylogenetic affinities and taphonomy of Brooksella from the Cambrian of Georgia and Alabama, USA. Palaeoworld 15: 256–265. Crossref
Crimes, T.P. and Crossley, J.D. 1991. A diverse ichnofauna from Silurian flysch of the Aberystwyth Grist Formation, Wales. Geological Journal 26: 27–64. Crossref
D’Alessandro, A. 1982. Processi tafonomici e distribuzione delle tracce fossili net Flysch di Gorgolione (Appenino Meridionale). Rivista Italiana di Paleontologia e Stratigrafia 87: 511–560.
D’Alessandro, A. and Bromley, RG. 1986. Trace fossils in Pleistocene sandy deposits from Gravina area Southern Italy. Rivista Italiana di Paleontologia e Stratigrafia 92: 67–102.
Dam, G. 1990. Taxonomy of trace fossils from the shallow marine Lower Jurassic Neill Klinter Formation, East Greenland. Bulletin of the Geological Society of Denmark 38: 119–144. Crossref
Ekdale, A.A. and Bromley, R.G. 1984. Comparative ichnology of shelf-sea and deep-sea chalk. Journal of Paleontology 58: 322–332.
Fitch, A. 1850. A historical, topographical and agricultural survey of the County of Washington. Part 5. New York Agricultural Society, Transactions 9 (for 1849): 753–944.
Fu, S. 1991. Funktion, Verhalten und Einteilung fucoider und lophocteniider Lebensspuren. Courier Forschungsinstitut Senckenberg 135: 1–79.
Fürsich, F.T. and Bromley, R.G. 1985. Behavioural interpretation of a rosetted spreite trace fossil: Dactyloidites ottoi (Geinitz). Lethaia 18: 199–207. Crossref
Gaillard, C. 1991. Recent organism traces and ichnofacies on the deep-sea floor off New Caledonia, Southwestern Pacific. Palaios 6: 302–315. Crossref
García-Ramos, J.C., Mángano, M.G., Buatois, L.A., and Rodríguez-Tovar, F.J. 2014. The ichnogenus Tubotomaculum: an enigmatic pellet-filled structure from upper Cretaceous to Miocene deep-marine deposits of Southern Spain. Journal of Paleontology 88: 1189–1198. Crossref
Geinitz, H.B. 1849. Das Quadersandsteingebirge oder Kreidegebirge in Deutschland. 292 pp. Craz and Gerlach, Freiberg. Crossref
Hall, J. 1886. Note on some obscure organisms in the roofing slate of Washington County, New York. New York State Museum of Natural History, Annual Report 39: 1–160.
Hu, B., Song, H., Liu, S., and Zhang, L. 2010. Sedimentary facies, ichnofossils and storm deposits in the Lower Permian Taiyuan Formation, Jiaozuo city, Henan Province, Central China. Acta Geologica Polonica 60: 45–52.
Huggett, Q.J. 1987. Mapping of hemipelagic versus turbiditic muds by feeding traces observed in deep-sea photographs. In: P.P.E. Weaver and J. Thomson (eds.), Geology and Geochemistry of Abyssal Plains. Geological Society Special Publication 31: 105–112. Crossref
Izumi, K. and Yoshizawa, K. 2016. Star-shaped trace fossils and Phymatoderma from Neogene deep-sea deposits in central Japan: probable echiuran feeding and fecal traces. Journal of Paleontology 90: 1169–1180. Crossref
Jurkowska A., Uchman, A., and Banaś, M. 2017. Life beneath ammonite shells—A unique Late Cretaceous habitat for the trace maker of Chondrites and its impact on taphonomy of the shells. Cretaceous Research 72: 151–160. Crossref
Jurkowska, A., Uchman, A., and Świerczewska-Gładysz, E. 2018. A record of sequestration of plant material by marine burrowing animals as a new feeding strategy under oligotrophic conditions evidenced by pyrite microtextures. Palaios 33: 312–322. Crossref
Kędzierski, M., Uchman, A., Sawlowicz, Z., and Briguglio, A. 2015. Fossilized bioelectric wire—the trace fossil Trichichnus. Biogeosciences 12: 2301–2309. Crossref
Kotake, N. 1989. Paleoecology of the Zoophycos producers. Lethaia 22: 327–341. Crossref
Książkiewicz, M. 1968. On some problematic organic traces from the Flysch of the Polish Carpathians, Part 3. Rocznik Polskiego Towarzystwa Geologicznego 38: 3–17.
Książkiewicz, M. 1977. Trace fossils in the Flysch of the Polish Carpathians. Acta Palaeontologica Polonica 36: 1–208.
Kundal, S.N., Kundal, P., Kumar, S., Chowdhary, N., and Prasad, G.V.R. 2022. Late Pliocene–Early Pleistocene ichnofauna from bentonitized tuff band and associated mudstone horizons, Jammu and Kashmir, India, and their palaeoenvironmental implications. Arabian Journal of Geosciences 15: 269. Crossref
Landing, E. 2012. The great American carbonate bank in eastern Laurentia: Its births, deaths, and linkage to paleooceanic oxygenation (Early Cambrian–Late Ordovician). In: J.R. Derby, R.D. Fritz, S.A. Longacre, W.A. Morgan, and C.A. Sternbach (eds.), The Great American Carbonate Bank: The Geology and Economic Resources of the Cambrian–Ordovician Sauk Megasequence of Laurentia. AAPG Memoir 98: 451–492. Crossref
Le Roux, J.P., Nielsen, S.N., and Henríquez, Á. 2008. Depositional environment of Stelloglyphus llicoensis isp. nov.: a new radial trace fossil from the Neogene Ranquil Formation, south-central Chile. Revista Geológica de Chile 35: 307–319. Crossref
Linnaeus, C. 1758. Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tenth edition. 824 pp. Laurentius Salvius, Holmiae. Crossref
Massalongo, A. 1855. Zoophycos, novum genus plantarum fossilium. 52 pp. Typis Antonellianis, Veronae.
Mikuláš, R., Fatka, O., and Szabad, M. 2012. Paleoecologic implications of ichnofossils associated with slightly skeletonized body fossils, middle Cambrian of the Barrandian Area, Czech Republic. Ichnos 19: 199–210. Crossref
Muñoz, D.F., Mángano, M.G., and Buatois, L.A. 2019. Unravelling Phanerozoic evolution of radial to rosette trace fossils. Lethaia 52: 350–369. Crossref
Němcová, A. 1973. Klasifikace a význam bioglyfů při výzkumu sedimentů karpatského flyše. 124 pp. Unpublished Ph.D. Thesis, Faculty of Science, Jan Evangelista Purkyně University (Masaryk University), Brno.
Nicholson, H.A. 1873. Contributions to the study of the errant annelids of the older Palaeozoic rock. Proceedings of the Royal Society of London 21: 288–290. Crossref
Olivero, D. 2007. Zoophycos and the role of type specimens in ichnotaxonomy. In: W. Miller III. (ed.), Trace Fossils: Concepts, Problems, Prospects, 219–231. Elsevier, Amsterdam. Crossref
Olivero, D. and Gaillard, C. 1996. Paleoecology of Jurassic Zoophycos from south-eastern France. Ichnos 4: 249–260. Crossref
Olivero, D. and Gaillard, C. 2007. A constructional model for Zoophycos, In: W. Miller III. (ed.), Trace Fossils: Concepts, Problems, Prospects, 466–477. Elsevier, Amsterdam. Crossref
Otto, E. von 1854. Additamente zur Flora des Quadergebirges in Sachsen. 2. Heft. 54 pp. G. Mayer, Leipzig.
Pickerill, R.K., Donovan, S.K., and Dixon, H.L. 1993. The trace fossil Dactyloidites ottoi (Geinitz, 1849) from the Neogene August Town Formation of South-Central Jamaica. Journal of Paleontology 67: 1070–1074. Crossref
Plička, M. 1989. Rotundusichnium zumayensis ichnog. n. a new trace fossil from the Outer Carpathian Flysch of West Slovakia (Czechoslovakia). Západne Karpaty, séria paleontológia 13: 69–79.
Quatrefages, M.A. de 1849. Note sur la Scolicia prisca (A. de Q.) Annelide fossile de la Craie. Annales des Sciences Naturelles, 3 série, Zoologie 12: 265–266.
Richter, R. 1850. Aus der Thüringischen Grauwacke. Zeitschrift der Deutschen Geologischen Gesellschaft 2: 198–206.
Rijken, M. 1979. Food and food uptake in Arenicola marina. Netherlands Journal of Sea Research 13: 406–421. Crossref
Rindsberg, A.K. and Chowns, T.M. 1986. Ringgold Gap: Progradational sequences in the Ordovician and Silurian of northwest Georgia. In: T.L. Neathery (ed.), Geology of North America, Geological Society of America Centennial Field Guide, Vol. 6, 159–162. Southeastern Section of the Geological Society of America, Boulder. Crossref
Rodríguez-Tovar, F.J., Miguez-Salas, O., Dorador, J., and Duarte, L.V. 2019. Opportunistic behaviour after the Torcian Oceanic Anoxic Event: The trace fossil Halimedides. Palaeogeography, Palaeoclimatology, Palaeoecology 520: 240–250. Crossref
Savi, P. and Meneghini, G.G. 1850. Osservazioni stratigrafiche e paleontologiche concernenti la geologia della Toscana e dei Paesi Limitrofi. 259 pp. Stemparia Granducale, Firenze.
Seilacher, A. 1990. Aberrations in bivalve evolution related to photo- and chemosymbiosis. Historical Biology 3: 289–311. Crossref
Seilacher, A. 2000. Ordovician and Silurian arthrophycid ichnostratigraphy. In: M.A. Sola and D. Worsley (eds.), Geological Exploration in Murzuq Basin, 237–258. Elsevier, Amsterdam. Crossref
Seilacher, A. 2007. Trace Fossil Analysis. 226 pp. Springer-Verlag, Berlin.
Seilacher, A. 2008. Fossil Art: An Exhibition of the Geologisches Institut, Tübingen University, Germany. 98 pp. CBM-publishing, Laasby.
Sternberg, G. K. 1833. Versuch einer geognostisch-botanischen Darstellung der Flora der Vorwelt. IV Heft. 48 pp. C.E. Brenck, Regensburg.
Stráník, Z., Bubík, M., Krejčí, O., Marschalko, R., Švábenická, L., and Vujta, M. 1995. New Lithostratigraphy of the Hluk Development of the Bílé Karpaty unit. Geologické práce, Správy 100: 57–69.
Tchoumatchenco, P. and Uchman, A. 2001. The oldest deep-sea Ophiomorpha and Scolicia and associated trace fossils from the Upper Jurassic–Lower Cretaceous deep-water turbidite deposits of SW Bulgaria. Palaegeography, Palaeoclimatology, Palaeoecology 169: 85–99. Crossref
Turnewitsch, R., Witte, U., and Graf, G. 2000. Bioturbation in the abyssal Arabian Sea influence of fauna and food supply. Deep-Sea Research Part II: Topical Studies in Oceanography 47: 2877–2911. Crossref
Uchman, A. 1998. Taxonomy and ethology of flysch trace fossils: revision of the Marian Książkiewicz collection and studies of complementary material. Annales Societatis Geologorum Poloniae 68: 105–218.
Uchman, A. and Pervesler, P. 2007. Palaeobiological and palaeonvironmental significance of the Pliocene trace fossil Dactyloidites peniculus. Acta Palaeontologica Polonica 52: 799–808.
Uchman, A. and Rattazzi, B. 2017. The trace fossil Polykampton cabellae isp. nov. from the Pagliaro Formation (Paleocene), Northern Apennines, Italy: A record of nutritional sediment sequestration by a deep sea invertebrate. Ichnos 25: 1–10. Crossref
Uchman, A. and Wetzel, A. 1999. An aberrant, helicoidal trace fossil Chondrites Sternberg. Palaeogeography, Palaeoclimatology, Palaeoecology 146: 165–169. Crossref
Uchman, A. and Wetzel, A. 2016. Sequestrichnia—a new ethological category of trace fossils in oligotrophic deep-sea environments. In: A. Baucon, C.N. de Carvalho, and J. Rodrigues (eds.), Ichnia 2016. Abstract Book, 190. UNESCO Geopark Naturtejo/International Ichnological Association, Castelo Branco.
Uchman, A. and Wetzel, A. 2017. Hidden subsurface garden on own faeces—the trace fossil Tubulichnium rectum (Fischer-Ooster, 1858) from the Cretaceous–Palaeogene deep-sea sediments. Palaeontologia Electronica 20.2.40A: 1–18. Crossref
Uchman, A., Hanken, N.-M., and Binns, R. 2005. Ordovician bathyal trace fossils from metasiliciclastics in Central Norway and their sedimentological and paleogeographical implications. Ichnos 12: 105–133. Crossref
Uchman, A., Lebanidze, Z., Beridze, T., Kobakhidze, N., Lobzhanidze, K., Khutsishvili, S., Chagelishvili, R., Makadze, D., Koiava, K., and Khundadze, N. 2020. Abundant trace fossil Polykampton in Palaeogene deep-sea flysch deposits of the Lesser Caucasus in Georgia: Palaeoecological and palaeoenvironmental implications. Palaeogeography, Palaeoclimatology, Palaeoecology 558: 109958. Crossref
Uchman, A., Wetzel, A., and Rattazzi, B. 2019. Alternating stripmining and sequestration in deep-sea sediments: The trace fossil Polykampton—an ecologic and ichnotaxonomic evaluation. Palaeontologia Electronica 22.2.21A: 1–18. Crossref
Vallon, L.H., Rindsberg, A.K., and Bromley, R.G. 2016. An updated classification of animal behaviour preserved in substrates. Geodinamica Acta 28: 5–20. Crossref
Vialov, O.S. 1964. Star-shaped hieroglyphs from the Triassic of north-eastern Siberia [in Russian with English summary]. Geologiâ i Geofizika 5: 112–115.
Vialov, O.S. 1989. Paleoichnological studies [in Russian with English summary]. Paleontologičeskij Sbornik 26: 72–78.
Vinn, O., Bendella, M., Benyoucef, M., Zhang, L.-J., Bouchemla, I., Ferré, B., and Lagnaoui, A. 2020. Abundant Zoophycos and Chondrites from the Messinian (Upper Miocene) of northwest Algeria. Journal of African Earth Sciences 171: 103921. Crossref
Walcott, C.D. 1896. Fossil jellyfishes from the Middle Cambrian terrane. Proceedings of the United States National Museum 18: 611–615. Crossref
Wetzel, A. 2008. Recent bioturbation in the deep South China Sea: A uniformitarian ichnologic approach. Palaios 23: 601‒615. Crossref
Wetzel, A. and Bromley, R.G. 1996. Re-evaluation of the ichnogenus Helminthopsis—A new look at the type material. Palaeontology 39: 1–19.
Wetzel, A. and Uchman, A. 2012. Hemipelagic and pelagic basin plains. In: D. Knaust and R.G. Bromley (eds.), Trace fossils as indicators of sedimentary environments. Developments in Sedimentology 64: 673–701. Crossref
Wetzel, A., Tjallingii, R., and Wiesner, M.G. 2011. Bioturbational structures record environmental changes in the upwelling area off Vietnam (South China Sea) for the last 150,000 years. Palaeogeography, Palaeoclimatology, Palaeoecology 311: 256–267. Crossref
Wilmsen, M. and Niebuhr, B. 2014. The rosetted trace fossil Dactyloidites ottoi (Geinitz, 1849) from the Cenomanian (Upper Cretaceous) of Saxony and Bavaria (Germany): ichnotaxonomic remarks and palaeoenvironmental implications. Paläontologische Zeitschrift 88: 123–138. Crossref
Acta Palaeontol. Pol. 67 (3): 767–779, 2022
https://doi.org/10.4202/app.00965.2021