

A new needle stonefly with parasitic mites from the Eocene Baltic amber
ZHI-TENG CHEN and HONG-LING LIU
Baltileuctra dewalti Chen sp. nov., a new extinct species of needle stoneflies (Leuctridae, Plecoptera), is described and illustrated from the Eocene Baltic amber and compared to the other leuctrids. Baltileuctra dewalti Chen sp. nov. differs from both extant and extinct representatives of Leuctridae by a combination of morphological characters including wing venation, unique terminalia structures, and cercal modification. The most distinguishable character for the new taxon is the presence of two dentate long spines lateral to a well-developed subanal probe. Several parasitic mites are also found on the new stonefly. This is the earliest known fossil evidence for mite parasitism in Plecoptera.
Introduction
The order Plecoptera (stoneflies) comprises a relatively small group of aquatic insects and the oldest representative is dated back to the late Carboniferous (Béthoux et al. 2011). The lack of sufficient well-preserved stonefly fossils and inaccurate taxonomic assignment of some of them make it difficult to correctly time-calibrate the phylogenetic tree of Plecoptera (but see Cui et al. 2019). Five families of Plecoptera are already known from the Eocene Baltic amber, including Leuctridae, Nemouridae, Perlidae, Perlodidae, and Taeniopterygidae (Caruso and Wichard 2010, 2011). Among these families, Leuctridae (needle stoneflies) is most frequently reported, with six known genera including Leuctra Stephens, 1836, Megaleuctra Neave, 1934, Zealeuctra Ricker, 1952, Palaeopsole Caruso and Wichard, 2011, Baltileuctra Chen, 2018a, and Euroleuctra Chen, 2018b; the former three genera are extant with fossil species, whereas the latter three are exclusively fossil (Caruso and Wichard 2010, 2011; Chen 2018a, b).
This study examines a well-preserved piece of Eocene Baltic amber and discovers a new needle stonefly as the second known species of Baltileuctra which is parasitized by mites. New information is provided for Baltileuctra foraminis Chen, 2018a, and Euroleuctra gillesi Chen, 2018b, for morphological comparison.
Institutional abbreviations.—ICJUST, Insect Collection of Jiangsu University of Science and Technology, Jiangsu Province, China.
Other abbreviations.—AA1/A2, first/second anterior analis; CuA/P, anterior/posterior cubitus; M, media; RA/P, anterior/posterior radius; ra-rp, crossvein between RA and RP; ScP, posterior subcosta; T, abdominal tergum; ST, abdominal sternum.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid: zoobank.org:pub: B9E23181-7496-4167-AC0B-AF21140822F9
Material and methods
The Baltic amber studied below is obtained from Lithuania, with an age of ca. 40–50 million years (Larsson 1978). The material is deposited in the Insect Collection of Jiangsu University of Science and Technology, Jiangsu Province, China (ICJUST, No. CZT-PLE-BA8). The specimen was polished with sandpaper and polishing powder. Observation and measurements were performed with a SDPTOP SZM45 stereo microscope. Photos were taken with a Canon EOS 6D digital camera with a Canon MP-E 65 mm macro lens. Figures were optimized and assembled with Adobe Photoshop CS6. Line drawings were made manually by tracing the photographs with tracing papers and colored in Adobe Photoshop CS6. Terminology of wing venation follows Béthoux (2005); terminology of body parts follows Chen (2018b).
Systematic palaeontology
Class Insecta Linnaeus, 1758
Order Plecoptera Burmeister, 1839
Family Leuctridae Klapálek, 1905
Genus Baltileuctra Chen, 2018a
Type species: Baltileuctra foraminis Chen, 2018a; Eocene, Lithuania
Baltileuctra dewalti Chen sp. nov.
Figs. 1–3.
ZooBank LSID: urn:lsid:zoobank.org:act:662DFF67-70D3-4085-ADE7 -12DFF421C526
Etymology: Named for R. Edward DeWalt, who has made substantial contributions to the knowledge of Plecoptera.
Holotype: ICJUST CZT-PLE-BA8, male, a complete specimen in a clear and well-preserved piece of Baltic amber from Lithuania, Eocene (ca. 40–50 Ma).
Type locality: Unknown locality in Lithuania.
Type horizon: Eocene Baltic Amber.
Material.—Holotype only.
Diagnosis.—The ra-rp crossvein located distal to the fork of RP in forewings but basal to it in hind wing; MP partially fused with CuA in hind wing. Median area of terga 8–9 membranous. Tergum 8 with a deep median notch on posterior margin. Tergum 9 with two semicircular posterior sclerites. Tergum 10 medially with a longitudinal, narrow split, posteriorly followed by a fusiform sclerite. Sternum 9 with an elliptical vesicle, length of which 1.5 times longer than wide. Sternum 9 medially and posterolaterally membranous, posteriorly projected as a rounded lobe with two slender oblique sclerites. Subanal probe elongated and curved ventrad, apex enlarged ventrally. Sternum 10 developed innerly, forming two long spines lateral to subanal probe, the spines denticulate ventrally, apex pointed and slightly curved. Cercus elongated and cylindrical, apically with a membranous area and a stout spine.
Description.—Macropterous (Fig. 1); body length ca. 5.0 mm, generally dark brown; ventral surface covered by unknown white substance. Head rounded and entirely dark brown, much wider than pronotum. Triocellate; compound eyes large and bulbous. Antennae dark and filiform, subequal in length to body, with 36 segments preserved, each segment covered with dense short setae. Maxillary palp four-segmented, apical segment longest; labial palp very short and hardly visible. Pronotum elongated, near rectangular, corners obtuse. Cervical gills absent; cervix with small lateral nubs. Meso- and metathorax much wider than prothorax. Legs generally dark brown; forelegs shortest, hindlegs longest; femur slightly shorter but much thicker than tibia; tibia with two giant ventroapical spurs; tarsus three-segmented, each segment covered with dense setae, second segment shortest; claws short and sharp. Ventral sclerites of thorax invisible. Wings sub-hyaline and fringed with short setae, without any characteristic patterns; veins dark brown (Figs. 1, 2). Left forewing length ca. 5.5 mm (Fig. 2A1, A2); ScP reaches RA before ra-rp, distally almost touching anterior wing margin; one basal humeral crossvein present between anterior margin and ScP; RP and M with separate origins; RP originated from basal 1/5 of RA, forked near apical 1/3 of forewing; ra-rp located distal to the fork of RP; Cu basally forked into CuA and CuP; four crossveins present between M and CuA; area between CuA and CuP with eight crossveins, terminal one jointing posterior margin of the wing; AA1 curved at half length, apical half divergent from CuP; AA2 forked. Visible venation of right forewing similar to left forewing (Fig. 2A1, A3), terminal crossvein between CuA and CuP jointing CuP near apex. Hind wings length ca. 5.0 mm (Fig. 2A4, A5); ScP reaches RA before ra-rp; ra-rp located basal to the fork of RP; MA simple; MP fused with CuA for a short distance; basal parts of CuP, AA1, and AA2 invisible; anal area small and folded, with very few anal veins. Abdomen generally dark brown (Fig. 1), terga 9–10 darker; terga and sterna of abdominal segments 1–8 divided by conspicuous membrane. Abdominal terga without sclerotized, paired processes. Median area of terga 8–9 completely membranous (Fig. 3A1, A2). Posterior margin of tergum 8 with a deep median notch. Tergum 9 with two extra semi-circular sclerites at posterior margin. Tergum 10 short, darkly sclerotized, medially with a longitudinal, narrow split; a fusiform sclerotized plate present posterior to tergum 10 (Fig. 3A1, A2). Ventral vesicle of sternum 9 elliptical (Fig. 3A3–A5), length 1.5 times longer than wide. Sternum 9 with a median membranous area which posteriorly connected with the circular posterior margin of sternum 9 (Fig. 3A3–A5); sternum 9 projected backwards, forming a rounded posteromedial lobe with two slender oblique sclerites, the two sclerites originated from half length of sternum 9, gradually converged backwards but never touched; posterolateral areas of sternum 9 completely membranous, with several transverse folds. Subanal probe elongated and slightly curved ventrad from lateral view, apex apparently enlarged ventrally; ventral surface somewhat concaved medially and longitudinally (Fig. 3A3–A5). Sternum 10 seems cleft, each hemisternum developed innerly, respectively forming a long spine lateral to subanal probe; the spines slightly longer than cerci but much shorter than subanal probe, denticulate along ventral margin, apex pointed and slightly curved ventrad (Fig. 3A3–A5). Cercus hairy, elongated and cylindrical, much shorter than subanal probe, apically with a membranous circular area and a stout, dark spine (Fig. 3).
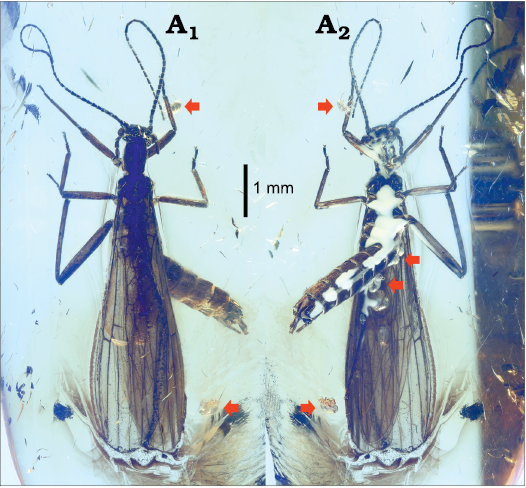
Fig. 1. Needle stonefly Baltileuctra dewalti Chen sp. nov. (holotype, ICJUST CZT-PLE-BA8) from Eocene Baltic Amber from unknown locality in Lithuania. Photomicrographs of male adult, habitus, in dorsal (A1) and ventral (A2) views. Sites of parasitic mites indicated by red arrowheads.
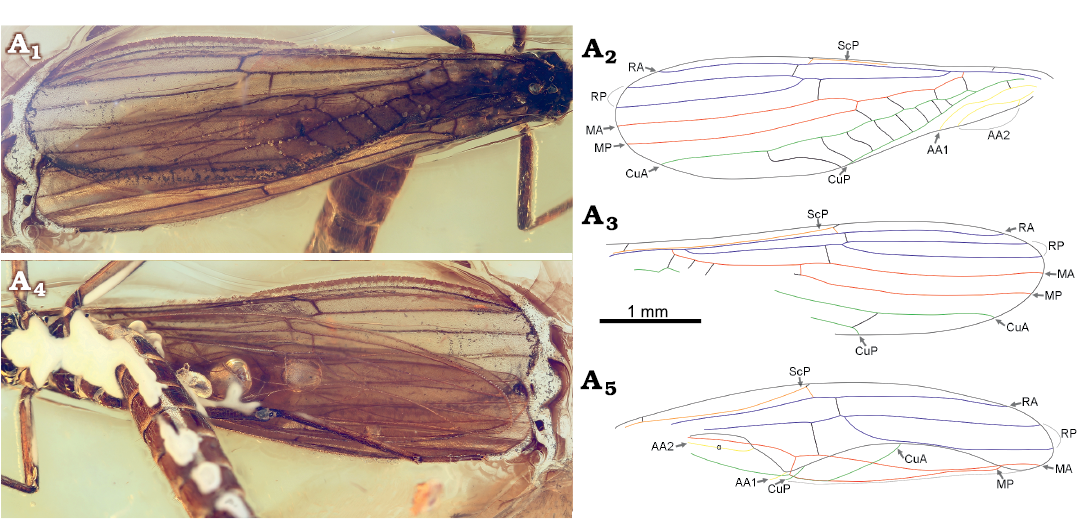
Fig. 2. Needle stonefly Baltileuctra dewalti Chen sp. nov. (holotype, ICJUST CZT-PLE-BA8) from Eocene Baltic Amber from unknown locality in Lithuania. Male adult, left (A1, A2) and right (A3) forewings in dorsal view, left hind wing in ventral view (A4, A5). Photomicrographs (A1, A4), explanatory drawings (A2, A3, A5).
Remarks.—The new needle stonefly undoubtedly belongs to the family Leuctridae due to its needle-like habitus, absence of gills, apical segment of labial palp similar to other segments, absence of X-patterned crossveins in forewings, second tarsal segment shorter than first and third segments, and one-segmented cerci (Stewart and Stark 2008; Chen and Du 2018). Additionally, in forewing CuA is simple, and the antero-distal cell lacks a cross-vein, a combination of traits which, within Systellognatha, indicate a Leuctridae (Béthoux et al. 2015). At the subfamilial level, the new stonefly is unlikely to fit in the single genus of Megaleuctrinae, Megaleuctra Neave, 1934, which is characterized by large body size (about 15 mm), presence of six anal veins in hind wings, elaborate epiproct and abruptly recurved subanal probe (Zwick 2000; Ham and Bae 2002; Baumann and Stark 2013). When compared with the extant species of Leuctrinae, the ra-rp crossvein of the new stonefly is located distal to the fork of RP in forewings but basal to it in hind wing, which is uncommon among Leuctridae and can easily distinguish it from species of Calileuctra Shepard and Baumann, 1995 (Shepard and Baumann 1995), Paraleuctra Hanson, 1941 (Stark and Kyzar 2001; Chen 2019b), Perlomyia Banks, 1906 (Nelson and Hanson 1973), Pomoleuctra Stark and Kyzar, 2001 (Stark and Kyzar 2001), Rhopalopsole Klapálek, 1912 (Kawai 1968; Chen 2019a), and Zealeuctra (Caruso and Wichard 2010). For the species of remaining extant genera without detailed wing venation description, the new stonefly differs from species of Despaxia Ricker, 1943, and Moselia Ricker, 1943, by the presence of a well-developed subanal probe (Kondratieff and Lechleitner 2002; Stark and Harrison 2016); of Pachyleuctra Despax, 1929, by the medially cleft tergum 10 and absence of strongly recurved paraprocts (Stark and Nelson 2019); of Tyrrhenoleuctra Consiglio, 1957, by presence of paired lateral spines along subanal probe (or namely paraprocts) (Stark and Nelson 2019); of Leuctra Stephens, 1836 by presence of a much longer, medially cleft abdominal tergum 10, and absence of an elevated, club-shaped epiproct (or namely supraanal organ, process, lobe, etc.) (Ricker and Ross 1969; Pardo and Zwick 1993). The wing venation and especially the male terminalia of the new species is very similar to that of B. foraminis Chen, 2018a (Fig. 4) and support its attribution to Baltileuctra Chen, 2018a (Chen 2018a). The original illustration of B. foraminis neglected the hind wings and paired lateral spines of terminalia, which are provided herein (Fig. 4). The presence of apical cercal spines, absence of subapical lateral dentation on subanal probe, and presence of isolated, slender, lateral spines near subanal probe can distinguish species of Baltileuctra from Euroleuctra (Fig. 5; Chen 2018a, b). Within Baltileuctra, the newly described species can be separated from B. foraminis by the following terminalia characters: posteromedial sclerites of sternum 9 interrupted (connected in B. foraminis); subanal probe curved ventrad (curved upward in B. foraminis); lateral spines of subanal probe dentate (smooth in B. foraminis). The partially fused MP with CuA in hind wings of Baltileuctra corresponds with the defining character state of Collaleuctrida as defined in the phylogenetic and nomenclatural framework of needle stoneflies proposed by Béthoux et al. (2015).
Stratigraphic and geographic range.—Type locality and horizon only.
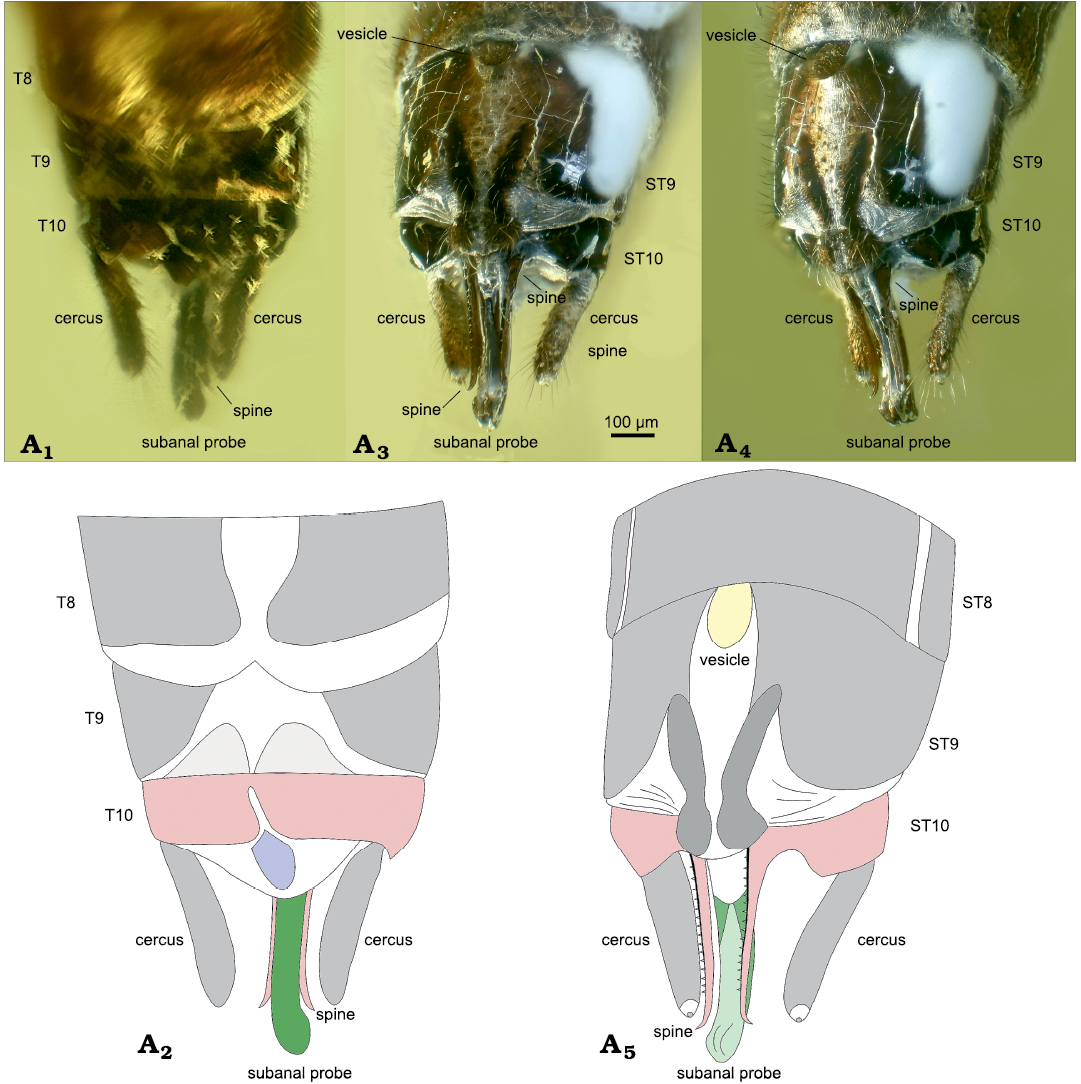
Fig. 3. Needle stonefly Baltileuctra dewalti Chen sp. nov. (holotype, ICJUST CZT-PLE-BA8) from Eocene Baltic Amber from unknown locality in Lithuania. Male adult terminalia in dorsal view (A1, A2), ventral view (A3), ventrolateral view (A4, A5). Photomicrographs (A1, A3, A4), drawing (A2, A5).
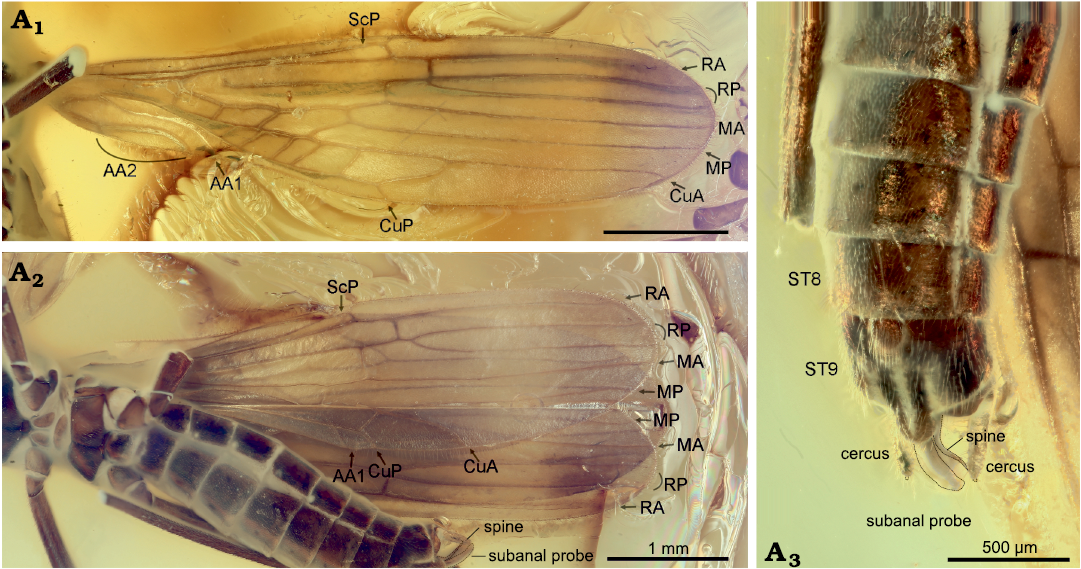
Fig. 4. Needle stonefly Baltileuctra foraminis Chen, 2018a (ICJUST CZT-PLE-BA2) from Eocene Baltic Amber from unknown locality in Lithuania. Male adult, photomicrographs of left forewing in dorsal view (A1), hind wings in ventral view (A2), and terminalia in ventral view (A3). Outlines of subanal probe, lateral spines, and apical cercal spines indicated with black lines.

Fig. 5. Needle stonefly Euroleuctra gillesi Chen, 2018b (ICJUST CZT-PLE-BA3) from Eocene Baltic Amber from unknown locality in Poland. Male adult, photomicrographs of left forewing in dorsal view (A1) and terminalia in ventral view (A2).
Association with parasitic mites
At least five parasitic mites are found in the amber (Fig. 1): three visible mites attached on abdominal tergum 1–2 of the stonefly; one attached on abdominal tergum 4; one separated from the abdomen; one touching the apex of right antenna. These mites are probably water mites. The mites are entirely pale, ca. 0.3 mm long, near rugby ball-shaped, dorsally humped and ventrally compressed. Three slender legs of the mites are present, being located mostly on anterior half of idiosoma, almost glabrous but occasionally with several long bristles on terminal segments. Basal abdominal tergites of stoneflies apparently represent one of the most frequent attachment sites for these mites, which is identical to the situation in extant stoneflies (Yasick et al. 2003).
Concluding remarks
The discovery of Baltileuctra dewalti Chen sp. nov. and re-examination of Baltileuctra type species holotype in this study increases our knowledge of the diversity of Leuctridae and provides more precise diagnostic characters of the genus. The characters of male genitalic structures are found to be the most informative in delimitating taxa of needle stoneflies (Leuctridae).
Acknowledegments.—The authors are grateful to Olivier Béthoux (MNHN-CNRS – Sorbonne Université, Paris, France) and an anonymous reviewer for helpful comments and manuscript improvement. This work is funded by the Natural Science Foundation of Jiangsu Province (Grant No. BK20201009) and the Start-up Funding of Jiangsu University of Science and Technology (Grant No. 1182931901).
References
Banks, N. 1906. New species of Perlidae. The Canadian Entomologist 38: 335–338. Crossref
Baumann, R.W. and Stark, B.P. 2013. The genus Megaleuctra Neave (Plecoptera: Leuctridae) in North America. Illiesia 9: 65–93.
Béthoux, O. 2005. Wing venation pattern of Plecoptera (Neoptera). Illiesia 1: 52–81.
Béthoux, O., Cui, Y.Y., Kondratieff, B., Stark, B., and Ren, D. 2011. At last, a Pennsylvanian stem-stonefly (Plecoptera) discovered. BMC Evolutionary Biology 11: 248. Crossref
Béthoux, O., Kondratieff, B., Grímsson, F., Ólafsson, E., and Wappler, T. 2015. Character state‐based taxa erected to accommodate fossil and extant needle stoneflies (Leuctridae–Leuctrida tax. n.) and close relatives. Systematic Entomology 40: 322–341. Crossref
Burmeister, H. 1839. Zweite Halfte. Neuroptera. Handbuch der Entomologie. 863 pp. Reimer, Berlin.
Caruso, C.E. and Wichard, W.I. 2010. Overview and descriptions of fossil stoneflies (Plecoptera) in Baltic Amber. Entomologie Heute 22: 85–97.
Caruso, C.E. and Wichard, W.I. 2011. Paleogeographic distribution of Leuctridae and Nemouridae genera preserved in Baltic amber, with the description of Palaeopsole weiterschani n. gen., n. sp. (Plecoptera). Entomologie Heute 23: 69–77.
Chen, Z.T. 2018a. Baltileuctra gen. nov., a new genus of Leuctridae (Insecta: Plecoptera) in Baltic amber. Zootaxa 4407: 281–287. Crossref
Chen, Z.T. 2018b. Description of Euroleuctra gen. nov., a new fossil genus of Leuctridae (Insecta: Plecoptera) in Eocene Baltic amber. Zootaxa 4462: 291–295. Crossref
Chen, Z.T. 2019a. Redescription of Rhopalopsole hamata Yang & Yang, 1995 (Plecoptera: Leuctridae) based on the male, female, and nymph, with notes on the R. dentata group from China. Zootaxa 4613: 172–180. Crossref
Chen, Z.T. 2019b. The first formal report of brachypterous stonefly of Leuctridae (Plecoptera) from China. Zootaxa 4624: 230–240. Crossref
Chen, Z.T. and Du, Y.Z. 2018. A checklist and adult key to the Chinese stonefly (Plecoptera) genera. Zootaxa 4375: 59–74. Crossref
Consiglio, C. 1957. Tyrrhenoleuctra Nomen Novum. Bollettino della Società Entomologica Italiana 87: 1–2.
Cui, Y., Ren, D., and Béthoux, O. 2019. The pangean journey of “south forestflies” (Insecta: Plecoptera) revealed by their first fossils. Journal of Systematic Palaeontology 17: 255–268. Crossref
Despax, R. 1929. Description sommaire d’un sous-genre nouveau et de deux espèces nouvelles de genre Leuctra Steph (Plecoptera). Bulletin de la Societe Entomologique de France 34: 298–301.
Ham, S.A. and Bae, Y.J. 2002. The stonefly genus Megaleuctra (Plecoptera: Leuctridae) new to East Palearctic region, with description of Megaleuctra saebat new species. Entomological News 113: 336–341.
Hanson, J.F. 1941. Studies on the Plecoptera of North America, II. Bulletin of the Brooklyn Entomological Society 36: 57–66.
Kawai, T. 1968. Stoneflies (Plecoptera) from the Ryukyu Islands in the Bishop Museum, Honolulu. Pacific Insects 10: 231–239.
Klapálek, F. 1905. Conspectus Plecopterorum Bohemiae. Casopis Ceskoslovenské Spolecnosti Entomologické 2: 27–32.
Klapálek, F. 1912. H. Sauter’s Formosa-Ausbeute. Plecoptera. Entomologische Mitteilungen 1: 342–351. Crossref
Kondratieff, B.C. and Lechleitner, R.A. 2002. Stoneflies (Plecoptera) of Mount Rainier National Park, Washington. Western North American Naturalist 62: 385–404.
Larsson, S.G. 1978. Baltic amber—a palaeobiological study. Entomonograph 1: 1–192.
Linnaeus, C. 1758. Systema natura per regna tria naturae secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. 10th Edition. Vol. 1. 824 pp. Laurentius Salvius, Holmiae. Crossref
Neave, F. 1934. Stoneflies from the Purcell Range. The Canadian Entomologist 66: 1–6. Crossref
Nelson, C.H. and Hanson, J.F. 1973. The genus Perlomyia (Plecoptera: Leuctridae). Journal of the Kansas Entomological Society 46: 187–199.
Pardo, I. and Zwick, P. 1993. Contribution to the knowledge of Mediterranean Leuctra (Plecoptera: Leuctridae). Mitteilungen der Schweizersichen Entomologischen Gesellschaft 66: 417–434.
Ricker, W.E. 1943. Stoneflies of southwestern British Columbia. Indiana University Publications Science Series 12: 1–145.
Ricker, W.E. 1952. Systematic studies in Plecoptera. Indiana University Publications Science Series 18: 1–200.
Ricker, W.E. and Ross, H.H. 1969. The genus Zealeuctra and its position in the family Leuctridae (Plecoptera, Insecta). Canadian Journal of Zoology 47: 1113–1127. Crossref
Shepard, W.D. and Baumann, R.W. 1995. Calileuctra, a new genus, and two new species of stoneflies from California (Plecoptera: Leuctridae). The Great Basin Naturalist 55: 124–134. Crossref
Stark, B.P. and Harrison, A.B. 2016. The banded-wing Moselia (Plecoptera: Leuctridae) revisited. Illiesia 12: 42–58.
Stark, B.P. and Kyzar, J.W. 2001. Systematics of Nearctic Paraleuctra with description of a new genus (Plecoptera: Leuctridae). Tijdschrift voor Entomologie 144: 119–135. Crossref
Stark, B.P. and Nelson, C.H. 2019. First SEM micrographs of representatives of Pachyleuctra Despax, 1929 and Tyrrhenoleuctra Consiglio, 1957 (Plecoptera: Leuctridae). Illiesia 15: 98–106.
Stephens, J.F. 1836. Family II.—Perlidae, Leach. Illustrations of British Entomology 6: 134–145.
Stewart, K.W. and Stark, B.P. 2008. Plecoptera. In: R.W. Merritt, K.W. Cummins, and M.B. Berg (eds.), An Introduction to the Aquatic Insects of North America. 4th Edition, 311–384. Kendall/Hunt Publishing Company, Dubuque.
Yasick, A.L., Simmons, T.W., and Earle, J.I. 2003. Parasitic water mite larvae (Acari: Stygothrombidiidae) associated with stonefly adults (Insecta: Plecoptera) from an Allegheny mountain stream in western Pennsylvania, USA. In: I.M. Smith (ed.), ;An Acarological Tribute to David R. Cook(From Yankee Springs to Wheeny Creek), 323–330. Indira Publishing House, West Bloomfield.
Zwick, P. 2000. Phylogenetic system and zoogeography of the Plecoptera. Annual Review of Entomology 45: 709–746. Crossref
Zhi-Teng Chen [741208116@qq.com], School of Grain Science and Technology, Jiangsu University of Science and Technology, Zhenjiang, 212004, China.
Hong-Ling Liu [wstcczt@outlook.com] (corresponding author), Institute of Plant Protection, Sichuan Academy of Agricultural Sciences, Key Laboratory of Integrated Pest Management of Southwest Crops, Ministry of Agriculture, Chengdu, 610066, China.
Received 9 February 2022, accepted 12 May 2022, available online 29 August 2022.
Copyright © 2022 Z.-T. Chen and H.-L. Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Acta Palaeontol. Pol. 67 (3): 649–654, 2022
https://doi.org/10.4202/app.00984.2022