

Molar morphology and occlusion of the Early Jurassic mammaliaform Erythrotherium parringtoni
KAI R.K. JÄGER, PAMELA G. GILL, THOMAS MARTIN, and IAN J. CORFE
Jäger, K.R.K., Gill, P.G., Martin, T., and Corfe, I.J. 2022. Molar morphology and occlusion of the Early Jurassic mammaliaform Erythrotherium parringtoni. Acta Palaeontologica Polonica 67 (4): 975–982.
The South African Early Jurassic morganucodontan Erythrotherium is considered by some authors to be potentially synonymous with Morganucodon, due to similar tooth morphology. However, despite their similar dental morphology, the occlusal pattern of Erythrotherium parringtoni has been described as embrasure occlusion, close to the mode of Megazostrodon rudnerae, rather than that of Morganucodon. In this study the molars of Erythrotherium were re-examined and the two alternative occlusal hypotheses were tested using the Occlusal Fingerprint Analyser (OFA). Morphological comparison of the molars of Erythrotherium parringtoni to those of Morganucodon watsoni showed similarities in cusp height and shape in lingual/buccal views, but the molars and individual cusps of Erythrotherium parringtoni are considerably narrower linguo-buccally, and more gracile. With cusps a and c close together in Erythrotherium parringtoni, cusp positioning differs from that of Morganucodon watsoni and shows similarities to the pattern in Megazostrodon rudnerae. Also, the upper molars of Erythrotherium parringtoni are aligned in a straight row and lack the angle, relative to the longitudinal axis, between the first and second upper molars that is present in Morganucodon watsoni. This results in embrasure occlusion being the only viable occlusal mode for Erythrotherium parringtoni, which was confirmed by the OFA analysis. A Morganucodon-like occlusion would allow only the main cusps a/A to contact their antagonists and thus major gaps would be present, causing considerable reduction of functionality of the dentition. Based on the morphological evidence and the differing occlusal mode, the perpetuation of Erythrotherium parringtoni as a separate genus is confirmed.
Key words: Mammalia, Morganucodonta, dental morphology, occlusion, dental function, Mesozoic, Lesotho, South Africa.
Kai R.K. Jäger [jaegerk@uni-bonn.de] (corresponding author) and Thomas Martin [tmartin@uni-bonn.de], Section of Palaeontology, Institute of Geosciences, Rheinische Friedrich-Wilhelms-Universität Bonn, Nussallee 8, D-53115, Bonn, Germany.
Pamela G. Gill [pam.gill@bristol.ac.uk], School of Earth Sciences, University of Bristol, Wills Memorial Building, Queen’s Road, Bristol, BS8 1RJ, UK; Earth Sciences Department, Natural History Museum, Cromwell Road, London, SW7 5BD, UK.
Ian J. Corfe [ian.corfe@gtk.fi] (corresponding author), Geological Survey of Finland, Vuorimiehentie 2K, FI-02150 Espoo, Finland; Institute of Biotechnology, University of Helsinki, P.O. Box 56, FIN-00014 Helsinki, Finland.
Received 25 March 2022, accepted 8 September 2022, available online 29 November 2022.
Copyright © 2022 K.R.K. Jäger et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Morganucodonta are an insectivorous clade of small early Mammaliaformes that are characterized by a mobile mandibular symphysis, triconodont molars, diphyodonty, and precise occlusion, relative to non-mammaliaform mammaliamorphs (e.g., Kielan-Jaworowska et al. 2004; Luo 2007; Gill et al. 2014; Jäger et al. 2019; Newham et al. 2020). The order is represented by two families, Morganucodontidae and Megazostrodontidae, named after their most prominent representatives, Morganucodon and Megazostrodon (Kielan-Jaworowska et al. 2004). Morganucodon is the best-known genus, being represented by thousands of isolated teeth and jaw fragments from the Glamorgan (UK) fissure fillings belonging to Morganucodon watsoni, as well as other species such as the Chinese Morganucodon oehleri and Morganucodon heikuopengensis (Kermack et al. 1973; Whiteside et al. 2016).
Megazostrodon
is represented by two species, with Megazostrodon
rudnerae from the Upper Triassic–Lower
Jurassic of South Africa and Megazostrodon
chenali from the Upper Triassic of France (Crompton and Jenkins 1968; Debuysschere et
al. 2015). Though M. rudnerae
was initially assigned to Morganucodontidae (Crompton
1974), Gow (1986) proposed the family
Megazostrodontidae based on mandibular characters (Kielan-Jaworowska
et al. 2004; Montellano et al. 2008; Close
et al. 2015).
In this study, references to Morganucodon
generally refer to the material of M. watsoni, Megazostrodon to
the two skulls (NHMUK PV M 26407 and BP/1/4983) of M.
rudnerae, and Erythrotherium
to Erythrotherium parringtoni (Crompton 1974; Gow 1986).
The morganucodontan E. parringtoni is known only from a single fragmentary juvenile skull, with mandibles and some associated postcranial elements, from the Early Jurassic of South Africa (Crompton 1964; Crompton and Jenkins 1968). The postcranial elements were described by Jenkins and Parrington (1976). Subsequent to its original description, Mills (1971) and Kermack et al. (1973) considered Erythrotherium to be a junior synonym of Morganucodon, with the observed differences falling within the range of variability of Morganucodon. Crompton (1974) described the dentition in detail and corroborated the validity of Erythrotherium despite its overall similarity to Morganucodon, pointing out that its ultimate upper premolar is smaller than M1 and that m1 lacks cusp g (= Kühnecone), in contrast to that of Morganucodon. Likewise, Kielan-Jaworowska et al. (2004) considered Erythrotherium to be a valid taxon.
In Morganucodonta, two occlusal modes are present. Embrasure occlusion, which is present in Megazostrodon, is characterized by cusps a and A, of the lower and upper molars, respectively, entering between two antagonistic molars during the power stroke (Crompton and Jenkins 1968). The other occlusal pattern is characterized by cusp a entering in the valley between the upper cusps A and B, and cusp A entering between lower cusps a and c (Crompton and Jenkins 1968). The best-known representative of this second pattern is Morganucodon. However, wear facets in Morganucodon exhibit variability (Crompton and Jenkins 1968), and Jäger et al. (2019) showed that the occlusion of cusp a in Morganucodon is subject to variation and in some cases occludes anterior to B between two upper molars. Thus, the most reliable difference between the two patterns is the occlusion of cusp A.
The occlusal pattern of Erythrotherium has been described as embrasure occlusion similar to that of Megazostrodon, and unlike that of Morganucodon, despite the similarities in molar morphology between Erythrotherium and Morganucodon (Crompton 1974). This interpretation was based on a single wear facet on the mesial side of cusp B of M1, which would have required the lower cusp a to enter mesially to M1 (Crompton 1974). In order to more fully address the occlusal relationships of Erythrotherium, we investigate in this paper additional lines of evidence.
Dental formulae of the three taxa discussed here are: 4.1.4.3?/3.1.4.4 for Erythrotherium (Crompton 1974) and 3.1.4.4/4.1.4.4–5 for Morganucodon watsoni (Mills 1971; Kermack et al. 1981). The two specimens of Megazostrodon differ in their molar count, with the one located at the Bernard Price Institute for Palaeontological Research having 4.1.5.5/4.1.5.5 (Gow 1984) and the holotype located at the Natural History Museum London (NHMUK PV M26407) having ?.?.5.4/?.?.5.4, respectively (Crompton 1974). However, it should be noted that the isolated left m4/M4 are no longer present in the collection, and the lower left dentary at the position of the alveoli of m4 has been damaged since figured in Crompton (1974). Further, Jäger et al. (2019: supplementary data 1) reinterpreted the molar positions on the right side, with m3 and M3 being the most posterior preserved, unlike Crompton (1974) who considered these positions on the right to be m4/M4. However, we tentatively agree with Crompton’s interpretation of an overall molar count of four upper and four lower molars for the holotype.
In this study micro-computed tomography (µ-CT) and 3D models are used to re-examine the molars of Erythrotherium parringtoni and to compare them to those of Morganucodon watsoni and Megazostrodon rudnerae. The hypothesis that Erythrotherium had embrasure type occlusion, similar to Megazostrodon is tested with the Occlusal Fingerprint Analyser software (OFA). With the OFA a hypothetical occlusal path can be simulated and tested via collision detection of contact between two polygonal 3D models of the tooth surfaces (e.g., Kullmer et al. 2009; Schultz et al. 2019; Jäger et al. 2019).
Institutional abbreviations.—NHMUK PV, Natural History Museum, London, UK; SAM-PK, Iziko South African Museum (ISAM), Cape Town, Africa; UMZC, Cambridge University Museum of Zoology, Cambridge, United Kingdom.
Other abbreviations.—OFA, Occlusal Fingerprint Analyser. We follow standard convention in abbreviating tooth families as I, C, P, and M, with upper and lower case letters referring to upper and lower teeth, respectively.
Material and methods
The holotype of Erythrotherium parringtoni (SAM-PK-K00359) is housed in the Iziko South African Museum (ISAM). The specimen was µ-CT scanned at 0.0067 mm resolution, and subsequently, the right lower m2 and m3 and upper M1 and M2 were segmented using Avizo (8.1, Visualization Sciences Group, France) and further processed using Polyworks (2014, InnovMetric Software Inc., Canada). Because the teeth are still in occlusion and the difference in density between the teeth and embedding matrix is small, the quality of the segmented 3D models is lower than the comparative digital models of Morganucodon and Megazostrodon, first introduced in Jäger et al. (2019). Nonetheless, these models are still sufficient for interpreting the morphological and OFA results with confidence.
The term “occlusal fingerprint” was introduced by Kullmer et al. (2009). It describes the orientation and position of wear facets on the occlusal surface. Within the Research Unit 771 of the German Research Foundation (DFG) the OFA software was developed and applied to analyze the chewing path of extinct and extant mammals (e.g., Kullmer et al. 2009, 2013; Benazzi et al. 2011, 2013; Koenigswald et al. 2013; Schultz and Martin 2014; Jäger et al. 2019; Schultz et al. 2019). The polygonal tooth models follow a user-defined pathway based on occlusal hypotheses, and the virtual collision of the teeth is simulated.
The most reliable OFA analyses are based on matching upper and lower dentitions, which is the case for the Erythrotherium specimen discussed here, and the molars chosen for the OFA are those that have suffered little damage. The µ-CT data also provides further information on the molar morphology, as molars have previously only been accessible in buccal view, and thus have not been fully figured.
For comparison with Erythrotherium parringtoni, the holotype of Megazostrodon rudnerae (NHMUK PV M26407) (Crompton 1974) and a well-preserved specimen of Morganucodon watsoni (UMZC Eo.CR.1) were examined (Parrington 1978). Both specimens were chosen because they have matching upper and lower dentitions, and were previously described in detail, together with OFA analysis (Jäger et al. 2019).
Results and discussion
Morphology.—The teeth of Erythrotherium parringtoni were described in detail by Crompton (1964, 1974). Therefore, this study focuses on morphological comparison of 3D models of the molars of E. parringtoni with those of Megazostrodon rudnerae and Morganucodon watsoni. When m2 and m3 of Erythrotherium are compared to the equivalent molars of Megazostrodon and Morganucodon, several morphological differences are apparent (Fig. 1A4–A6, B4–B6, C4–C6). In both the latter, the m2 is noticeably larger than the m3. In Erythrotherium the m3 is slightly larger than the m2. Another striking difference is the width of the lower molars in occlusal view. The lower molars of Erythrotherium are much more slender, relative to their length and height, than those of Morganucodon and Megazostrodon (Table 1). This is partially due to a weaker cingulid in Erythrotherium, a difference already noted by Crompton (1964), but the difference in width is primarily driven by the slender main cusps of Erythrotherium (Fig. 1A4, B4, C4). This is most notable in the mesial region of cusp a, which, despite being the tallest cusp, is almost as slender as cusps b and c (Fig. 1B4). In contrast, cusp a in Morganucodon has a wide base that noticeably surpasses that of cusps b and c, and almost corresponds with the total tooth width (Fig. 1C4). While cusp width differs between Erythrotherium and Morganucodon, the ratio of cusp height of a, b and c is almost equal (Fig. 1B5, C5). In Megazostrodon, cusp a is also the tallest cusp, but the height difference to the other cusps is less pronounced (Fig. 1A5).
Table 1. Length and width measurements (in mm) of the molars of the morganucodontids Morganucodon watsoni (UMZC Eo.CR.1), Erythrotherium parringtoni (SAM-PK-K00359, holotype), and the megazostrodontid Megazostrodon rudnerae (NHMUK PV M26407, holotype) examined in this study.
|
Taxon and tooth position |
Length |
Width |
Length/width ratio |
|
Morganucodon watsoni (UMZC Eo.CR.1) |
|||
|
m2 |
1.51 |
0.75 |
2.01 |
|
m3 |
1.15 |
0.63 |
1.83 |
|
M1 |
1.27 |
0.58 |
2.19 |
|
M2 |
1.47 |
0.77 |
1.91 |
|
Erythrotherium parringtoni (SAM-PK-K00359) |
|||
|
m2 |
1.28 |
0.47 |
2.72 |
|
m3 |
1.3 |
0.52 |
2.5 |
|
M1 |
1.06 |
0.35 |
3.03 |
|
M2 |
1.33 |
0.49 |
2.71 |
|
Megazostrodon rudnerae(NHMUK PV M26407) |
|||
|
m2 |
1.65 |
0.75 |
2.2 |
|
m3 |
1.3 |
0.76 |
1.71 |
|
M1 |
1.55 |
0.53 |
2.92 |
|
M2 |
1.3 |
0.71 |
1.83 |
Cusp positioning of the lower molars of Erythrotherium, on the other hand, is more similar to that of Megazostrodon than to Morganucodon. In Erythrotherium and Megazostrodon a relatively large gap is present between cusps a and b, while cusps a and c are positioned more closely. In contrast, in Morganucodon the space between cusps a and c is large enough to accommodate the upper molar cusp A during occlusion (Jäger et al. 2019) and cusps a and b are positioned relatively close to each other. Cusp d is more distinct and upwards facing in Erythrotherium than in the other taxa, where cusp d faces more posteriorly. Compared to Morganucodon, the small valley for interlocking with the next anterior molar, formed by cusps b and e, is more prominent in Erythrotherium, and thus similar to Megazostrodon.
In lingual and buccal views M1 and M2 of Erythrotherium and Morganucodon are very similar. Relative cusp height and positioning of the main cusps are almost identical in buccal or lingual view (Fig. 1B2, C2). However, the morphological differences between these taxa become apparent in occlusal view. Similar to the lower molars, the upper molars of Erythrotherium are much more slender than those of the other taxa (Fig. 1A1, B1, C1). The upper molars of Morganucodon, in particular, are both more massive, due to wider cusps and cingula. Megazostrodon has a relatively slender M1, almost as slender as that of Erythrotherium, but an M2 that is relatively wider than that of Morganucodon. In Morganucodon the more posterior upper molars, beginning with M2, are angled lingually, relative to the anterior portion of the tooth row, and therefore create space between M1 and M2 for the large central cusp a of m2 (Fig. 1C1). This enables the teeth to pass close to each other, despite the large size of the lower main cusp a (Jäger et al. 2019). This change in orientation is absent in Megazostrodon and Erythrotherium (Fig. 1A1, B1).
The buccal cingulum of Erythrotherium is restricted to the distal and mesial regions and is absent next to cusp A (Fig. 1B1). This division of the buccal cingulum is even more pronounced in Megazostrodon, whereas it is continuous in Morganucodon (Crompton 1974). Similar to the situation in the lower molars, upper molar cusp D is larger and more pronounced in Erythrotherium than in Megazostrodon and Morganucodon (Fig. 1A1–A3, B1–B3, C1–C3).
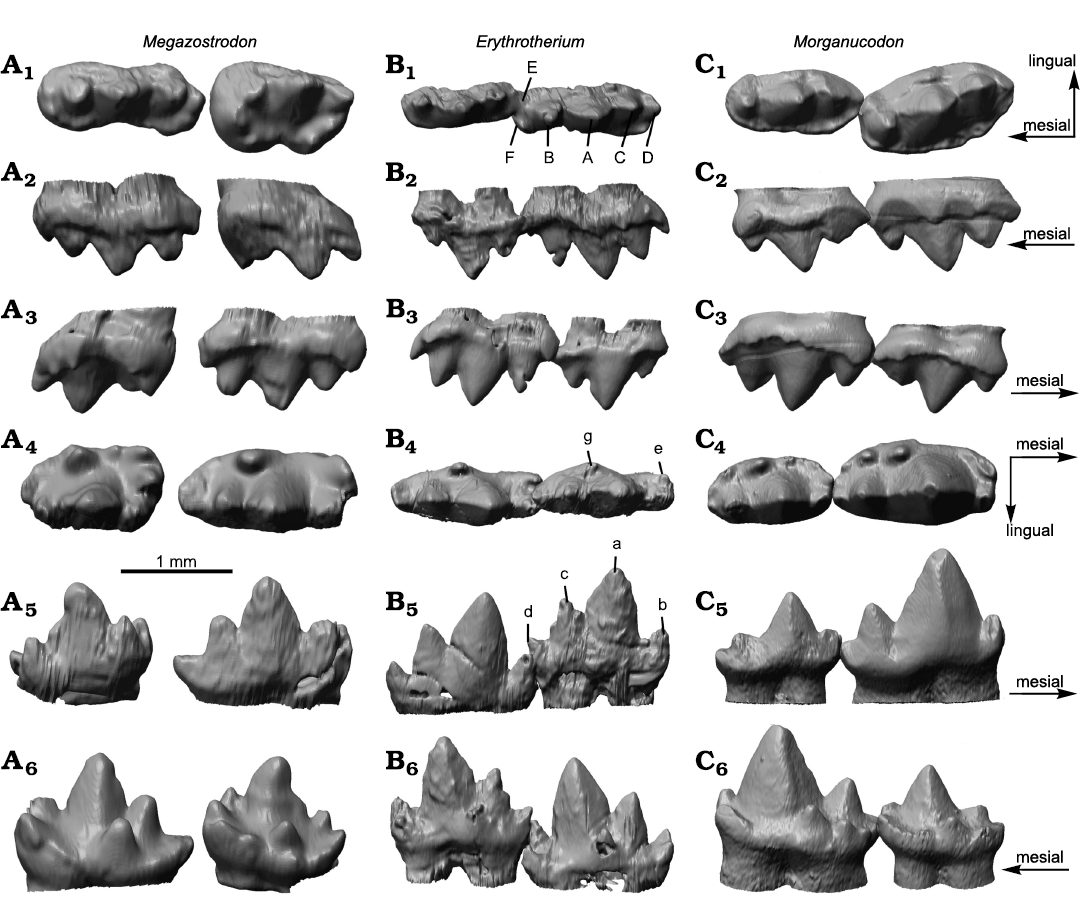
Fig. 1. Upper and lower molar comparison of morganucodontan mammaliaforms Megazostrodon rudnerae (Crompton and Jenkins, 1968), NHMUK PV M26407; Early Jurassic, Lesotho, Red Bed Series (A), Erythrotherium parringtoni (Crompton, 1964) SAM-PK-K00359; Late Triassic, South Africa, Mafeteng locality, Upper Red Beds (B), and Morganucodon watsoni (Kühne, 1949) UMZC Eo.CR.1; Early Jurassic, United Kingdom, Glamorgan fissure systems, Pontalun 3 (C). M1 and M2 in occlusal (A1, B1, C1), lingual (A2, B2, C2), and buccal (A3, B3, C3) views. m2 and m3 in occlusal (A4, B4, C4), buccal (A5, B5, C5), and lingual (A6, B6, C6) views.
Wear.—Little can be said regarding wear on the molars because the teeth of the holotype of Erythrotherium parringtoni are preserved in occlusion, and the 3D models provide limited information. Based on the small degree of wear that he observed, Crompton (1974) concluded that the individual was most likely a juvenile. He described a mesially oriented facet on cusp B of the left upper M1 and this observation is confirmed by the µCT data. Clear wear facets are absent on the right m2, m3, M1, and M2, and the only additional regions on which wear facets can be identified are the buccal side of cusp b of m3, and the lingual side of cusp C of M2.
OFA Analysis.—The OFA analysis was limited due to the lack of observable striations and wear facets, as well as the quality of the occlusal aspects of the 3D models. Nevertheless, the results clearly support the hypothesis by Crompton (1974) that Erythrotherium had embrasure occlusion, rather than a Morganucodon-like occlusal mode. When the occlusal mode described for Morganucodon (Crompton and Jenkins 1968) was applied, only the lower cusps a and c and upper cusps A and C were able to make contact (Fig. 2A5–A8). While cusps a and A entered in the valleys between cusps B and A, and cusps a and c, respectively, their size, relative to the space between the antagonistic cusps, was too large, thus they prevented the rest of the molars from making close contact. Gaps are especially apparent for cusps b/B and d/D (Fig. 2A8) with the Morganucodon-like occlusal mode. With the latter mode, the few observed wear facets in Erythrotherium are located in areas that do not come into close contact with their antagonists. As stated above, in Morganucodon the more posterior upper molars are angled to create space between M1 and M2 for the large central cusp a of m2, in order to provide space for the teeth to pass close to each other (Fig. 2C; Jäger et al. 2019). Apart from this, cusps a and c are further spaced antero-posteriorly in the lower molars of Morganucodon (see above) which apparently is necessary for a functioning occlusion with a Morganucodon-like occlusal mode (Fig. 2C).
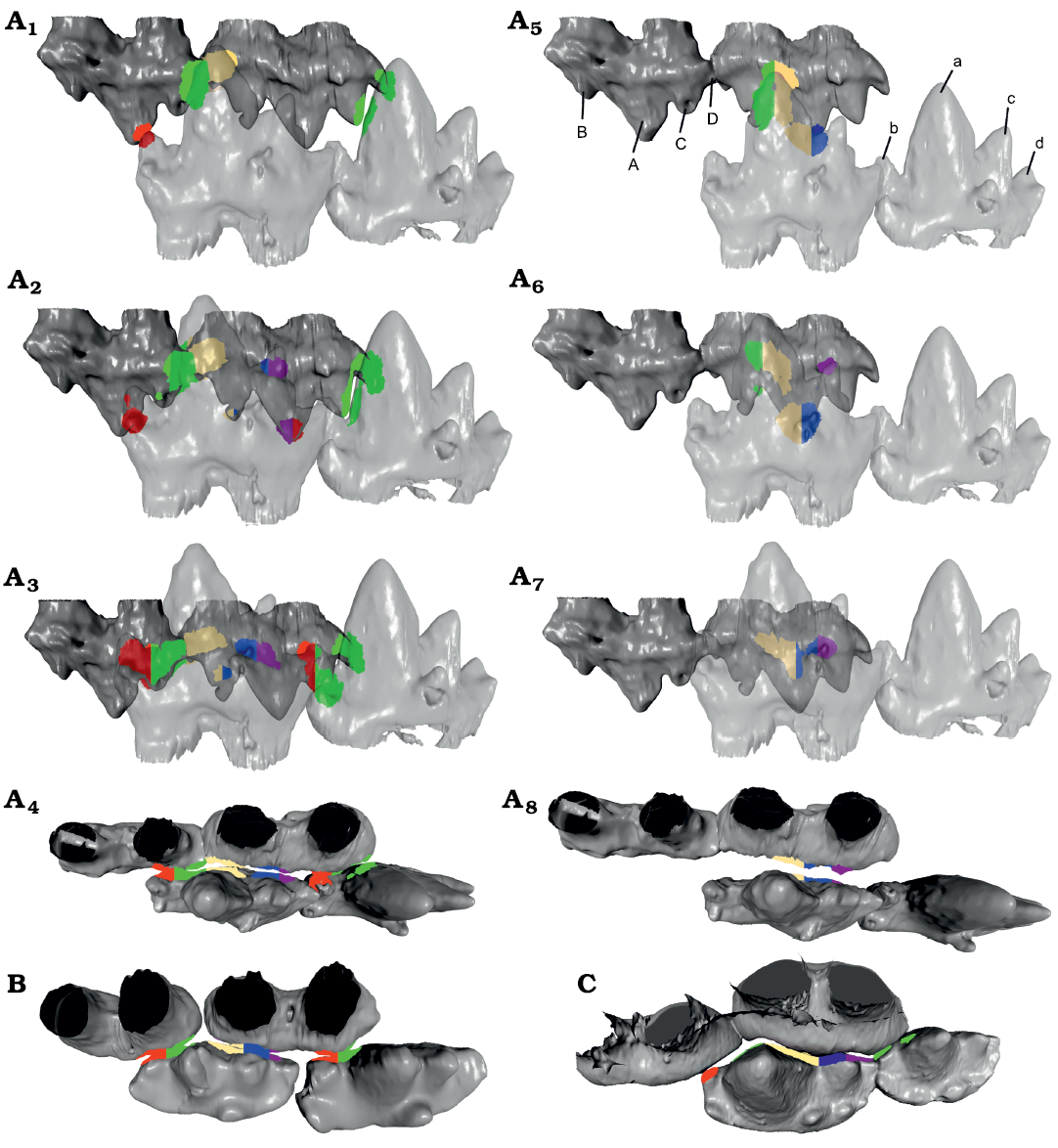
Fig. 2. Comparison of the occlusal hypothesis for m2–m3 and M1–M2 of morganucodontan mammaliaforms Erythotherium parringtoni (Crompton, 1964), SAM-PK-K00359; Late Triassic, South Africa, Mafeteng locality, Upper Red Beds (A), Morganucodon watsoni (Kühne, 1949), UMZC Eo.CR.1; Early Jurassic, United Kingdom, Glamorgan fissure systems, Pontalun 3 (B), and m1–m2, P5 and M1 of Megazostrodon rudnerae (Crompton and Jenkins, 1968), NHMUK PV M26407; Early Jurassic, Lesotho, Red Bed Series (C). Lingual view of Erythrotherium embrasure occlusion and Morganucodon-like occlusion, compared in early (A1, A5), middle (A2, A6), and late (A3, A7) stages of the power stroke. Occlusal view of late stages of the power stroke of Erythrotherium with embrasure occlusion (A4) with Morganucodon-like occlusion (A8), Megazostrodon (B) and Morganucodon (C), note sufficient space between cusps a and c to accommodate cusp A. Additionally, the upper molars are angled to provide space for cusp a, especially the very large cusp a of m2. In comparison, the space between cusps a and c in Erythrotherium is much smaller and both lower and upper molars are aligned in a straight line, similar to Megazostrodon (A4, B). Therefore, Morganucodon-like occlusion in Erythrotherium lacks contact for the b/B and d/D cusps (A8). Embrasure occlusion on the other hand results in collision detection on all major cusps and correlates with observed wear facets (A4). This becomes most apparent in the later stages of the power stroke (A3), where embrasure occlusion results in well-aligned teeth with the a/A cusps utilizing the space between two antagonists. With Morganucodon-like occlusion the direct contact of the a/A cusps prevents close contact of the smaller cusps (A7, A8). Colored regions represent contact areas during the OFA analysis, for comparison see Jäger et al. (2019). Mesial is to the left. Not to scale.
In contrast, when embrasure occlusion is applied for Erythrotherium, the teeth pass along each other in much closer proximity, resulting in more extensive collision surface detection on all cusps (Fig. 2A1–A4). Cusp a occludes into the space between two molars, thus allowing cusp c to enter between cusps A and B, and cusp C to enter between cusps b and a. The limited space between cusps A and C is sufficient for the small cusp b to enter. Unlike the simulation using a Morganucodon-like occlusal mode, embrasure occlusion brings the observed wear facets on the lingual side of cusp C of M2, and the buccal side of cusp b of m3, into contact over the course of the power stroke (Fig. 2A3). The difference with a Morganucodon-like occlusion becomes most apparent when the two occlusal modes are compared during the later phase of the power stroke from a dorsal view (Fig. 2A4, A8). Embrasure occlusion results in a better fit along all of the teeth involved (Fig. 2A4).
Bhullar et al. (2019) discussed the potential presence of mandibular roll during occlusion in early Mammaliaformes. For Erythrotherium the OFA was able to produce all observed wear facets without the application of roll. This does not exclude the presence of roll during the power stroke; however, it shows that, if roll was present, it was likely only a few degrees. This is in accordance with observations on Morganucodon (Jäger et al. 2019). Further analysis of non-mammalian Mammaliaformes is required to determine where in the mammalian phylogeny such extended jaw-roll first appeared (Bhullar et al. 2019; Grossnickle 2020).
Conclusions
We consider that the morphological differences between the molars of Erythrotherium parringtoni and Morganucodon spp. are sufficient to maintain Erythrotherium as a separate genus. While some aspects, such as cusp height and relative proportions are similar to Morganucodon, others, such as tooth width and the relative positions of the lower molar cusps, differ noticeably. While the dental morphology of Erythrotherium is more similar to that of Morganucodon, the occlusal mode (embrasure occlusion), verified via OFA analysis, resembles that of Megazostrodon, as previously observed by Crompton (1974). This supports the interpretation by Jäger et al. (2019) that the difference in the occlusal mode of Megazostrodon and Morganucodon is primarily driven by relative cusp positioning, and potentially by the angulation of the upper teeth within the maxillae, rather than by the exact position of the teeth along the longitudinal axis of the jaw (Crompton 1974).
Jäger et al. (2020) argued that Triconodontidae, which previously were considered to have had a Morganucodon-like occlusion (Mills 1971), instead also had embrasure occlusion. With embrasure occlusion confirmed for Erythrotherium, only Morganucodon and a few other members of Morganucodonta, such as Dinnetherium nezorum (Crompton and Luo 1993), Storchodon cingulatus (Martin et al. 2019), and a new morganucodontan from the Morrison Formation (Davis et al. 2022), exhibit a Morganucodon-like occlusal mode.
In non-mammaliaform cynodonts such as Thrinaxodon, upper and lower molariforms did not occlude, thus the postcanines usually lack wear facets (Crompton and Jenkins 1968; Crompton 1974). In Thrinaxodon, tooth positioning could vary considerably, with some specimens having the lower teeth positioned directly lingually to the uppers when the jaw is closed, and others having an alternating positioning of lower and upper teeth, more similar to an embrasure pattern (Crompton 1974; Abdala et al. 2013).
Mills (1971) argued that the mammaliamorph Sinoconodon rigneyi had embrasure occlusion similar to Megazostrodon rudnerae. A similar positioning was depicted by Crompton and Sun (1985; Fig. 1A1) with cusps a/A positioned between two antagonists. However, molariforms in S. rigneyi were replaced, and multiple studies pointed out that S. rigneyi lacked a constant occlusal pattern (Crompton 1974; Crompton and Sun 1985; Crompton and Luo 1993). Butler (1997) also suggested embrasure occlusion in the Rhaetian “symmetrodontan” Woutersia spp.
While neither of the two occlusal modes can be confirmed with certainty as the ancestral, plesiomorphic condition of Mammaliaformes, embrasure occlusion appears to be the more likely, since Morganucodon-like occlusion seems to have been limited to Morganucodon and a few other morganucodontan taxa. Embrasure occlusion, on the other hand, was present in some other members of Morganucodonta, “symmetrodontans”, Eutriconodonta, and also appears in non-mammaliaform cynodonts, although without occlusal contact (Crompton 1971, 1974; Butler 1997; Butler et al. 2012; Jäger et al. 2020).
Jäger et al. (2019) demonstrated that Morganucodon-like occlusion required teeth with sufficient spacing between cusps a and c; the massive a cusps most likely also required the rearrangement of tooth position and inclination in the upper molar row, the latter being also present in a new morganucodontan from the Morrison Formation (Davis et al. 2022). In that regard, it is noteworthy that Morganucodon was abundant (Gill et al. 2014), widespread (Kermack et al. 1973; Kielan-Jaworowska et al. 2004), and arguably a highly successful mammaliaform, and that this type of occlusal mode, while limited to a smaller number of taxa, persisted at least until the Late Jurassic (Martin et al. 2019; Davis et al. 2022). One potential explanation is that Morganucodon-like occlusion was derived from embrasure occlusion and provided increased food processing capabilities relative to other early Mammaliaformes, contributing to the success of the genus.
Acknowledgements
We thank Bhart-Anjan Bhullar (Yale University, New Haven, USA) and Roger Smith (ISAM), who kindly provided the scan of Erythrotherium parringtoni, as well as Pip Brewer (Natural History Museum of Denmark, Copenhagen, Denmark), Michael Day, Farah Ahmed, Amin Garbout (all Natural History Museum, London, UK), Robert Asher and Mathew Lowe (both UMZC) for scans and access to comparative material of Morganucodon and Megazostrodon. We are also grateful to Anton Du Plessis (ISAM), Fernando Abdala (Bernard Price Institute for Palaeontological Research, Johannesburg, South Africa) and Amin Garbout (Natural History Museum, London, United Kingdom), who scanned the material used in this study. We also thank Richard Cifelli (Sam Noble Museum, Norman, USA) and David Grossnickle (University of Washington, Seattle, USA) for their kind and helpful reviews. This study was funded by grants MA 1643/15-2 and MA 1643/20-1 of the Deutsche Forschungsgemeinschaft (DFG) to TM.
References
Abdala, F., Jasinoski, S.C., and Fernandez, V. 2013. Ontogeny of the early Triassic cynodont Thrinaxodon liorhinus (Therapsida): dental morphology and replacement. Journal of Vertebrate Paleontology 33: 1408–1431. Crossref
Benazzi, S., Kullmer, O., Grosse, I.R., and Weber, G.W. 2011. Using occlusal wear information and finite element analysis to investigate stress distributions in human molars. Journal of Anatomy 219: 259–272. Crossref
Benazzi, S., Kullmer, O., Schulz, D., Gruppioni, G., and Weber, G.W. 2013. Technical note: individual tooth macrowear pattern guides the reconstruction of Sts 52 (Australopithecus africanus) dental arches. American Journal of Physical Anthropology 150: 324–329. Crossref
Bhullar, B.A.S., Manafzadeh, A.R., Miyamae, J.A., Hoffmann, E.A., Brainerd, E.L., Musinsky, C., and Crompton, A.W. 2019. Rolling of the jaw is essential for mammalian chewing and tribosphenic molar function. Nature 566: 528–532. Crossref
Butler, P.M. 1997. An alternative hypothesis on the origin of docodont molar teeth. Journal of Vertebrate Paleontology 17: 435–439. Crossref
Butler, P.M., Sigogneau-Russell, D., and Ensom, P.C. 2012. Possible persistence of the morganucodontans in the Lower Cretaceous Purbeck Limestone Group (Dorset, England). Cretaceous Research 33: 135–145. Crossref
Close, R.A., Friedman, M., Lloyd, G.T., and Benson, R.B.J. 2015. Evidence for a mid-Jurassic adaptive radiation in mammals. Current Biology 25:2137–2142. Crossref
Crompton, A.W. 1964. A preliminary description of a new mammal from the Upper Triassic of South Africa. Journal of Zoology 142: 441–452. Crossref
Crompton, A.W. 1971. The origin of the tribosphenic molar. In: D.M. Kermack and K.A. Kermack (eds.), Early Mammals. Zoological Journal of the Linnean Society 50 (Supplement No. 1): 65–87.
Crompton, A.W. 1974. The dentitions and relationships of Southern African Triassic mammals, Erythrotherium parringtoni and Megazostrodon rudnerae. Bulletin of the British Museum (Natural History), Geology Series 24: 397–437.
Crompton, A.W. and Jenkins, F.A. 1968. Molar occlusion in Late Triassic mammals. Biological Reviews 43: 427–458. Crossref
Crompton, A.W. and Luo, Z.-X. 1993. Relationships of the Liassic mammals Sinoconodon, Morganucodon, and Dinnetherium. In: F.S. Szalay, M.J. Novacek, and M.C. McKenna (eds.), Mammal Phylogeny, Volume 1, Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials, 30–44. Springer-Verlag, Inc., New York. Crossref
Crompton A.W. and Sun, A.-L. 1985. Cranial structure and relationships of the Liassic mammal Sinoconodon. Zoological Journal of the Linnean Society 85: 99–119. Crossref
Davis, B.M., Jäger, K.R.K., Rougier, G.W., Trujillo, K., and Chamberlain, K. 2022. A morganucodontan mammal from the Upper Jurassic Morrison Formation, Utah, USA. Acta Palaeontologica Polonica 67: 77–93. Crossref
Debuysschere, M., Gheerbrant, E., and Allain, R. 2015. Earliest known European mammals: a review of the Morganucodonta from Saint-Nicolas-de-Port (Upper Triassic, France). Journal of Systematic Palaeontology 13: 825–855. Crossref
Gill, P.G., Purnell, M.A., Crumpton, N.K., Brown, R., Gostling, N.J., Stampanoni, M., and Rayfield, E.J. 2014. Dietary specializations and diversity in feeding ecology of the earliest stem mammals. Nature 512: 303–305. Crossref
Gow, C.E. 1986. A new skull of Megazostrodon (Mammalia: Triconodonta) from the Elliot Formation (Lower Jurassic) of southern Africa. Palaeontologia Africana 26: 13–23.
Grossnickle, D.M. 2020. Jaw roll and jaw yaw in early mammals. Nature 582: E6–E8. Crossref
Jäger, K.R.K, Cifelli, R.L., and Martin, T. 2020. Molar occlusion and jaw roll in early crown mammals. Scientific Reports 10: 22378. Crossref
Jäger, K.R.K., Gill, P.G., Corfe, I., and Martin, T. 2019. Occlusion and dental function of Morganucodon and Megazostrodon. Journal of Vertebrate Paleontology 39: e1635135. Crossref
Jenkins, F.A. and Parrington, F.R. 1976. The postcranial skeletons of the Triassic mammals Eozostrodon, Megazostrodon and Erythrotherium. Philosophical Transactions of the Royal Society of London 273: 387–431. Crossref
Kielan-Jaworowska, Z., Cifelli, R.L., and Luo, Z.-X. 2004. Mammals from the Age of Dinosaurs: Origins, Evolution and Structure. 630 pp. Columbia University Press, New York. Crossref
Koenigswald, W. von, Anders, U., Engels, S., Schultz, J.A., and Kullmer, O. 2013. Jaw movement in fossil mammals: analysis, description and visualization. Paläontologische Zeitschrift 87: 141–159. Crossref
Kermack, K.A., Musset, F., and Rigney, H.W. 1981. The skull of Morganucodon. Zoological Journal of the Linnean Society 71: 1–158. Crossref
Kermack, K.A., Musset, F., and Rigney, H.W. 1973. The lower jaw of Morganucodon. Zoological Journal of the Linnean Society 33: 87–175. Crossref
Kullmer, O., Benazzi, S., Fiorenza, L., Schulz, D., Bacso, S., and Winzen, O. 2009. Technical note: occlusal fingerprint analysis: quantification of tooth wear pattern. American Journal of Physical Anthropology 139: 600–605. Crossref
Kullmer, O., Benazzi, S., Schulz, D., Gunz, P., Kordos, L., and Begun, D.R. 2013. Dental arch restoration using tooth macrowear patterns with application to Rudapithecus hungaricus, from the late Miocene of Rudabánya. Journal of Human Evolution 64: 151–160. Crossref
Luo, Z.-X. 2007. Transformation and diversification in early mammal evolution. Nature 450: 1011–1019. Crossref
Newham, E., Gill, P.G., Brewer, P., Benton, Fernandez, V.M.J., Gostling, N.J., Haberthür, D., Jernvall, J., Kankaanpää, T., Kallonen, A., Navarro, C. Pacureanu, A., Richards, K., Robson Brown, K., Schneider, P., Suhonen, H., Tafforeau, P., Williams, K.A., Zeller-Plumhoff, B., and Corfe, I.J. 2020. Reptile-like physiology in Early Jurassic stem-mammals. Nature Communications 11: 1–13. Crossref
Martin, T., Averianov, A.O., Jäger, K.R.K., Schwermann, A.H., and Wings, O. 2019. A large morganucodontan mammaliaform from the Late Jurassic of Germany. Fossil Imprint 75: 504–509. Crossref
Mills, J.R.E. 1971. The dentition of Morganucodon. In: D.M. Kermack, and K.A. Kermack (eds.), Early Mammals. Zoological Journal of the Linnean Society 50 (Supplement 1): 29–63.
Montellano, M., Hopson, J.A., and Clark, J.M. 2008. Late Early Jurassic mammaliforms from Huizachal Canyon, Tamaulipas, Mexico. Journal of Vertebrate Paleontology 28: 1130–1143. Crossref
Parrington, F.R. 1978. A further account of the Triassic mammals. Philosophical Transactions Royal Society Series B, Biological Sciences 282 (989): 177–204. Crossref
Schultz, J.A. and Martin, T. 2014. Function of pretribosphenic and tribosphenic mammalian molars inferred from 3D animation. Naturwissenschaften 101: 771–781. Crossref
Schultz, J.A., Bhullar, B.-A., and Luo, Z.-X. 2019. Re-examination of the Jurassic mammaliaform Docodon victor by computed tomography and occlusal functional analysis. Journal of Mammalian Evolution 26: 9–38. Crossref
Whiteside, D.I., Duffin, C.J., Gill, P.G., Marshall, J.E.A., and Benton, M.J. 2016. The Late Triassic and Early Jurassic fissure faunas from Bristol and South Wales: Stratigraphy and setting. Palaeontologia Polonica 67: 257–287.
Acta Palaeontol. Pol. 67 (4): 975–982, 2022
https://doi.org/10.4202/app.00998.2022