

Dimorphism in tetragonitid ammonoid Tetragonites minimus from the Upper Cretaceous in Hokkaido, Northern Japan
DAISUKE AIBA
Aiba, D. 2022. Dimorphism in tetragonitid ammonoid Tetragonites minimus from the Upper Cretaceous in Hokkaido, Northern Japan. Acta Palaeontologica Polonica 67 (4): 949–961.
Mature modifications, ontogeny, and dimorphism of the small-sized tetragonitid ammonoid Tetragonites minimus were investigated in 43 specimens from the Santonian, Upper Cretaceous of the northwestern area of Hokkaido, Japan. Four types of mature modifications were recognised in the shell diameters of 11–13 mm and 16–19 mm, and two differently sized adults were regarded as microconchs and macroconchs respectively. The conch forms of dimorphic pairs were similar in juvenile but differ in the later stage. The supplementary analysis showed that the remarkable adult size differences in antidimorphs continued at least in the Turonian–Santonian. The mature size and size difference between dimorphic pairs decreased chronologically. 36 specimens (84% of examined specimens) were mature, and immature shells were rare in the Santonian. Most of the shells were remarkably well preserved, indicating that T. minimus assemblage fossilised quickly near their original habitat without long-distance post-mortem transport. Hence the bias in the fossil occurrence of adult T. minimus is unlikely to be due to taphonomy such as the bias of fossilisation potential and floatability in the bottom currents. Tetragonites minimus might have been changing their habitats during their life cycle.
Key words: Ammonoidea, Tetragonites, dimorphism, taphonomy, Late Cretaceous, Yezo Group, Hokkaido.
Daisuke Aiba [aiba698@city.mikasa.hokkaido.jp; ORCID: https://orcid.org/0000-0002-2940-0433], Mikasa City Museum, 1-212-1, Nishiki-machi, Ikushumbetsu, Mikasa City, Hokkaido 068-2111, Japan.
Received 8 April 2022, accepted 19 August 2022, available online 30 November 2022.
Copyright © 2022 D. Aiba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The genus Tetragonites Kossmat, 1895, belongs to the family Tetragonitidae, superfamily Lytoceratoidea (Hoffman 2015). This genus is characterized by involute sub-rectangular whorl section and smooth shell surface (Murphy 1967a, b; Wiedmann 1973; Shigeta 1989; Wright et al. 1996; Hoffmann 2010, 2015). The genus ranges from Aptian to Maastrichtian strata worldwide (e.g., Hoffmann 2015). Five species of Tetragonites are known from the Yezo Group cropping out in Hokkaido, northern Japan and Sakhalin, Russian Far East (Jimbo 1894; Yabe 1903; Matsumoto 1942; Shigeta 1989; Matsumoto and Toshimitsu 1991; Maeda et al. 2005). One remarkable species among them, Tetragonites minimus Shigeta, 1989, having small-sized shell with adorally convex aperture on the venter was established by Shigeta (1989). This species occurs from Turonian to Campanian strata in the north-western Pacific realm, but is thought to be derived from Tetragonites spathi (Fabre, 1940), which inhabited southern regions in the Cenomanian (Shigeta 1989, 2001).
Mature modifications are morphological changes that appear only in the adult shell of fossil molluscs and are clues to recognising dimorphism. Numerous cases of dimorphism have been reported among ammonoids across geologic eras and taxa (e.g., Callomon 1955, 1963; Makowski 1962; Davis et al. 1969, 1996; Klug et al. 2015). In several cases, species that had been assigned to different taxa were identified as the dimorphic pairs by later authors. Therefore, the detection of dimorphism is important for ammonoid taxonomy. Recognising the mature modification and dimorphism is important not only from the point of view of taxonomy, but also from the palaeoecological viewpoint such as reproduction and mating, because the concept that dimorphic pairs correspond to sex is widely accepted (Makowski 1962; Davis et al. 1996; Klug et al. 2015).
Dimorphism is known in Cretaceous ammonoids (e.g., Cobban 1969; Tanabe 1977, 2022; Futakami 1990; Cobban and Kennedy 1993; Landman and Waage 1993; Maeda 1993; Davis et al. 1996; Metzdorf and Sowiak 2003; Machalski 2005; Landman et al. 2012), but has not yet been reported in the genus Tetragonites. Davis et al. (1996) pointed out that the differences in adult sizes among some species of Tetragonites described by Wiedmann (1973), could indicate dimorphism. However, this hypothesis remained untested. In this paper, the mature modifications and dimorphism of T. minimus are described. In addition, the taphonomy and palaeoecology of this species is discussed.
Institutional abbreviations.—MCM, Mikasa City Museum, Hokkaido, Japan; UMUT, University Museum, University of Tokyo, Japan.
Other abbreviations.—B, whorl breadth; B/D, whorl breadth ratio to D; D, shell diameter; H1/D, whorl height ratio to D; H2/D, ventral whorl height ratio to D; [m], microconch; [M], macroconch; PD, phragmocone diameter; U/D, umbilical width ratio to D.
Geological setting
The Cretaceous (Aptian–Maastrichtian) forearc basin deposits, the Yezo Group, is widely distributed in a north to south direction, from Sakhalin, Russian Far East to Hokkaido, northern Japan (Matsumoto 1954; Takashima et al. 2004; Shigeta and Maeda 2005). The Yezo Group is well exposed in the Haboro, Kotanbetsu, and Tappu areas of northwestern Hokkaido, and its stratigraphy has been studied in detail (e.g., Tanabe et al. 1977; Toshimitsu 1985, 1988; Wani and Hirano 2000; Okamoto et al. 2003; Oizumi et al. 2005; Honda and Hirano 2014; Fig. 1). The Haborogawa Formation, widely distributed in these areas is composed of mudstone, sandy mudstone, and sandstone; and by correlation is assigned to the Turonian–lower Campanian stages (Toshimitsu 1985, 1988; Wani and Hirano 2000; Okamoto et al. 2003; Takashima et al. 2004). The strata with north-south strike dip generally westward, and are successively exposed ascending from east to west (Toshimitsu 1985, 1988; Wani and Hirano 2000; Aiba 2019). The strata consist of several cycles of upward-coarsening sequences ranging from sandy siltstone to sandstone, and from the sedimentary structures, it is assumed that the depositional environments correspond to an outer shelf and storm-dominated inner shelf to shoreface (Toshimitsu 1985, 1988; Wani 2003; Tsujino and Maeda 2007).
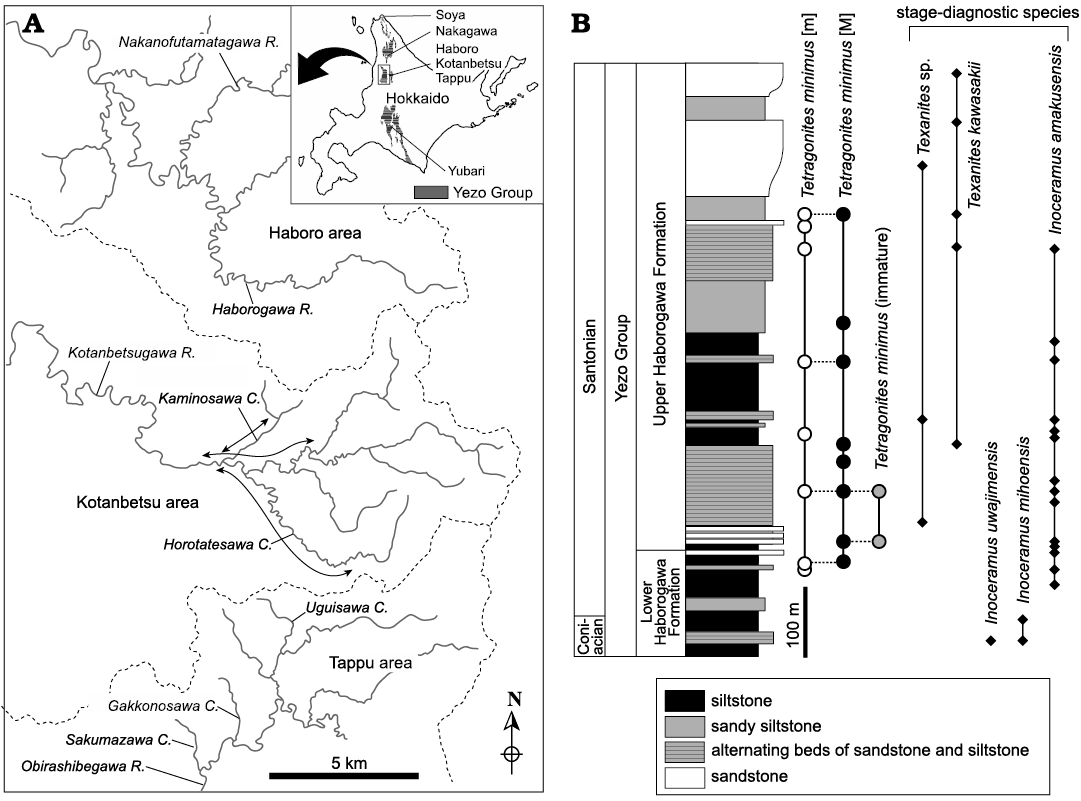
Fig. 1. A. Location map around studied areas in Hokkaido, Japan (inset shoving location of Yezo Group deposits). B. Generalized columnar section of the Santonian, Yezo Group in the Kotanbetsu area showing stratigraphic occurrence of dimorphic pairs of Tetragonites minimus Shigeta, 1989, and the selected stage-diagnostic mega-fossils. Double head arrows indicate the areas where the columnar sections were constructed. See Aiba (2019) for details of stratigraphy. Abbreviations: C., Creek; R., River.
Material and methods
Material.—43 specimens of Tetragonites minimus, collected from the Santonian in the Kotanbetsu, Tappu, and Haboro areas, Hokkaido, Japan were studied (Figs. 1, 2; Table 1). These specimens are housed in the Mikasa City Museum (Hokkaido, Japan). The numbering of localities used in this paper corresponds to that used in the previous studies in Kotanbetsu (Aiba 2019) and Tappu (Oizumi et al. 2005). Some of the Tappu specimens are listed as “Tetragonites glabrus” or “Tetragonites sp.” in Oizumi et al. (2005). All the specimens were extracted from calcareous concretions encased in siltstone or sandy siltstone beds. Some of them were preserved in the same concretion or horizon. 33 specimens were collected from calcareous concretions in situ in the outcrops, and the other ten were collected from calcareous concretions as floats in the river. The geological age of all specimens is Santonian, judging from the co-occurring stage-diagnostic ammonoids and inoceramid bivalves (Toshimitsu et al. 1995, 2007). In addition to the Santonian specimens of T. minimus, 30 specimens from the Turonian–Coniacian and the Campanian were examined (Table 2). The locality numbers are shown in the following publications: UMUT specimens, Shigeta (1989 and references therein); MCM specimens from Kotanbetsu area, Aiba (2019); and MCM specimens from Tappu area, Funaki and Hirano (2004).
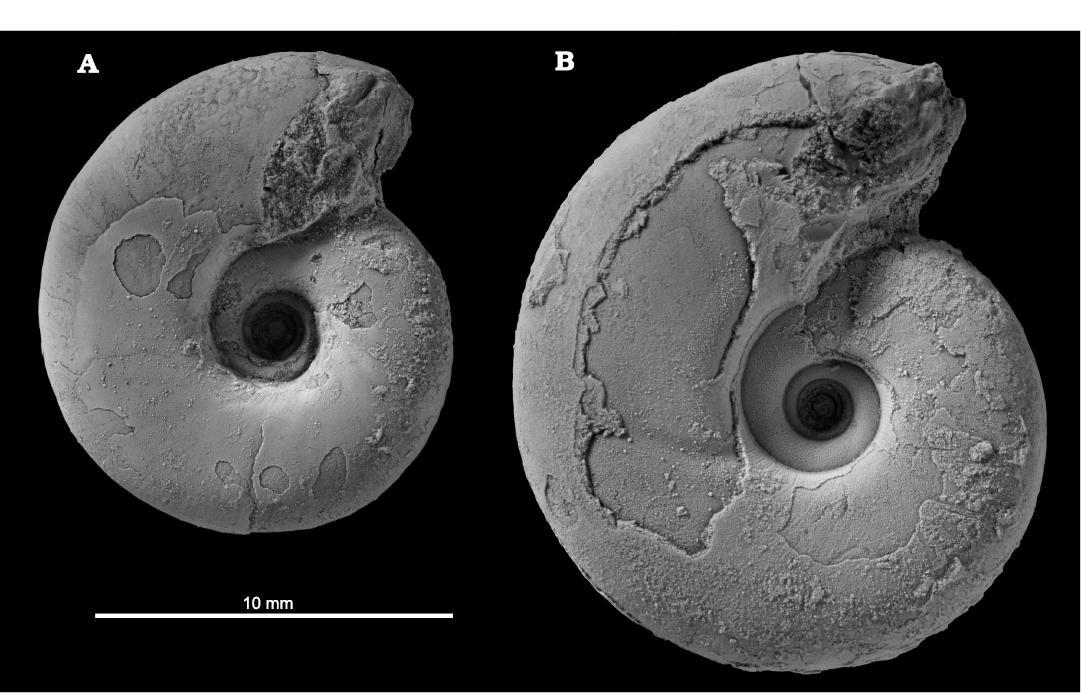
Fig. 2. Photographs of microconch and macroconch in lateral view of tetragonitid ammonoid Tetragonites minimus Shigeta, 1989, from the Santonian, Yezo Group, Horotatesawa Creek (A, outcrop HR001, and B, HR109) the Kotanbetsu area, Hokkaido, Japan. A. MCM-W1560 [m]. B. MCM-W1572 [M].
Table 1. List of Santonian specimens of tetragonitid ammonoid Tetragonites minimus Shigeta, 1989, from the Yezo Group in Hokkaido, Japan. Measurements in mm. Abbrevitions: C., Creek; D, shell diameter; [m], microconch; [M], macroconch; PD, phragmocone diameter; R., River; + satisfied; – unsatisfied; ?, unmeasurable or undecidable. The preservation categories: A, complete or almost intact; B, incomplete.
|
Specimen number |
Locality |
Area |
D |
PD |
Adult |
Mature modifications |
Antidimorphs |
Preservation |
|||
|
(i) |
(ii) |
(iii) |
(iv) |
||||||||
|
MCM–W0239-1 |
Sakumazawa C. (OB33013y) |
Tappu |
15.0 |
10.3 |
+ |
+ |
? |
? |
? |
[M] |
A |
|
MCM-W0249-1 |
Gakkonosawa C. (OB35017b) |
Tappu |
17.7 |
11.3 |
+ |
+ |
+ |
+ |
– |
[M] |
A |
|
MCM-W0252-1 |
Gakkonosawa C. (OB35021a) |
Tappu |
16.5 |
10.4 |
+ |
+ |
+ |
– |
– |
[M] |
A |
|
MCM-W0256-1 |
Gakkonosawa C. (OB35041a) |
Tappu |
16.3 |
10.6 |
+ |
+ |
? |
– |
– |
[M] |
A |
|
MCM-W0499-1 |
Uguisawa C. (OB40599a) |
Tappu |
19.3 |
11.3 |
+ |
+ |
? |
+ |
– |
[M] |
A |
|
MCM-W1549 |
Kotanbetsugawa R. (float) |
Kotanbetsu |
15.4 |
10.2 |
+ |
+ |
+ |
+ |
+ |
[M] |
A |
|
MCM-W1550 |
Kotanbetsugawa R. (KT045k) |
Kotanbetsu |
13.0 |
8.3 |
+ |
+ |
? |
+ |
– |
[m] |
A |
|
MCM-W1551 |
Kotanbetsugawa R. (KT093b) |
Kotanbetsu |
17.9 |
11.2 |
– |
? |
? |
? |
? |
? |
A |
|
MCM-W1553 |
Kotanbetsugawa R. (KT093a) |
Kotanbetsu |
12.7 |
8.9 |
+ |
+ |
? |
? |
? |
[m] |
A |
|
MCM-W1554 |
Kotanbetsugawa R. (KT093g) |
Kotanbetsu |
11.6 |
7.5 |
+ |
+ |
+ |
? |
– |
[m] |
A |
|
MCM-W1555 |
Kotanbetsugawa R. (KT093y) |
Kotanbetsu |
? |
7.9 |
+ |
+ |
? |
+ |
+ |
[m] |
B |
|
MCM-W1556 |
Kotanbetsugawa R. (KT093d) |
Kotanbetsu |
18.2 |
10.9 |
+ |
+ |
? |
? |
? |
[M] |
A |
|
MCM-W1557 |
Kotanbetsugawa R. (KT113b) |
Kotanbetsu |
? |
11.9 |
+ |
? |
? |
+ |
+ |
[M] |
B |
|
MCM-W1558 |
Kotanbetsugawa R. (KT121a) |
Kotanbetsu |
13.1 |
8.6 |
+ |
+ |
? |
– |
? |
[m] |
A |
|
MCM-W1559 |
Kotanbetsugawa R. (KT121a) |
Kotanbetsu |
12.3 |
8.3 |
+ |
+ |
? |
+ |
? |
[m] |
A |
|
MCM-W1560 |
Horotatesawa C. (HR001y) |
Kotanbetsu |
13.0 |
8.5 |
+ |
+ |
+ |
+ |
– |
[m] |
A |
|
MCM-W1561 |
Horotatesawa C. (HR001y) |
Kotanbetsu |
18.1 |
11.2 |
+ |
+ |
? |
+ |
– |
[M] |
A |
|
MCM-W1562 |
Horotatesawa C. (HR003a) |
Kotanbetsu |
13.1 |
8.4 |
+ |
+ |
? |
+ |
+ |
[m] |
A |
|
MCM-W1563 |
Horotatesawa C. (HR015c) |
Kotanbetsu |
17.1 |
11.5 |
+ |
+ |
? |
? |
? |
[M] |
A |
|
MCM-W1564 |
Horotatesawa C. (HR023e) |
Kotanbetsu |
12.1 |
8.0 |
+ |
? |
? |
+ |
+ |
[m] |
A |
|
MCM-W1565 |
Horotatesawa C. (HR023c) |
Kotanbetsu |
? |
11.7 |
+ |
? |
? |
+ |
– |
[M] |
A |
|
MCM-W1567 |
Horotatesawa C. (HR057y) |
Kotanbetsu |
12.2 |
8.3 |
+ |
+ |
? |
- |
– |
[m] |
A |
|
MCM-W1568 |
Horotatesawa C. (HR109a) |
Kotanbetsu |
18.1 |
11.4 |
+ |
+ |
+ |
+ |
– |
[M] |
A |
|
MCM-W1569 |
Horotatesawa C. (HR109a) |
Kotanbetsu |
? |
11.1 |
– |
? |
? |
? |
? |
? |
B |
|
MCM-W1570 |
Horotatesawa C. (HR109a) |
Kotanbetsu |
17.4 |
11.5 |
+ |
+ |
? |
+ |
+ |
[M] |
A |
|
MCM-W1571 |
Horotatesawa C. (HR109b) |
Kotanbetsu |
? |
11.4 |
– |
? |
? |
? |
? |
? |
B |
|
MCM-W1572 |
Horotatesawa C. (HR109c) |
Kotanbetsu |
16.6 |
10.6 |
+ |
+ |
- |
+ |
– |
[M] |
A |
|
MCM-W1573 |
Horotatesawa C. (HR109c) |
Kotanbetsu |
11.3 |
7.6 |
+ |
+ |
? |
+ |
+ |
[m] |
A |
|
MCM-W1574 |
Horotatesawa C. (HR109c) |
Kotanbetsu |
? |
7.4 |
+ |
? |
? |
+ |
– |
[m] |
A |
|
MCM-W1575 |
Horotatesawa C. (HR119b) |
Kotanbetsu |
? |
10.0 |
+ |
? |
? |
+ |
? |
[M] |
A |
|
MCM-W1576 |
Horotatesawa C. (HR119b) |
Kotanbetsu |
? |
11.0 |
+ |
+ |
? |
? |
? |
[M] |
A |
|
MCM-W1577 |
Horotatesawa C. (HR119b) |
Kotanbetsu |
? |
11.0 |
+ |
+ |
? |
+ |
? |
[M] |
B |
|
MCM-W1578 |
Kotanbetsugawa R. (float) |
Kotanbetsu |
? |
10.6 |
+ |
? |
? |
- |
? |
[M] |
A |
|
MCM-W1579 |
Kotanbetsugawa R. (float) |
Kotanbetsu |
15.9 |
9.9 |
+ |
+ |
+ |
+ |
? |
[M] |
A |
|
MCM-W1580 |
Kaminosawa C. (float) |
Kotanbetsu |
14.5 |
8.9 |
– |
– |
– |
– |
– |
? |
A |
|
MCM-W1581 |
Kotanbetsugawa R. (float) |
Kotanbetsu |
13.2 |
8.7 |
+ |
+ |
– |
? |
? |
[m] |
A |
|
MCM-W1582 |
Kotanbetsugawa R. (float) |
Kotanbetsu |
? |
11.3 |
– |
? |
? |
– |
– |
? |
A |
|
MCM-W1588 |
Nakanofutamatagawa R. (float) |
Haboro |
14.1 |
9.0 |
– |
– |
– |
? |
? |
? |
A |
|
MCM-W1591 |
Kotanbetsugawa R. (KT093) |
Kotanbetsu |
17.2 |
11.3 |
+ |
+ |
? |
– |
+ |
[M] |
A |
|
MCM-W1593 |
Nakanofutamatagawa R. (float) |
Haboro |
17.5 |
12.0 |
+ |
+ |
? |
+ |
– |
[M] |
A |
|
MCM-W1594 |
Kotanbetsugawa R. (float) |
Kotanbetsu |
16.1 |
10.7 |
+ |
+ |
? |
+ |
– |
[M] |
A |
|
MCM-W1595 |
Kotanbetsugawa R. (float) |
Kotanbetsu |
? |
9.5 |
– |
? |
? |
– |
– |
? |
B |
|
MCM-W1596 |
Horotatesawa C. (HR023f) |
Kotanbetsu |
? |
11.4 |
+ |
+ |
? |
+ |
+ |
[M] |
A |
Methods.—Each specimen was polished near the median plane, and investigated for the mature modifications on the outer and inner structures. For the specimens with mature modifications, the size distribution, ratio of numbers and stratigraphic occurrences of microconchs and macroconchs were examined.
The size distribution of the matured specimens was statistically tested by Silverman’s test. The “VISUAL-SILVERMAN”, programmed and provided by Kusuhashi and Okamoto (2015) was used for the test (http://www.palaeo-soc-japan.jp/publications/fossil/vol97/).
Twenty three specimens polished exactly along the median plane were measured for the conch dimensions (Fig. 3A). Parameter “B” was calculated by multiplying the original parameter “B/2” by 2. Each parameter was measured at maximum of two points (near the aperture and last septum) on the preserved last whorl. These parameter measurements were not carried out on the points with significant deformation. Furthermore, two medially sectioned specimens (identified as macroconch and microconch, respectively) were polished along the perpendicular plane on the top of the caecum, and examined for ontogenetic changes of the conch geometries (Fig. 3B). In addition to the measurements in the perpendicular section, each parameter was measured near the aperture in the two specimens. The purpose was to record the ontogenetic changes until the end of the growth, as far as possible.
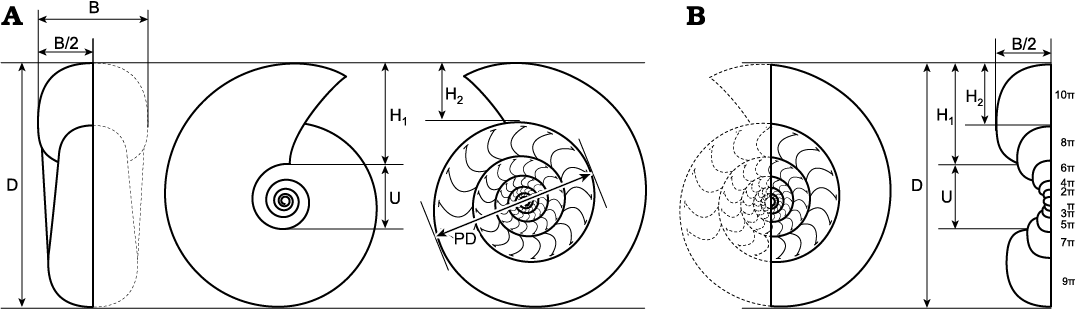
Fig. 3. Measurements of the conch geometries of tetragonitid ammonoid Tetragonites minimus Shigeta, 1989, from the Yezo Group in Hokkaido, Japan. A. Measured parameters on the specimens polished along the median plane. B. Measured ontogenetic changes of parameters on the specimens polished along the median plane, followed by the perpendicular plane. Abbreviations: B, whorl breadth; D, shell diameter; H1, whorl height; H2, ventral whorl height; PD, phragmocone diameter; U, umbilical width.
To investigate their earlier taphonomic histories (postmortem to final burial) and palaeoecology, the damage to the body chamber was examined. In addition, the conditions of the air chamber and sediment infill in the body chamber were also observed.
The above methods were carried out on the 43 Santonian specimens. Turonian, Coniacian, and Campanian-aged specimens of T. minimus were morphologically investigated to verify the chronological continuity of the dimorphism. Therefore, unless otherwise noted, the following results and discussion of the analyses are based on the Santonian specimens only.
Results
Mature modifications.—Four types of mature modifications were recognised in Tetragonites minimus; (i) changes in the shape of the body chamber, (ii) shell thickening in the aperture, (iii) septal crowding, and (vi) increase in septal thickness (Fig. 4). In some specimens, more than one of the four types were combined simultaneously (Fig. 4D).
(i) Changes in the shape of the body chamber (Figs. 2, 4A, B). The whorl rapidly deviated inward and constricted in the venter (Figs. 2, 4A) and the umbilicus became slightly wider (Figs. 2, 4B). In some specimens, the whorl reduced the expansion rate, which is deviated from the previous logarithmic spiral before forming of a rapid constriction. These changes were also shown in the ontogenetic changes of the parameters H1/D and U/D, as discussed later in the text. The apertural edge is turned slightly up on the umbilical seam (Fig. 4B). These terminal apertural modifications were significantly more obvious than the constrictions appearing sometimes in the immature conchs. Type (i) changes in the shape of the body chamber were observed in 30 specimens (69.8%).
(ii) Shell thickening in the aperture was characteristic (Fig. 4C); the shell thickness rather gradually increases and suddenly decreases toward the apertural edge. On the specimens with peeled shell, the terminal shell thickening appeared like a constriction in the internal mold. It was also obvious than the constrictions sometimes appearing on the immature conch. This was recognised in seven specimens (16.3%). Regardless of the change in shell thickness and the subsequent constriction, the deviation of the aperture inward appeared at the venter. Thus, types (i) changes in the shape of the body chamber and (ii) shell thickening in the aperture are independent phenomena.
(iii) Septal crowding is a reduction in the angle between the last two septa (Fig. 4D). Compared to the spacing in previous growth, the angle narrowed up to about half. It was also observed on the suture lines in some specimens. This was observed in 23 specimens (53.5%).
(iv) Septal thickness increased in last septa (Fig. 4D). In the most prominent case, thickness of the last septum was about twice the thickness of the previous septa. This was especially visible on the dorsal side of the septa. This was noted in nine specimens (20.9%).
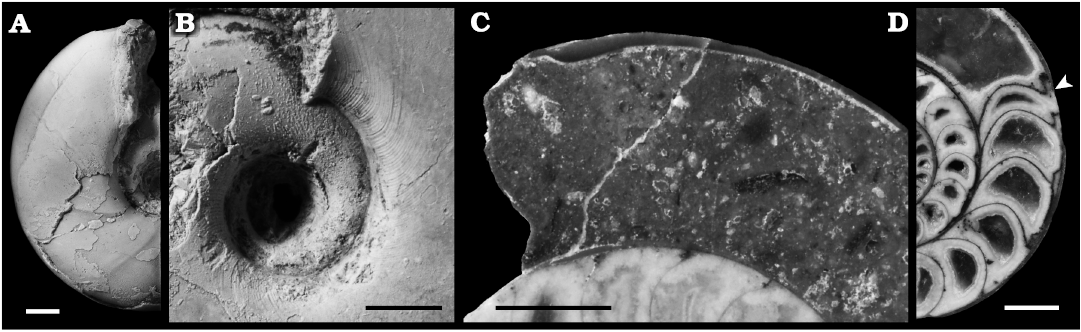
Fig. 4. Mature modifications recognised in tetragonitid ammonoid Tetragonites minimus Shigeta, 1989, from the Yezo Group in the Haboro, Kotanbetsu, Tappu, Nakagawa, Soya, and Yubari areas, Hokkaido, Japan. A, B. Changes in the shape of the body chamber: modification appeared at venter (A, MCM-W1591) and umbilicus (B, MCM-W1556). C. Shell thickening in the aperture, MCM-W1560. D. Septal crowding and increase in septal thickness, MCM-W1565; arrowhead indicates the last septum. Scale bars 2 mm.
Types (iii) septal crowding and (vi) increase in septal thickness of T. minimus were reported and figured by Shigeta (1989, 2001). All types have previously been found in other ammonoids (Klug et al. 2015), but type (iii) might also appear in the middle of growth in some cases and is a weaker criterion for maturity (e.g., Bucher et al. 1996; Klug et al. 2015), while change in the shape of the body chamber (type (i)) is a more robust criterion for maturity (e.g., Parent 2021). The types (i), (iii), and (iv) have also been observed in the recent Nautilus (Collins and Ward 1987; Ward 1987; Klug 2004; Klug et al. 2015).
Size distribution, ratio of numbers and stratigraphic occurrence of dimorphic pair.—Among the specimens examined, 36 specimens (84%) had any type of mature modification (Fig. 5A, Table 1). The compilation histogram of all mature size (specimens with mature modifications) demonstrated two peak distributions (Fig. 5B). Note that this histogram is displayed by the diameter at the last septum (Fig. 3A) because the body chambers of some specimens were lacking or deformed. The results of the Silverman’s test for the adult size distribution showed that the null hypothesis of unimodal distribution was rejected (p-value: 0.0441; 95% CI: 0.0402~0.0483), and the null hypothesis of bimodal distribution was not rejected (p-value: 0.8425; 95% CI: 0.8352~0.8495). The size distribution was also examined in the specimens yielded by a single horizon, outcrop HR109 in the Horotatesawa Creek of the Kotanbetsu area. These specimens are considered to constitute a population sample. The histogram also showed a two-peak trend in spite of the small sample size (Fig. 6). Small mature shells can be regarded as microconchs and large mature shells as macroconchs (Callomon 1955, 1963; Fig. 2).
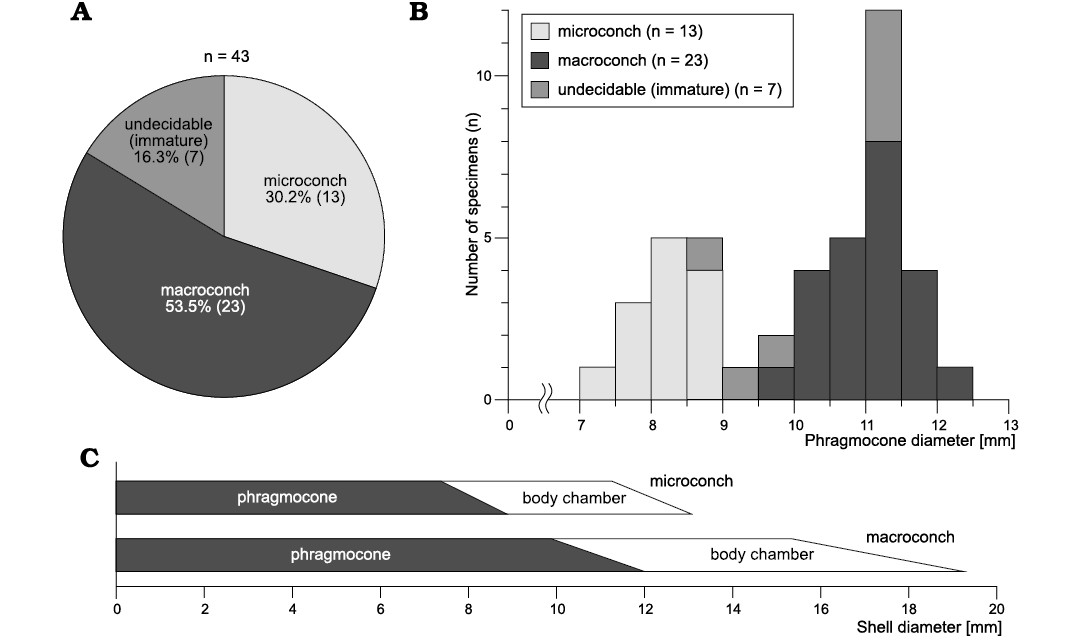
Fig. 5. Characteristics of ratio of numbers and size of dimorphism in tetragonitid ammonoid Tetragonites minimus Shigeta, 1989, from the Yezo Group in Hokkaido, Japan. The specimens treated here were collected from various stratigraphic levels in the Santonian in the Kotanbetsu, Tappu, and Haboro areas. A. The ratio of numbers of dimorphic pairs. B. Compilation of the size distribution in phragmocone diameter (PD). C. Comparative representation of the size of dimorphic pairs with indication of the phragmocone and body chamber.
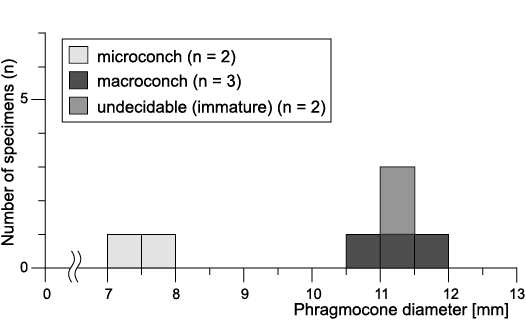
Fig. 6. The size distribution in a single horizon: outcrop HR109 (Santonian) in the Horotatesawa Creek of the Kotanbetsu area, Hokkaido, Japan.
Each mature shell size (body chamber diameter) did not overlap; 11–13 mm for microconchs and 16–19 mm for macroconchs (Figs. 3, 5C). Macroconchs are approximately 1.4 times larger than microconchs. The number of dimorphic pairs was 13 [m] and 23 [M] respectively (Fig. 5A). In the Santonian strata in the Kotanbetsu area, there were no significant differences in the stratigraphic occurrences of dimorphic pairs (Fig. 1B). In four of the 12 horizons, dimorphic pairs occur.
Morphological analysis.—Comparisons of the conch forms between microconchs and macroconchs in the four parameters are shown in Fig. 7. Each comparison demonstrates that the conch forms are similar in juvenile (approximately less than 10 mm in D) but clearly differ in the later part (approximately more than 10 mm in D). Especially in the parameters H1/D and U/D, the ontogenetic changes of the later stage in microconchs (the whorl height becomes smaller; the umbilicus becomes wider) precede those in macroconchs (Fig. 7A, C). Ontogenetic changes on these parameters could also be observed in actual specimens as changes in the shape of the body chamber (Fig. 2, 4A, B).
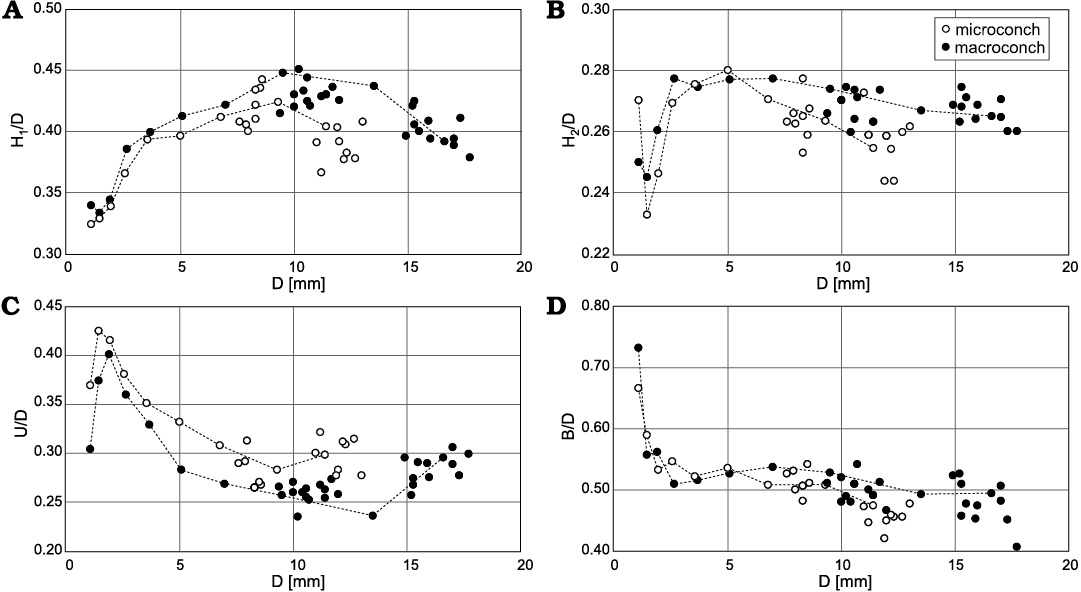
Fig. 7. Scatter diagrams of four parameters versus shell diameter (D) of tetragonitid ammonoid Tetragonites minimus Shigeta, 1989, from the Santonian, Yezo Group in Hokkaido, Japan. A. H1/D (whorl height ratio). B. H2/D (ventral whorl height ratio). C. U/D (umbilical width ratio). D. B/D (whorl breadth ratio). Abbreviations: H1, whorl height; H2, ventral whorl height; U, umbilical width; B, whorl breadth.
Dimorphism in Turonian, Coniacian, and Campanian specimens.—Mature modifications were also identified in Turonian, Coniacian, and Campanian-aged specimens (Table 2). Turonian and Coniacian specimens were matured at the shell diameters of approximately 20 and 35 mm (macroconch), and approximately 16 and 28 mm (microconch), respectively. These seem to be discontinuous and likely dimorphic pairs. On the other hand, mature size differences are obscure in the Campanian specimens.
Table 2. List of Turonian, Coniacian, and Campanian specimens of tetragonitid ammonoid Tetragonites minimus Shigeta, 1989, from the Yezo Group in Hokkaido, Japan. Measurements in mm. Abbreviations: C., Creek; D, shell diameter; [m], microconch; [M], macroconch; PD, phragmocone diameter; R., River; + satisfied; – unsatisfied; ?, unmeasurable or undecidable.
|
Specimen number |
Locality |
Area |
D |
PD |
Adult |
Antidimorphs |
|
Turonian specimens |
||||||
|
MCM-TS0023 |
Pankemoshuparogawa R. (float) |
Yubari |
35.0 |
? |
+ |
[M] |
|
MCM-TS0029 |
Pankemoshuparogawa R. (float) |
Yubari |
19.0 |
? |
+ |
[m] |
|
MCM-W0414-1 |
Okufutamatazawa C. (OB40103a) |
Tappu |
18.3 |
12.3 |
+ |
[m] |
|
MCM-W1583 |
Horotatesawa C. (HR171) |
Kotanbetsu |
20.6 |
? |
+ |
[m] |
|
MCM-W1584 |
Horotatesawa C. (HR185) |
Kotanbetsu |
20.3 |
14.9 |
+ |
[m] |
|
MCM-W1587 |
Horotatesawa C. (HR199) |
Kotanbetsu |
14.2 |
9.0 |
– |
? |
|
MCM-W1597 |
Horotatesawa C. (HR185) |
Kotanbetsu |
10.3 |
? |
– |
? |
|
MCM-W1598 |
Horotatesawa C. (HR185) |
Kotanbetsu |
26.8 |
16.7 |
– |
[M]? |
|
MCM-W1599 |
Horotatesawa C. (HR185) |
Kotanbetsu |
29.4 |
16.6 |
– |
[M]? |
|
UMUT-MM18671-1 (paratype) |
Obirashibegawa R. (R4018) |
Tappu |
? |
18.4+ |
– |
[M]? |
|
UMUT-MM18671-2 (paratype) |
Obirashibegawa R. (R4018) |
Tappu |
18.4 |
? |
+ |
[m] |
|
UMUT-MM18678-2 (paratype) |
Obirashibegawa R. (R2110) |
Tappu |
21.3 |
15.4 |
+ |
[m] |
|
UMUT-MM18678-3 (paratype) |
Obirashibegawa R. (R2110) |
Tappu |
18.0 |
11.2 |
+ |
[m] |
|
UMUT-MM18678-4 (paratype) |
Obirashibegawa R. (R2110) |
Tappu |
? |
14.5 |
+ |
[m] |
|
UMUT-MM18681-1 (paratype) |
Higashiura |
Soya |
22.6 |
? |
+ |
[m] |
|
UMUT-MM18682-3 (paratype) |
Nakakinenbetsugawa R. (R6394) |
Tappu |
17.9 |
? |
+ |
[m] |
|
Coniacian specimens |
||||||
|
MCM-W0115-1 |
Obirashibegawa R. (OB11189b) |
Tappu |
15.8 |
? |
+ |
[m] |
|
MCM-W0115-2 |
Obirashibegawa R. (OB11189b) |
Tappu |
15.5 |
? |
+ |
[m] |
|
MCM-W0118-1 |
Obirashibegawa R. (OB11193c) |
Tappu |
14.6 |
? |
+ |
[m] |
|
MCM-W1592 |
Obirashibegawa R. (OB11181) |
Tappu |
21.2 |
? |
– |
[M]? |
|
UMUT-MM18667 (holotype) |
Obirashibegawa R. (T1220 (= OB11189)) |
Tappu |
28.1 |
? |
+ |
[M] |
|
Campanian specimens |
||||||
|
UMUT-MM18642-2 (paratype) |
Wakkaenbetsugawa R. (T313) |
Nakagawa |
13.6 |
? |
+ |
? |
|
UMUT-MM18642-3 (paratype) |
Wakkaenbetsugawa R. (T313) |
Nakagawa |
12.9 |
8.6 |
– |
? |
|
UMUT-MM18642-4 (paratype) |
Wakkaenbetsugawa R. (T313) |
Nakagawa |
13.0 |
8.8 |
+ |
? |
|
UMUT-MM18642-5 (paratype) |
Wakkaenbetsugawa R. (T313) |
Nakagawa |
? |
8.9 |
+ |
? |
|
UMUT-MM18642-6 (paratype) |
Wakkaenbetsugawa R. (T313) |
Nakagawa |
13.5 |
9.0 |
+ |
? |
|
UMUT-MM18642-7 (paratype) |
Wakkaenbetsugawa R. (T313) |
Nakagawa |
8.5 |
5.8 |
– |
? |
|
UMUT-MM18642-8 (paratype) |
Wakkaenbetsugawa R. (T313) |
Nakagawa |
10.7 |
7.5 |
– |
? |
|
UMUT-MM18642-9 (paratype) |
Wakkaenbetsugawa R. (T313) |
Nakagawa |
? |
9.6 |
+ |
? |
|
UMUT-MM18644 (paratype) |
Osoushinaisawa C. (T205) |
Nakagawa |
? |
8.5 |
+ |
? |
Taphonomic analysis.—The damage of the body chamber was classified into two categories: A, completely or almost intact; B, incomplete (Fig. 8). The numbers and ratios of each category are following: category A, n = 37, 86%; category B, n = 6, 14%. The interior of the body chamber was filled with sparry calcite in 16 specimens (37%) (Fig. 8A). The air chambers showed occasional intrusion of sediments, but most were intact or internally fractured with no punctures. The epifauna were not attached to any of the specimens. Jaw apparatuses were not observed in any specimens.
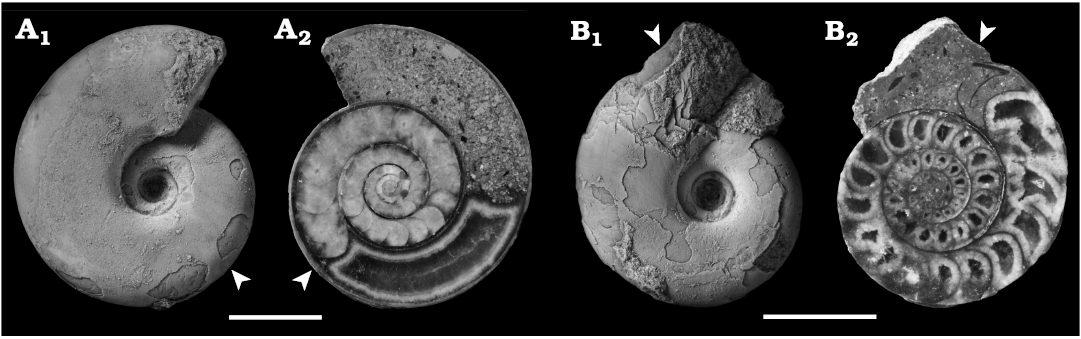
Fig. 8. Two categories of the preservation pattern of the body chamber of tetragonitid ammonoid Tetragonites minimus Shigeta, 1989, from the Santonian, Yezo Group in the Haboro, Kotanbetsu, and Tappu areas, Hokkaido, Japan. A. MCM-W1549, complete or almost intact specimen, note the interiors of the body chamber filled with sparry calcite. B. MCM-W1555, incomplete specimen. In lateral (A1, B1) and median section (A2, B2) views. Arrowheads indicate the last septum. Scale bars 5 mm.
Discussion
Characteristics of dimorphism of Tetragonites minimus.—The results show it is reasonable to regard the two different adult-size groups in T. minimus as dimorphs, since many of the characteristics identified as the criteria for dimorphism of ammonoids are met (Klug et al. 2015). The idea of dimorphism of ammonoids as sexual is widely accepted. However, as in many other cases, no clues have been obtained to determine whether microconchs or macroconchs are female or male for T. minimus.
Size differences between other known dimorphic pairs varied among taxa. In some Jurassic and Cretaceous cases, macroconchs were much larger than microconchs (Makowski 1962; Cobban and Kennedy 1993). On the other hand, in other cases there is only a slight difference and in other cases the sizes overlap (Landman and Waage 1993). Among the modern cephalopods, the size difference between the sexes might be extremely large like in species of Argonauta (Roper et al. 1984; Nesis 1987), or slight like in species of Nautilus (Willey 1902; Saunders and Spinosa 1978; Saunders and Ward 1987; Hayasaka et al. 1987; Dunstan et al. 2011a). The difference in the conch size of dimorphic pairs of T. minimus of the Santonian was approximately 1.4 times, which could be considered relatively small. The size differences of antidimorphs in the Turonian and Coniacian are larger than that of the Santonian, at approximately 1.8 times. Same cases in which the dimorphic size difference has changed in the linage, have been reported (Dzik 1994; Parent 1998; Schweigert and Dietze 1998). On the other hand, no remarkably discontinuous maturity size difference was observed in Campanian specimens. Considering the trend of decreasing mature conch size and size difference between dimorphic pairs from the Turonian–Coniacian to the Santonian, the trend might have continued to the Campanian and the size overlap might occur at that time. This should be verified with sufficient numbers of specimens by the statistical testing in the future work.
It is common for the ratio of the number of ammonoid dimorphic pairs to not be equal (Makowski 1962; Cobban 1969; Kennedy 1988; Cobban and Kennedy 1993; Landman and Waage 1993; Davis et al. 1996; Machalski 2005). Even in the Recent Nautilus pompilius, population differences can be noted depending on the season and region (Hayasaka et al. 1987). Therefore, approximately 1 [m]: 2 [M] of T. minimus is a possible ratio.
The similarity at the juvenile stage, but different at the adult stage of the conch form has been confirmed in many cases of dimorphism of ammonoids (e.g., Palframan 1966, 1967; Tanabe 1977, 2022; Parent 1997; Zatoń 2008; Parent and Zatoń 2016). The ontogenetic pattern of dimorphs in shell morphology of T. minimus is similar to these cases (Fig. 7).
Taphonomic history.—Understanding the taphonomic processes of fossils is essential for reconstructing their palaeoecology (Maeda and Seilacher 1996; Wani and Gupta 2015). The present analyses showed a somewhat peculiar trend in which the mature or nearly mature conchs were predominant. In this section, the mode of occurrence is discussed from the aspect of taphonomy.
The shells of dead ammonoids followed variable paths before become finally buried in the sea floor. Sometimes, the dead shells surfaced and drifted away on the sea surface, thereby losing the chance of being fossilised (Maeda and Seilacher 1996; Maeda 1999). First, the body chamber might be damaged or lost during post-mortem transport on both the sea surface and the sea floor (Maeda 1991, 1999; Maeda and Seilacher 1996; Wani 2004; Maeda et al. 2010). There is an example of an ammonoid assemblage consisting of more than 600 specimens that transported on the sea surface and beached, with none preserving body chamber (Maeda et al. 2003). Therefore, based on the preservation of the body chamber, it is possible to estimate the degree of post-mortem transport of the shell. The results of this study showed that preservation of the body chamber of T. minimus from the Yezo Group, Hokkaido, was remarkably good. In particular, specimens with almost complete body chambers belonging to the category A are unlikely to have experienced long-distance post-mortem transport (Maeda and Seilacher 1996; Maeda 1999; Wani 2001). The results showed that the part of the body chamber was partially filled with sparry calcite in 37% of the specimens. It has been suggested that the body chamber might have contained un-decayed soft parts, suggesting that they were buried quickly after death (Tanabe and Fukuda 1987; Landman et al. 2015; Cherns et al. 2021). Additionally, a previous field experiment revealed that the smaller conchs of ectocochleate cephalopods (e.g., those of Recent Nautilus) tend to sink sooner than the large conchs of the same species (Wani et al. 2005). As discussed above, it is highly likely that T. minimus assemblage examined here have fossilised quickly near their original habitat without long-distance post-mortem transport. However, if so, the jaw apparatus preserved in the body chamber would be expected to be found (Maeda and Seilacher 1996; Tanabe and Landman 2002; Klug and Jerjen 2012; see also Wani 2007 for an alternative opinion), but were not identified in this study. In the future studies, a more detailed examination should be performed utilizing CT (computed tomography) or other techniques for the present specimens (such as the analysis performed in Kruta et al. 2011).
On the other hand, it is necessary to understand why juvenile conchs are rare. One possible cause is the taphonomic due to different fossilisation potential of small juvenile conchs. It is possible that the small-sized shells are less likely to be fossilized than large shells because of the dissolution and other factors (Maeda 1991). This is supported by the selective dissolution of the early growth stages of large ammonoid shells during post-mortem exposition to seawater (Maeda 1987; Maeda and Seilacher 1996; Maeda et al. 2010). However, the other coiled ammonoids which are smaller than T. minimus (<10 mm), are common in the Yezo Group, and are particularly abundant in the Santonian (DA unpublished data). Therefore, there is no convincing explanation for the nearly absent small-sized T. minimus, unless they were selectively dissolved. Another possibility is that the difference in the floatability in bottom currents of differently-sized shells causes a bias in occurrence (Wani and Ikeda 2006). Smaller shells might have been more easily carried away by the bottom currents, and assuming this case, the scenario is that the small juvenile T. minimus were selectively carried, even though they were inhabiting the study area during the Late Cretaceous. However, this scenario is also implausible. If floatability by size is considered, then the equal-sized conchs of microconchs and “juvenile” macroconchs should show the same behaviour to currents, and both are expected to occur. However, even though 11–13 mm in diameter microconchs were found, juvenile macroconchs of almost the same size were not obtained. In addition, if small-sized (D <10 mm) T. minimus were to be carried away in the bottom currents, then the same-sized shells of the other coiled ammonoids should have also been carried away. However, small-sized (D <10 mm) conchs of the other coiled ammonoids are common in the study area. Thus, the absence of the small-sized T. minimus cannot be explained by the difference in floatability for conch sizes. It is reasonable to interpret that the absence of juvenile shells was not caused by the taphonomic factors but instead by primary reasons, i.e., they did not originally inhabit the area.
Predominance of adult conchs and speculative palaeoecology.—The reason for absence of juvenile conch of T. minimus in the study area is possibly connected to the palaeoecology of the species. If individuals of T. minimus changed their habitat during their life cycle and became fossilised in their respective habitats, this would result in bias in the proportion of variously aged specimens. Ontogenetic active migration is assumed among some Mesozoic ammonoids and well known in many modern cephalopods (ammonoids; Morton 1988; Lukeneder et al. 2010; Ikeda and Wani 2012; Brayard and Escarguel 2013; Lukeneder 2015; Moriya 2015; modern cephalopods: Clarke 1970; Crick 1988; Landman 1988; Oba et al. 1992; Westermann 1996; Rexfort and Mutterlose 2006; Lukeneder et al. 2008; Price et al. 2009; Warnke et al. 2010; Dunstan et al. 2011b; Ritterbush et al. 2014).
Here, two working hypotheses are proposed to explain the predominance of adult T. minimus in the Santonian of the Yezo Group. Working hypothesis A: T. minimus inhabits the sea outside the Yezo sedimentary basin at juvenile stage, migrates geographically during growth, and inhabits the Yezo sedimentary basin at adult stage. Working hypothesis B: T. minimus inhabits the shallow-water column at juvenile stage, migrates vertically to deeper parts of the water column during growth, and inhabits bottom waters at the adult stage. In the working hypothesis B, the bias in the occurrence of adult and juvenile specimens is attempted to explain by the difference in fossilisation potentials depending on the concept of the limit depth on post-mortem surfacing (Maeda and Seilacher 1996).
However, at this time it is not possible to determine which working hypothesis is more plausible. Stable isotope analysis is effective in clarifying detailed habitat changes during the life cycle (Lukeneder 2015; Moriya 2015), and the hybrid analyses of the actual fossil occurrence and the stable isotope analysis might provide new insights into the ammonoid’s palaeoecology and taphonomy (Landman et al. 2015).
Conclusions
Mature modifications, ontogeny, and dimorphism of Tetragonites minimus from the Santonian, the Upper Cretaceous in the northwestern area of Hokkaido, were examined. The conch form of the dimorphs is similar at the juvenile stage, but differs at the adult stage in microconch and macroconch. The mature shell size of microconchs and macroconchs did not overlap; 11–13 mm for microconchs and 16–19 mm for macroconchs. The approximate ratio of dimorphic pairs was 1 [m]: 2 [M]. Dimorphism was recognised by shell size differences of the dimorphs at least in the Turonian–Santonian in the supplementary analysis. The mature conch size and size difference between dimorphic pairs decreased chronologically.
More than 80% of the examined specimens were adults. Taphonomic analysis focused on shell preservation showed that the T. minimus assemblages fossilised quickly near their original habitat. Hence, the bias in the fossil occurrence of T. minimus was not caused by the taphonomic factors such as the bias of fossilisation potential and floatability in the bottom currents, and was interpreted by the ontogenetic active migration. Two working hypotheses of migration (geographical migration and vertical migration) were proposed to explain the predominance of adults, but it was not possible to determine which working hypothesis was more plausible.
Acknowledgements
I thank Ryoji Wani (Yokohama National University, Kanagawa, Japan) for encouragement, fruitful suggestions and discussions; Yasunari Shigeta (National Museum of Nature and Science, Tsukuba, Japan) for fruitful suggestions of analysis procedure and species identification; Takenori Sasaki (UMUT) for access to the type specimens; Tetsuro Suekane (Tokai University Sagami Junior & Senior High School, Kanagawa, Japan) for donating the Yubari specimens; Amane Tajika (American Museum of Natural History, New York, USA; UMUT), Tomoki Karasawa (MCM), Yasuhisa Nakajima (Tokyo City University, Tokyo, Japan), Tatsuo Oji (Nagoya University Museum, Aichi, Japan) and Ren Hirayama (Waseda University, Tokyo, Japan) for fruitful discussions and suggestions; Horacio Parent (Universidad Nacional de Rosario, Argentina) and Haruyoshi Maeda (The Kyushu University Museum, Fukuoka, Japan) for their valuable comments to improve the manuscript. Thanks are extended to Fumiko Murakami and Mamoru Murakami (both Murakami Lodging House, Haboro Town) and Kotanbetsu, Tappu, and the Northern Rumoi forestry offices and Hokkaido Government Rumoi Subprefectural Bureau. This study was partly supported by the Fukada Research Grant from the Fukada Geological Institute and the Sasakawa Scientific Research Grant from the Japan Science Society.
References
Aiba, D. 2019. A possible phylogenetic relationship of two species of Hyphantoceras (Ammonoidea, Nostoceratidae) in the Cretaceous Yezo Group, northern Japan. Paleontological Research 23: 65–79. Crossref
Brayard, A. and Escarguel, G. 2013. Untangling phylogenetic, geometric and ornamental imprints on Early Triassic ammonoid biogeography: a similarity-distance decay study. Lethaia 46: 19–33. Crossref
Bucher, H., Landman, N.H., Klofak, S.M., and Guex, J. 1996. Mode and rate of growth in ammonoids. In: N.H. Landman, K. Tanabe, and R.A. Davis, (eds.), Ammonoid Paleobiology, 408–461. Plenum Press, New York. Crossref
Callomon, J.H. 1955. The ammonite succession in the Lower Oxford Clay and Kellaways Beds at Kidlington, Oxfordshire and the zones of the Callovian Stage. Philosophical Transactions of the Royal Society of London. Series B 239: 215–264. Crossref
Callomon, J.H. 1963. Sexual dimorphism in Jurassic ammonites. Transactions of the Leicester Literary and Philosophical Society 57: 21–56.
Cherns, L., Spencer, A.R.T., Rahman I.A., Garwood, R.J., Reedman C., Burca G., Turner, M.J., Hollingworth N.T.J., and Hilton J. 2021. Correlative tomography of an exceptionally preserved Jurassic ammonite implies hyponome-propelled swimming. Geology 50: 397–401. Crossref
Clarke, M.R. 1970. Growth and development of Spirula spirula. Journal of the Marine Biological Association of the United Kingdom 50: 53–64. Crossref
Cobban, W.A. 1969. The Late Cretaceous ammonites Scaphites leei Reeside and Scaphites hippocrepis (DeKay) in the Western Interior of the United States. United States Geological Survey Professional Paper 619: 1–27. Crossref
Cobban, W.A. and Kennedy, W.J. 1993. The Upper Cretaceous Dimorphic Pachydiscid Ammonite Menuites in the Western Interior of the United States. U.S. Geological Survey Professional Paper 1533: 1–14. Crossref
Collins, D. and Ward, P.D. 1987. Adolescent growth and maturity in Nautilus. In: W.B. Saunders and N.H. Landman (eds.), Nautilus, 421–432. Plenum Press, New York. Crossref
Crick, R.E. 1988. Buoyancy regulation and macroevolution in nautiloid cephalopods. Senckenbergiana Lethaea 69: 13–42.
Davis, R.A., Furnish, W.M., and Glenister, B.F. 1969. Mature modification and dimorphism in late Paleozoic ammonoids. In: G.E.G. Westermann (ed.), Sexual Dimorphism in Fossil Metazoa and Taxonomic Implications, 101–110. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart.
Davis, R.A, Landman, N.H., Dommergues, J.L., Marchand, D., and Bucher, H. 1996. Mature modifications and sexual dimorphism in ammonoids. In: N.H. Landman, K. Tanabe, and R.A. Davis (eds.), Ammonoid Paleobiology, 463–539. Plenum Press, New York. Crossref
Dzik, J. 1994. Sexual dimorphism in the virgatitid ammonites. Palaeopelagos Special Publication 1: 129–141.
Dunstan, A.J., Ward, P.D., and Marshall, N.J. 2011a. Nautilus pompilius life history and demographics at the Osprey Reef Seamount, Coral Sea, Australia. PLoS ONE 6: e16312. Crossref
Dunstan, A.J., Ward, P.D., and Marshall, N.J. 2011b. Vertical distribution and migration patterns of Nautilus pompilius. PLoS ONE 6: e16311. Crossref
Fabre, S. 1940. Le Crétacé supérieur de la Basse-Provence occidentale. 1. Cénomanien et Turonien. Annales de la Faculté des Sciences de Marseille Second Série 14: 1–355.
Funaki, H. and Hirano, H. 2004. Cretaceous stratigraphy in the northeastern part of the Obira area, Hokkaido, Japan [in Japanese, with English abstract]. Bulletin of the Mikasa City Museum 8: 17–35.
Futakami, M. 1990. Turonian collignoniceratid ammonites from Hokkaido, Japan—stratigraphy and paleontology of the Cretaceous in the Ishikari province, central Hokkaido, Part 3. Journal of Kawamura Gakuen Woman’s University 1: 235–260.
Hayasaka, S., Oki, K., Tanabe, K., Saisho, T., and Shinomiya, A. 1987. On the habitat of Nautilus pompilius in Tañon Strait (Philippines) and the Fiji Islands. In: W.B. Saunders and N.H. Landman (eds.), Nautilus, 179–200. Plenum Press, New York. Crossref
Hoffmann, R. 2010. New insights on the phylogeny of the Lytoceratoidea (Ammonitina) from the septal lobe and its functional interpretation. Revue de Paléobiologie 29: 1–156.
Hoffmann, R. 2015. Treatise Online 70, Part L, Revised, Volume 3B, Lytoceratoidea. 34 pp. Paleontological Institute the University of Kansas, Lawrence. Crossref
Honda, B. and Hirano, H. 2014. Megafossil biostratigraphy and carbon isotope stratigraphy of the Upper Cretaceous Yezo Group in the Obira area, Hokkaido, Japan [in Japanese, with English abstract]. Fossils (Kaseki) 95: 19–37.
Ikeda, Y. and Wani, R. 2012. Different modes of migration within Late Cretaceous ammonoids in northwestern Hokkaido, Japan: evidence from the analyses of shell whorls. Journal of Paleontology 86: 605–615. Crossref
Jimbo, K. 1894. Beiträge zur Kenntniss der Fauna der Kreideformation von Hokkaido. Paläontologische Abhandlungen, Neue Folge 2: 149–194.
Kennedy, W.J. 1988. Late Cenomanian and Turonian Ammonite faunas from north-east and central Texas. Special Papers in Palaeontology 39: 1–131.
Klug, C. 2004. Mature modifications, the black band, the black aperture, the black stripe, and the periostracum in cephalopods from the Upper Muschelkalk (Middle Triassic, Germany). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universitat Hamburg 88: 63–78.
Klug, C. and Jerjen, I. 2012. The buccal apparatus with radula of a ceratitic ammonoid from the German Middle Triassic. Geobios 45: 57–65. Crossref
Klug, C., Zatoń, M., Parent, H., Hostettler, B., and Tajika, A. 2015. Mature modifications and sexual dimorphism. In: C. Klug, D. Korn, K. De Baets, I. Kruta, and R.H. Mapes (eds.), Ammonoid Paleobiology: From Anatomy to Ecology, 253–320. Springer, Dordrecht. Crossref
Kossmat, F. 1895. Untersuchungen über die südindische Kreideformation. Beiträge zur Paläontologie und Geologie Österreich-Ungarns und des Orients 9: 97–203.
Kruta, I., Landman, N., Rouget, I., Cecca, F., and Tafforeau, P. 2011. The role of ammonites in the Mesozoic marine food web revealed by jaw preservation. Science 331: 70–72. Crossref
Kusuhashi, N. and Okamoto, T. 2015. A nonparametric multimodality test−Silverman’s test−and its introduction into paleontology [in Japanese]. Fossils (Kaseki) 97: 23–37.
Landman, N.H. 1988. Early ontogeny of Mesozoic ammonites and nautilids. In: J. Wiedmann and J. Kullmann (eds.), Cephalopods, Present and Past, 215–228. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart.
Landman, N.H. and Waage, K.M. 1993. Scaphitid ammonites of the Upper Cretaceous (Maastrichtian) Fox Hills Formation in South Dakota and Wyoming. Bulletin of the American Museum of Natural History 215: 1–257.
Landman, N.H., Cobban, W.A., and Larson, N.L. 2012. Mode of life and habitat of scaphitid ammonites. Geobios 45: 87–98. Crossref
Landman, N.H., Grier, J.C., Grier, J.W., Cochran, J.K., and Klofak, S.M. 2015. 3-D orientation and distribution of ammonites in a concretion from the Upper Cretaceous Pierre Shale of Montana. Swiss Journal of Paleontology 134: 257–279. Crossref
Lukeneder, A. 2015. Ammonoid habitats and life history. In: C. Klug, D. Korn, I. Kruta, K. De Baets, and R.H. Mapes (eds.), Ammonoid Paleobiology: From Anatomy to Ecology, 689–791. Springer, Dordrecht. Crossref
Lukeneder, A., Harzhauser, M., Müllegger, S., and Piller, W. 2008. Stable isotopes (δ18O and δ13C) in Spirula spirula shells from three major oceans indicate developmental changes paralleling depth distributions. Marine Biology 154: 175–182. Crossref
Lukeneder, A., Harzhauser, M., Müllegger, S., and Piller, W. 2010. Ontogeny and habitat change in Mesozoic cephalopods revealed by stable isotopes (δ18O, δ13C). Earth and Planetary Science Letters 296: 103–114. Crossref
Machalski, M. 2005. Late Maastrichtian and earliest Danian scaphitid ammonites from central Europe: Taxonomy, evolution, and extinction. Acta Palaeontologica Polonica 50: 653–696.
Maeda, H. 1987. Taphonomy of ammonites from the Cretaceous Yezo Group in the Tappu area, northwestern Hokkaido, Japan. Transaction and Proceedings of the Palaeontological Society of Japan, New Series 148: 285–305.
Maeda, H. 1991. Sheltered preservation: a peculiar mode of ammonite occurrence in the Cretaceous Yezo Group, Hokkaido, north Japan. Lethaia 24: 69–82. Crossref
Maeda, H. 1993. Dimorphism of two late Cretaceous false-puzosiine ammonites, Yokoyamaoceras Wright and Matsumoto, 1954 and Neopuzosia Matsumoto, 1954. Transaction and Proceedings of the Palaeontological Society of Japan, New Series 169: 97–128.
Maeda, H. 1999. Did ammonoid carcasses surface or sink? [in Japanese, with English abstract] Memoirs, Geological Society of Japan 54: 131–140.
Maeda, H. and Seilacher, A. 1996. Ammonoid taphonomy. In: N.H. Landman, K. Tanabe, and R.A. Davis (eds.), Ammonoid Paleobiology, 543–578. Plenum Press, New York. Crossref
Maeda, H., Kumagae. T., Matsuoka, H., and Yamazaki, Y. 2010. Taphonomy of large Canadoceras (ammonoid) shells in the Upper Cretaceous Series in South Sakhalin, Russia. Paleontological Research 14: 56–68. Crossref
Maeda, H., Mapes, R.H., and Mapes, G. 2003. Taphonomic features of a Lower Permian beached cephalopod assemblage from central Texas. Palaios 18: 421–434. Crossref
Maeda, H., Shigeta, Y., Fernando, A.G.S., and Okada, H. 2005. Stratigraphy and fossil assemblages of the Upper Cretaceous System in the Makarov area, southern Sakhalin, Russian Far East. National Science Museum Monographs 31: 25–120.
Makowski, H. 1962. Problem of sexual dimorphism in ammonites. Palaeontologia Polonica 12: 1–92.
Matsumoto, T. 1942. Fundamentals in the Cretaceous stratigraphy of Japan, Part I. Memoirs of the Faculty of Science, Kyushu University, Series D, Geology 1: 129–280.
Matsumoto, T. 1954. The Cretaceous System in the Japanese Islands. 324 pp. Japan Society for the Promotion of Science, Tokyo.
Matsumoto, T. and Toshimitsu, S. 1991. A find of a Cenomanian ammonite from Tomiuchi, Hobetsu distinct, Hokkaido. The Bulletin of the Hobetsu Museum 7: 1–8.
Metzdorf, R. and Sowiak, M. 2003. Neue Erkentnisse über die Gehäusegestalt von Hyphantoceras reussianum (D’Orbigny, 1850) aus dem Bereich des Hyphantoceras-Event (Ober-Turonium) von Halle/Westf.). Osnabrücker Naturwissenschaftliche Mitteilungen 29: 45–52.
Moriya, K. 2015. Isotope Signature of Ammonoid Shells. In: C. Klug, D. Korn, K. De Baets, I. Kruta, and R.H. Mapes, (eds.), Ammonoid Paleobiology: From Anatomy to Ecology, 793–836. Springer, Dordrecht. Crossref
Morton, H. 1988. Segregation and migration patterns in some Graphoceras populations (Middle Jurassic). In: J. Wiedmann and J. Kullmann (eds.), Cephalopods, Present and Past, 377–385. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart.
Murphy, M.A. 1967a. Aptian and Albian Tetragonitidae (Ammonoidea) from Northern California. University of California Publications in Geological Sciences 70: 1–32.
Murphy, M.A. 1967b. The Aptian–Cenomanian members of the ammonite genus Tetragonites. University of California Publications in Geological Sciences 69: 1–78.
Nesis, K.N. 1987. Cephalopods of the World. 351 pp. TFH Publications, New Jersey.
Oba, T., Kai, M., and Tanabe, K. 1992. Early life history and habitat of Nautilus pompilius inferred from oxygen isotope examinations. Marine Biology 113: 211–217. Crossref
Oizumi, M., Kurihara, K., Funaki, H., and Hirano, H. 2005. Upper Cretaceous stratigraphy in the Obira area, Hokkaido, Japan [in Japanese, with English abstract]. Bulletin of the Mikasa City Museum 9: 11–26.
Okamoto, T., Matsunaga, T., and Okada, M. 2003. Restudy of the Upper Cretaceous stratigraphy in the Haboro area, northwestern Hokkaido [in Japanese, with English abstract]. Journal of the Geological Society of Japan 109: 363–382. Crossref
Palframan, D.F.B. 1966. Variation and ontogeny of some Oxfordian ammonites: Taramelliceras richei (De Loriol) and Creniceras renggeri (Oppel), from Woodham, Buckinghamshire. Palaeontology 9: 290–311.
Palframan, D.F.B. 1967. Variation and ontogeny of some Oxford Clay ammonites: Distichoceras bicostatum (Stahl) and Horioceras baugieri (d’Orbigny), from England. Palaeontology 10: 60–94.
Parent, H. 1997. Ontogeny and sexual dimorphism of Eurycephalites gottschei (Tornquist) (Ammonoidea) of the Andean lower Callovian (Argentine–Chile). Geobios 30: 407–419. Crossref
Parent, H. 1998. Upper Bathonian and lower Callovian ammonites from Chacay Melehué (Argentina). Acta Palaeontologica Polonica 43: 69–130.
Parent, H. 2021. The evolution and variation of the Jurassic ammonoid Stehnocephalites gerthi (Spath). Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 301: 85–107. Crossref
Parent, H. and Zatoń, M. 2016. Sexual dimorphism in the Bathonian morphoceratid ammonite Polysphinctites tenuiplicatus. Acta Palaeontologica Polonica 61: 875–884. Crossref
Price, G.D., Twitchett, R.J., Smale, C., and Marks, V. 2009. Isotopic analysis of the life history of the enigmatic squid Spirula spirula with implications for studies of fossil cephalopods. Palaios 24: 273–279. Crossref
Rexfort, A. and Mutterlose, J. 2006. Stable isotope records from Sepia officinalis—a key to understand the ecology of belemnites? Earth and Planetary Science Letters 247: 212–221. Crossref
Ritterbush, K.A., Hoffmann, R., Lukeneder, A., and De Baets, K. 2014. Pelagic palaeoecology: the importance of recent constraints on ammonoid palaeobiology and life history. Journal of Zoology 292: 229–241. Crossref
Roper, C.F.E., Sweeney, M.J., and Nauen, C.E. 1984. FAO species catalogue, vol 3: cephalopods of the world. An annotated and illustrated catalogue ofs of interest to fisheries. FAO Species Synopsis 125: 1–277.
Saunders, W.B. and Spinosa, C. 1978. Sexual dimorphism in Nautilus from Palau. Paleobiology 4: 349–358. Crossref
Saunders, W.B. and Ward, P.D. 1987. Ecology, distribution, and population characteristics of Nautilus. In: W.B. Saunders and N.H. Landman (eds.), Nautilus, 137–162. Plenum Press, New York. Crossref
Schweigert, G. and Dietze, V. 1998. Revision der dimorphen Ammonitengattungen Phlycticeras Hyatt-Oecoptychius Neumayr (Strigoceratidae, Mitteljura). Stuttgarter Beiträge zur Naturkunde B 269: 1–59.
Shigeta, Y. 1989. Systematics of the ammonite genus Tetragonites from the Upper Cretaceous of Hokkaido. Transaction and Proceedings of the Palaeontological Society of Japan, New Series 156: 319–342.
Shigeta, Y. 2001. Ammonitology [in Japanese]. 155 pp. Tokai University Press, Tokyo.
Shigeta, Y. and Maeda, H. 2005. Yezo Group research in Sakhalin—a historical review. National Science Museum Monographs 31: 1–24.
Takashima, R., Kawabe, F., Nishi, H., Moriya, K., Wani, R., and Ando, H. 2004. Geology and stratigraphy of forearc basin sediments in Hokkaido, Japan: Cretaceous environmental events on the north-west Pacific margin. Cretaceous Research 25: 365–390. Crossref
Tanabe, K. 1977. Functional evolution of Otoscaphites puerculus (Jimbo) and Scaphites planus (Yabe), Upper Cretaceous ammonites. Memoirs of the Faculty of Science Kyushu University, Series D, Geology 23: 367–407. Crossref
Tanabe, K. 2022. Late Cretaceous dimorphic scaphitid ammonoid genus Yezoites from the circum-North Pacific regions. Paleontological Research 26: 233–269. Crossref
Tanabe, K. and Fukuda, Y. 1987. The jaw apparatus of the Cretaceous ammonite Reesidites. Lethaia 20: 41–48. Crossref
Tanabe, K. and Landman, N.H. 2002. Morphological diversity of the jaws of Cretaceous Ammonoidea. Abhandlungen der Geologischen Bundesanstalt 57: 157–165.
Tanabe, K., Hirano, H., Matsumoto, T., and Miyata, Y. 1977. Stratigraphy of the Upper Cretaceous deposits in the Obira area, northwestern Hokkaido [in Japanese, with English abstract]. Science Reports, Department of Geology, Kyushu University 12: 181–202.
Toshimitsu, S. 1985. Biostratigraphy and depositional facies of the Cretaceous in the upper reaches of the Haboro River in Hokkaido [in Japanese, with English abstract]. Journal of the Geological Society of Japan 91: 599–618. Crossref
Toshimitsu, S. 1988. Biostratigraphy of the Upper Cretaceous Santonian Stage in northwestern Hokkaido. Memoirs of the Faculty of Science, Kyushu University, Series D, Geology 26: 125–192. Crossref
Toshimitsu, S., Hasegawa, T., and Tsuchiya, K. 2007. Coniacian–Santonian stratigraphy in Japan: a review. Cretaceous Research 28: 128–131. Crossref
Toshimitsu, S., Matsumoto, T., Noda, M., Nishida, T., and Maiya, S. 1995. Towards an integrated mega-, micro-, and magnetostratigraphy of the Upper Cretaceous in Japan [in Japanese, with English abstract]. Journal of the Geological Society of Japan 101: 19–29. Crossref
Tsujino, Y. and Maeda, H. 2007. Fossil bivalve assemblages and depositional environments of the upper part of the Cretaceous Yezo Supergroup, Kotanbetsu-Haboro area, Hokkaido, Japan. Paleontological Research 11: 251–264. Crossref
Wani, R. 2001. Reworked ammonoids and their taphonomic implications in the Upper Cretaceous of northwestern Hokkaido, Japan. Cretaceous Research 22: 615–625. Crossref
Wani, R. 2003. Taphofacies models for Upper Cretaceous ammonoids from the Kotanbetsu area, northwestern Hokkaido, Japan. Palaeogeography, Palaeoclimatology, Palaeoecology 199: 71–82. Crossref
Wani, R. 2004. Experimental fragmentation patterns of modern Nautilus shells and the implications for fossil cephalopod taphonomy. Lethaia 37: 113–123. Crossref
Wani, R. 2007. How to recognize in situ fossil cephalopods: evidence from experiments with modern Nautilus. Lethaia 40: 305–311. Crossref
Wani, R. and Gupta, N.S. 2015. Ammonoid taphonomy. In: C. Klug, D. Korn, K. De Baets, I. Kruta, and R.H. Mapes (eds.), Ammonoid Paleobiology: From Macroevolution to Paleogeography, 555‒597. Springer, Dordrecht. Crossref
Wani, R. and Hirano, H. 2000. Upper Cretaceous biostratigraphy in the Kotanbetsu area, northwestern Hokkaido [in Japanese, with English abstract]. Journal of the Geological Society of Japan 106: 171–188. Crossref
Wani, R. and Ikeda, H. 2006. Planispiral cephalopod shells as a sensitive indicator of modern and ancient bottom currents: new data from flow experiments with modern Nautilus pompilius. Palaios 21: 289–297. Crossref
Wani, R., Kase, T., Shigeta, Y., and De Ocampo, R. 2005. New look at ammonoid taphonomy, based on field experiments with modern chambered nautilus. Geology 33: 849–852. Crossref
Ward, P.D. 1987. The Natural History of Nautilus. 267 pp. Allen & Unwin, Boston.
Warnke, K., Oppelt, A., and Hoffmann, R. 2010. Stable isotopes during ontogeny of Spirula and derived hatching temperatures. Ferrantia 59: 191–201.
Westermann, G.E.G. 1996. Ammonoid life and habitat. In: N.H. Landman, K. Tanabe, and R.A. Davis (eds.), Ammonoid Paleobiology, 607–707. Plenum Press, New York. Crossref
Wiedmann, J. 1973. The Albian and Cenomanian Tetragonitiae (Cretaceous Ammonoidea), with special reference to the Circum-Indic species. Eclogae Geologicae Helvetiae 66: 585–616.
Willey, A. 1902. Zoological Results Based on Material from New Britain, New Guinea, Loyalty Islands and Elsewhere Collected during the Years 1895, 1896 and 1897. Part VI. Contribution to the Natural History of the Pearly Nautilus, II. Special Contribution. 736–826. Cambridge University Press, Cambridge. Crossref
Wright, C.W., Callomon, J.H., and Howarth, M.K. 1996. Treatise on Invertebrate Paleontology, Part L, Mollusca 4, Revised, Vol. 4, Cretaceous Ammonoidea. 362 pp. Geological Society of America, Boulder, and University of Kansas Press, Lawrence.
Yabe, H. 1903. Cretaceous Cephalopoda from the Hokkaido. Journal of the College of Science, Imperial University of Tokyo, Japan 18: 1–55.
Zatoń, M. 2008. Taxonomy and palaeobiology of the Bathonian (Middle Jurassic) tulitid ammonite Morrisiceras. Geobios 41: 699–717. Crossref
Acta Palaeontol. Pol. 67 (4): 949–961, 2022
https://doi.org/10.4202/app.01000.2022