


Suspected foraminiferan parasitism on a Late Cretaceous echinoid host recorded by the new attachment trace fossil Solichnus aestheticus
MAX WISSHAK, CHRISTIAN NEUMANN, GIOVANNI SANNA, KURT S.S. NIELSEN, and JESPER MILÀN
Wisshak, M., Neumann, C., Sanna, G., Nielsen, K.S.S., and Milàn, J. 2023. Suspected foraminiferan parasitism on a Late Cretaceous echinoid host recorded by the new attachment trace fossil Solichnus aestheticus. Acta Palaeontologica Polonica 68 (1): 13–22.
A critical reassessment of foraminiferan parasitism on echinoid hosts, past and present, identifies all previous records as doubtful and circumstantial evidence as being limited to possible foraminiferan bioerosion traces on a Late Cretaceous Echinocorys perconicus host from Northern Germany. Here, we report on a second type of putative foraminiferan attachment trace fossils found on a Late Cretaceous Echinocorys jaekeli from the Danish Basin, and establish the new ichnogenus and ichnospecies Solichnus aestheticus within the ichnofamily Centrichnidae. These delicate sun-shaped etchings are diagnosed as bowl-shaped circular depressions, wider than deep, from which numerous open canals radiate in a meandering fashion, ramify, and thin out. The canals indicate a mutual avoidance pattern with those of neighbouring specimens and they circumvent the areoles of the echinoid’s primary tubercles. We interpret the central depression as anchoring site of a foraminiferan test and the radiating canals, formed right at the interface of the stereom and epithelium, as the work of its long and ramifying pseudopodia. The symbiotic relationship was probably of parasitic nature (sensu stricto), with the foraminiferan feeding on the organic tissue of the epithelium (epithelium browsing) and profiting from protection offered by the host’s spines and defensive pedicellariae. The echinoid survived the infestation and formed skeletal regeneration textures that clearly identify the association as syn vivo. The high degree of specialisation required to infest an echinoid host and form the complex attachment trace might suggest that the pronounced rarity of the trace is not a case of a false host but of host specificity. The identity of the foraminiferan parasite remains unknown, although the bioerosion traces show some affinity to those of the extant species Cymbaloporella tabellaeformis and Gypsina vesicularis.
Key words: Echinoidea, Echinocorys, Foraminifera, 3D scanning, bioerosion, ichnotaxonomy, parasitism, trace fossil, symbiosis.
Max Wisshak [max.wisshak@senckenberg.de; ORCID: https://orcid.org/0000-0001-7531-3317 ] and Giovanni Sanna [giovanni.sanna@senckenberg.de; ORCID: https://orcid.org/0000-0002-9348-1522 ], Marine Research Department, Senckenberg am Meer, Wilhelmshaven, Germany.
Christian Neumann [christian.neumann@mfn-berlin.de; ORCID: https://orcid.org/0000-0001-9630-9624], Museum für Naturkunde, Leibniz Institute for Evolution and Biodiversity Science, Berlin, Germany.
Kurt S.S. Nielsen [knieslen@yahoo.dk; ORCID: https://orcid.org/0000-0002-8242-5541 ], Frederikssund Gymnasium, Frederikssund, Denmark.
Jesper Milàn [jesperm@oesm.dk; ORCID: https://orcid.org/0000-0002-9556-3177 ], Geomuseum Faxe, Østsjaellands Museum, Faxe, Denmark.
Received 5 October 2022, accepted 21 December 2022, available online 31 January 2023.
Copyright © 2023 M. Wisshak et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Attachment etchings are formed by a large variety of epibionts on dead or alive hard-substrates of many kinds to enhance adhesion for better protection from hydrodynamic forces, grazers, and predators (Bromley and Heinberg 2006) and allowing the epibiont to engage in symbiotic interactions with the host, if alive. Attachment etchings have a good fossilization potential and represent a category of bioerosion trace fossils currently including 27 ichnospecies established in 17 ichnogenera and grouped in three ichnofamilies (as reviewed by Wisshak et al. 2019). Based on the known or inferred underlying behaviour, they are categorized in an ethological class of their own, the fixichnia, proposed by Gibert et al. (2004). The morphology and size of attachment traces range from simple ring-shaped and fairly minute structures formed by diatoms (ichnogenus Ophthalmichnus Wisshak, Alexandrakis, and Hoppenrath, 2014) to relatively complex and large traces, such as those produced by anomiid bivalves and cirripeds (ichnogenus Centrichnus Bromley and Martinell, 1991).
While some types of attachment etchings are very common and their fossil or extant trace makers are well known, others are extremely rare and lack direct evidence (co-preservation) of the biological identity of their makers. Here, we present a new attachment etching of the latter category: delicate sun-shaped etchings only eight of which have so far been recognised in the fossil record, all preserved on a single fossil echinoid specimen found in the Upper Cretaceous chalk sediments of the Danish Basin. Intriguingly, these trace fossils were all formed during the lifetime of the host echinoid, thus identifying the association as symbiotic (sensu lato) in nature and stimulating discussion on this biotic interaction. Based on several lines of reasoning we suggest that the new trace fossil records a rare case of foraminiferan parasitism on an echinoderm host.
Institutional abbreviations.—DK, Danekræ Collection, Natural History Museum of Denmark, Copenhagen, Denmark; MGUH, Natural History Museum of Denmark, Copenhagen, Denmark.
Other abbreviations.—DSLR, digital single-lens reflex; H, height; L, length; W, width.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered with ZooBank under the urn:lsid:zoobank.org:pub:3B948D1E-496C-46D0-8885-8B1FC39D6BA3 (registered November 22, 2022).
Material and methods
The type specimens of the new trace fossil Solichnus aestheticus igen. et isp. nov. were identified on the aboral test surface of the echinoid Echinocorys jaekeli Nietsch, 1921 (Holasteroida, Echinocorytidae) from the Danekræ Collection (DK-1139) housed at the Natural History Museum of Denmark in Copenhagen under the inventory number MGUH 34117. This medium sized (L 70 mm, W 65 mm, H 55 mm) specimen comes from Hvideklint at the southern shore of the island Møn (Denmark) from a sequence of Upper Cretaceous chalk deposits of the Danish Basin, specifically the Boesdal Member of the Mandehoved Formation (see Surlyk et al. 2013 for detail). According to studies of micromorphic brachiopods (Surlyk 1984) and on coccolithophores (Nicolas Thibault personal communication 2021), these strata are late Campanian to early Maastrichtian in age; the specimen under study comes from the uppermost Campanian part of the Boesdal Member.
An extensive museum query was undertaken in an unsuccessful attempt to identify additional specimens of this rare trace on several thousands of Campanian to Maastrichtian Echinocorys tests from boreal chalk deposits of the Danish Basin and the North German Basin. Among these were hundreds of conspecific specimens from the type locality at Hvideklint (Møn, Denmark) as well as from equivalent strata of the upper Kronsmoor Formation of northern Germany. The investigated specimens include material from the Natural History Museum in Berlin (Germany), Geomuseum Faxe (Denmark), GeoCenter Møns Klint (Denmark), Natural History Museum of Denmark, as well as several private collections.
The echinoid and the eight attachment etchings were photographed with a Nikon D800 DSLR equipped with a Micro Nikkor 60 mm 1:2.8 G ED macro lens and extension tubes, applying a Cognisys StackShot Macro Rail for extended focal imaging with the software Helicon Focus Pro. Photographs were taken before and after coating with ammonium chloride for enhancing even the most delicate details. In addition, scanning electron micrographs (SEMs) of the traces were captured with a Tescan VEGA3 XMU using backscatter electrons (BSE) in low-vacuum mode at 20 keV, allowing the visualization of the holotype and paratypes without prior sputter coating. Morphometrical measurements were taken in the VEGA SEM software.
The three-dimensional surface of the specimen was digitised using the structured-light 3D scanner Artec Space Spider and the processing software Artec Studio 15. A polygonal 3D mesh with a resolution of 0.05 mm was generated and simplified (by reducing the number of polygons) to optimize the file size while retaining accuracy. The model was then textured using the information captured by the scanner’s colour camera. The resulting 3D model in PLY format is provided as Supplementary Online Mata (SOM, available at http://app.pan.pl/SOM/app68-Wisshak_etal_SOM.pdf), and in addition it has been uploaded into the Sketchfab 3D library for interactive online viewing at https://skfb.ly/oAEIA.
Systematic palaeoichnology
Ichnofamily Centrichnidae Wisshak, Knaust, and Bertling, 2019
Ichnogenus Solichnus nov.
Zoobank LSID: urn:lsid:zoobank.org:act:C60A3E00-F5B7-4021-ACB 9-E13898CA1DF1
Type ichnospecies: Solichnus aestheticus isp. nov., monoichnospecific; see below.
Etymology: Compound of the Latin sol, sun and ichnus (the Latinised form of Ancient Greek ἴχνος, ichnos), foot print, track, trace; referring to the distinctly sun-shaped morphology of the trace. An additional connotation is that given by Sól being the sun personified in Germanic mythology, thus referring to the Scandinavian type locality of the new trace fossil.
Diagnosis.—Circumradial surface etchings with a central depression and radiating open canals in calcareous skeletal substrates.
Ethological category.—Fixichnia (Gibert et al. 2004; attachment traces).
Remarks.—The new ichnogenus deemed necessary to clearly separate the morphology of Solichnus with its diagnostic radiating canals from the sole circular depressions of various kinds in echinoderm skeletons comprised within the ichnogenus Tremichnus Brett, 1985. While the ichnogenera Entobia Bronn, 1837, and Planobola Schmidt, 1992, include some ichnospecies with a vague similarity to Solichnus aestheticus (see below), they would not have been an appropriate parent ichnogenus, because the former concerns interconnected chambers below the substrate surface and the latter addresses bowl-shaped microborings that usually lack radiating grooves.
Solichnus aestheticus isp. nov.
Figs. 2, 3.
Zoobank LSID: urn:lsid:zoobank.org:act:82AE4741-8B0E-41D2-9E 02-0B2151E32219
Etymology: Adopts the Latin aestheticus, aesthetic; emphasizing the delicate nature and aesthetical value of the trace fossil.
Type material: The Echinocorys jaekeli test carrying the type specimens of Solichnus aestheticus igen. et isp. nov. (Fig. 1) MGUH 34117. The holotype of Solichnus aestheticus igen. et isp. nov. is registered as MGUH 34117a, and the seven paratypes as MGUH 34117b–h (Table 1).
Type locality: Hvideklint (54.930658°N, 12.272882°E) at the southern shore of the island Møn, Denmark.
Type horizon: Uppermost Campanian of the Boesdal Member, Mandehoved Formation, Upper Cretaceous (see Surlyk et al. 2013 for detail).
Diagnosis.—Bowl-shaped circular depression, wider than deep, from which numerous open canals radiate in a meandering fashion, ramify, and thin out.
Description.—A bowl-shaped circular depression, 0.8–1.5 mm in diameter (mean = 1.2±0.3 mm; n = 8) and wider than deep, marks the centre of the trace. From this excavation 17–23 canals (mean = 20.5±2.3; n = 6) radiate with a meandering course and repeatedly bifurcate in random intervals. These open canals are wider than deep, proximally about 0.1 mm wide (0.05–0.15 mm), and taper towards the indistinct periphery of the trace. The maximum diameter of the trace reaches 13.2–32.3 mm in diameter (mean = 19.5±5.7 mm; n = 8). There is no correlation between the diameter of the central depression and the trace as a whole (r2 of linear regression = 0.046). See Table 1 for detailed morphometrical data.
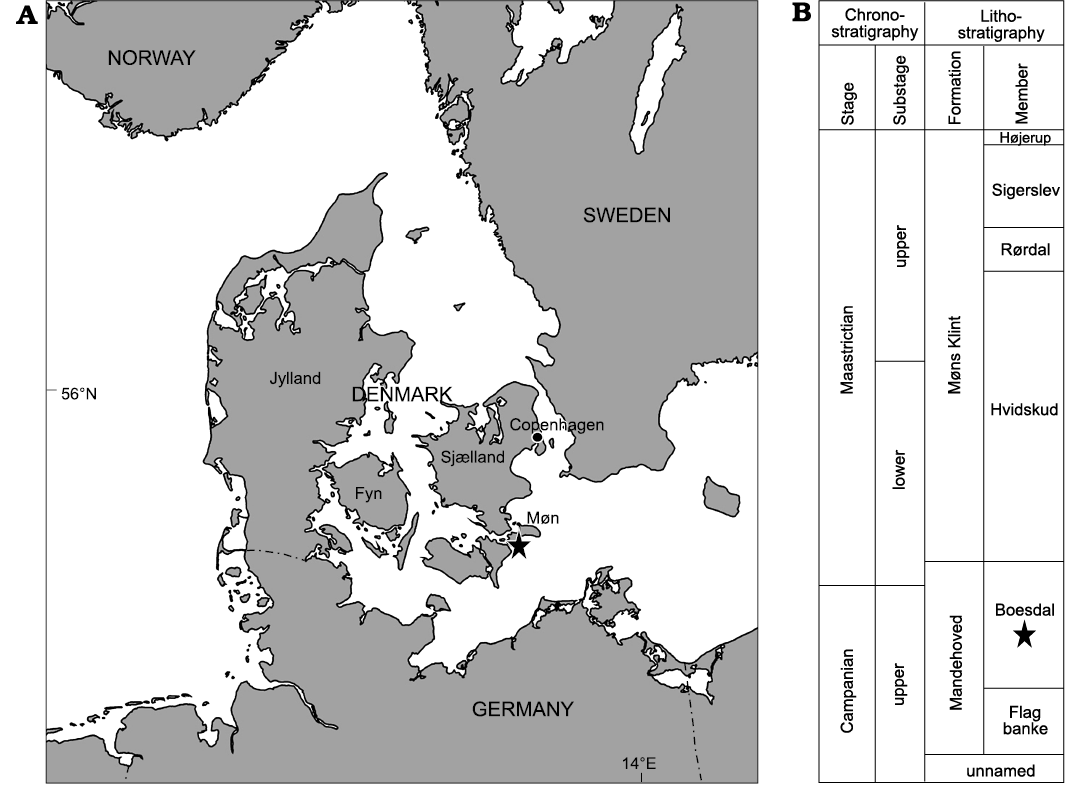
Fig. 1. Location and stratigraphy. A. Hvideklint is located on the southern shore of the island of Møn in eastern Denmark. B. Schematic representation of the Campanian to Maastrichtian stratigraphy of eastern Denmark (modified after Surlyk et al. 2013).
Table 1. Inventory numbers, Lovén’s position on the echinoid test, and morphometrical data for the holotype and the seven paratypes of Solichnus aestheticus igen. et isp. nov.
|
Type specimen |
Inventory number |
Lovén’s position |
ø trace [mm] |
ø depression [mm] |
n of initial rays |
|
holotype |
MGUH 34117a |
III + 3 |
17.7 |
1.5 |
23 |
|
paratype 1 |
MGUH 34117b |
1 |
19.1 |
0.8 |
20 |
|
paratype 2 |
MGUH 34117c |
2 |
21.3 |
0.8 |
17 |
|
paratype 3 |
MGUH 34117d |
III + 3 |
16.2 |
1.5 |
23 |
|
paratype 4 |
MGUH 34117e |
III + 3 |
13.2 |
1.0 |
oyster on margin |
|
paratype 5 |
MGUH 34117f |
3 |
32.3 |
1.5 |
oyster in centre |
|
paratype 6 |
MGUH 34117g |
3 + IV |
17.3 |
1.4 |
21 |
|
paratype 7 |
MGUH 34117h |
V |
18.7 |
0.9 |
19 |
|
mean±SD |
19.8±6.7 |
1.2±0.3 |
20.0±2.6 |
||
|
min–max |
13.2–32.3 |
0.8–1.5 |
17.0–23.0 |
||
Remarks.—The morphology of Solichnus aestheticus igen. et isp. nov. is unlike any other ichnospecies for attachment etchings or other bioerosion traces previously reported in the literature. The only traces with a vague similarity, albeit much larger in dimension, are some unicamerate sponge boring ichnospecies in the ichnogenus Entobia Bronn, 1837, including Entobia devonica (Clarke, 1921), E. solaris Mikuláš, 1992, E. astrologica Mikuláš, 1992, E. cracoviensis Bromley and Uchman in Bromley et al., 2009, and E. resinensis Santos, Mayoral, and Bromley, 2011. However, in the centre of these traces there is a globular chamber rather than a bowl-shaped depression, but hardground erosion can unroof this structure and also expose the radiating tunnels, which only then appear like open canals. There is also some similarity to one of the ichnospecies of the microboring ichnogenus Planobola Schmidt, 1992, namely P. radicata Schmidt, 1992, which is much smaller in dimension and has a central cavity of only 15 to 30 µm in diameter with a latitudinal contact to the substrate surface, from which thin tunnels, not canals, radiate. None of the ichnospecies within the related ichnogenus Tremichnus Brett, 1985, shows the radiating canals diagnostic for Solichnus aestheticus.
It shall be noted that there is some superficial resemblance to composite traces of borings (various ichnospecies) overprinted by the grazing/predation activity of echinoids (Gnathichnus pentax), such as those illustrated by Bromley (1970: fig. 6 and pl. 3/4) or Wisshak (2006: fig. 23D). These composite traces had erroneously led to the establishment of Asteriastoma Breton, 1992, and its type ichnospecies A. cretaceum Breton, 1992, both consequently rejected by Wisshak et al. (2019).
All specimens are loosely clustered on the lateral aboral surface of the same Echinocorys host echinoid, six on its anterior and two on the posterior part of the test (Fig. 2). All specimens show regeneration tissue (newly grown primary and secondary tubercles) formed by the echinoid (Fig. 3A1–A3, B1, B2, C1, C2). The canals seem to largely circumvent the areoles of the primary tubercles but cross plate sutures without any interference (Fig. 3B3, C3). They interfere, however, with canals of neighbouring specimens, in which case they turn before intersecting, forming a mutual avoidance pattern (Fig. 3B1, B2, C1, C2).
Geographic and stratigraphic range.—Type locality and horizon only.
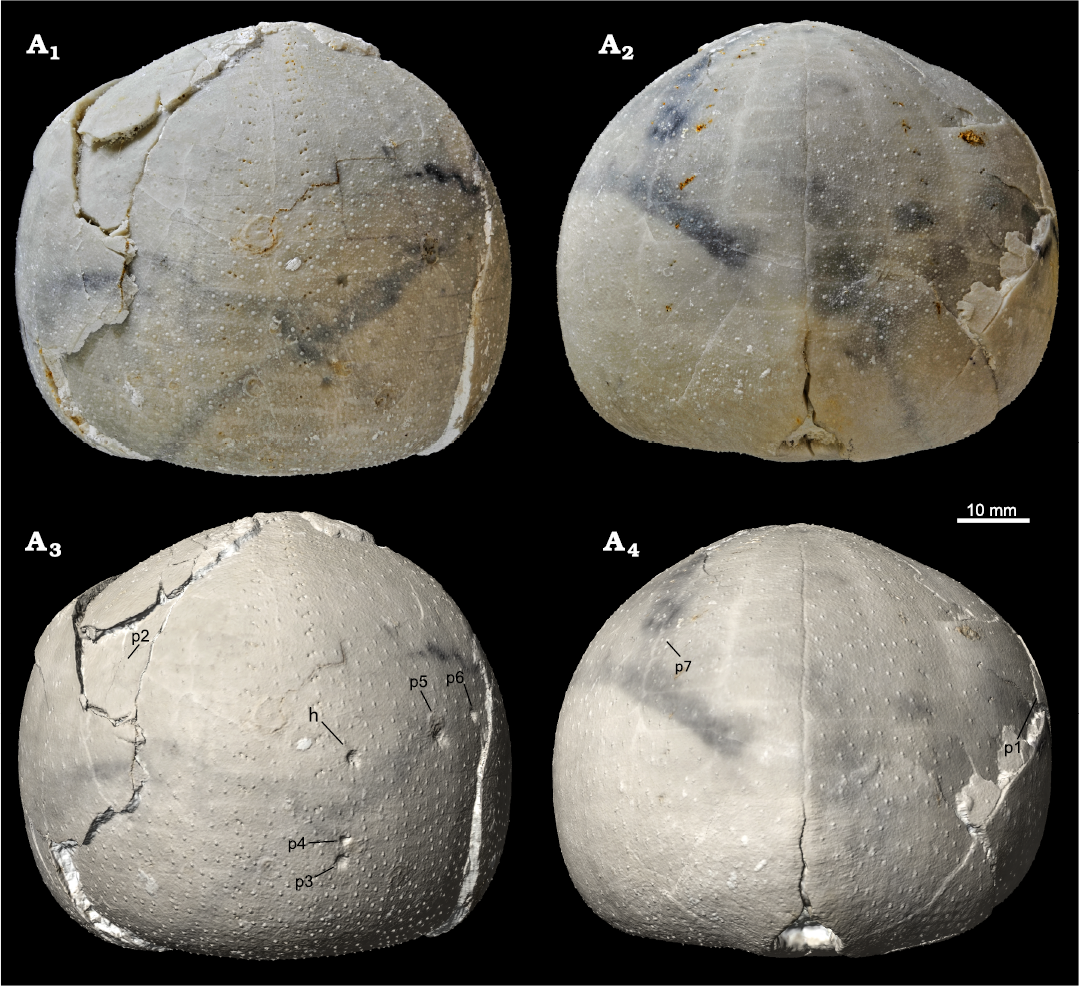
Fig. 2. Holasteroid echinoid Echinocorys jaekeli Nietsch, 1921 (MGUH 34117) from the upper Campanian of Hvideklint, Møn, Denmark; carrying the type series of the new foraminiferan attachment trace fossil Solichnus aestheticus igen. et isp. nov. Anterior (A1) and posterior (A2) views of the original specimen and the respective views (A3, A4) of a textured 3D digital surface model with the positions of the holotype (h; MGUH 34117a) and the seven paratypes (p1–7; MGUH 34117b–h) of Solichnus aestheticus igen. et isp. nov.; an interactive viewer with this digitype can be accessed online via Sketchfab at https://skfb.ly/oAEIA.
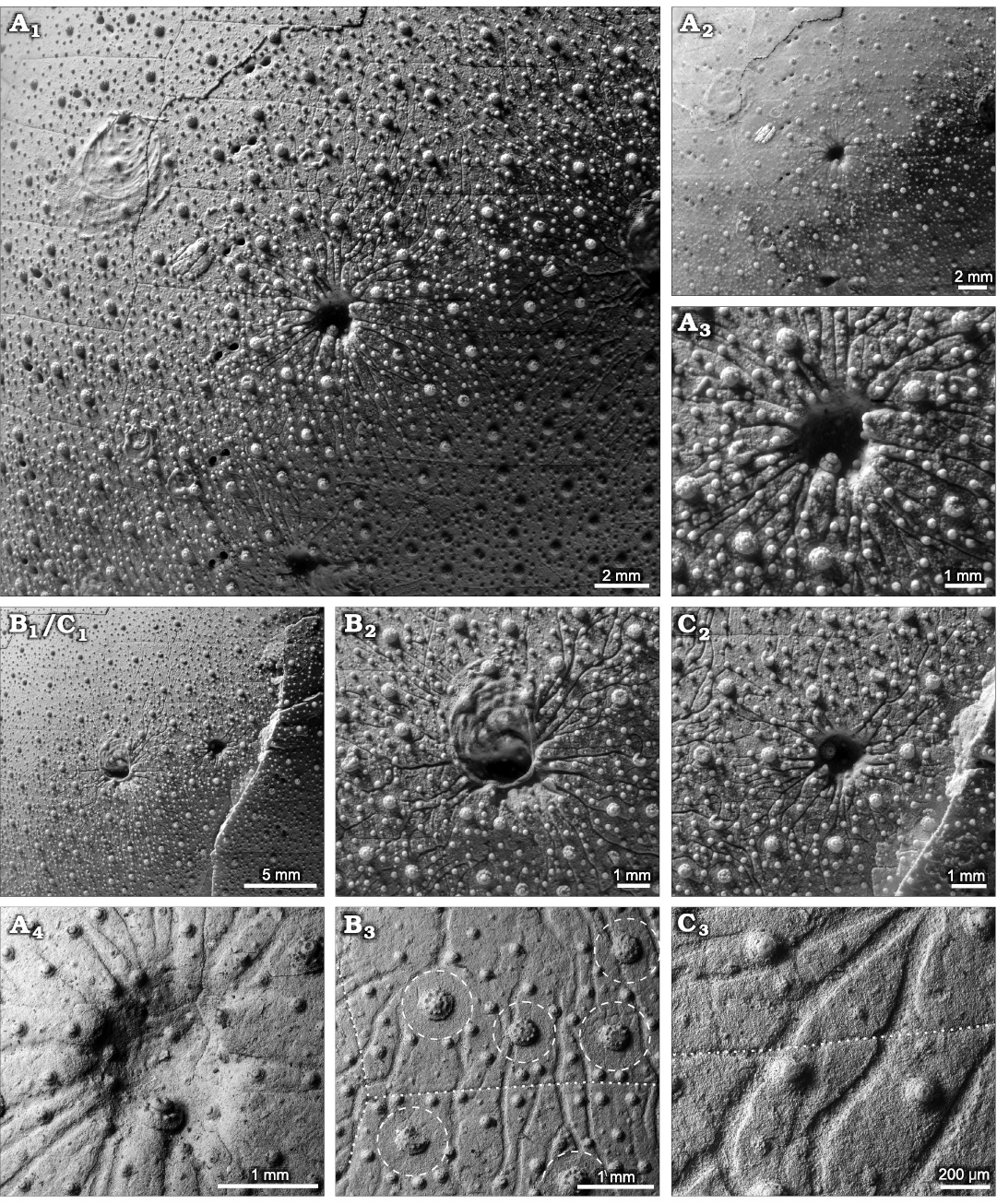
Fig. 3. Type specimens of the new foraminiferan attachment trace fossil Solichnus aestheticus igen. et isp. nov. from the upper Campanian of Hvideklint, Møn, Denmark. A. The holotype trace (MGUH 34117a), photographed after (A1) and before (A2) coating with ammonium chloride, showing the extent of the diagnostic radiating canals and their interference with those of neighbouring paratypes. Close-up of the central depression of the holotype (A3) with echinoid regeneration texture (newly formed tubercles). Backscatter electron SEM image of the central depression of the holotype (A4); note that the topography in these SEM images appears shallower than it is. B, C. Overview of paratypes 5, 6 (MGUH 34117f, g, respectively) demonstrating the mutual avoidance pattern of the radiating canals (B1, C1). The centre of MGUH 34117f (B2) overgrown by an oyster and the centre of MGUH 34117g (C2) with regeneration textures. SEM close-up of canals (B3), largely circumventing the tubercle areoles (i.e., spine muscle attachment areas; dashed lines) but crossing plate sutures (dotted lines) without interference. SEM close-up (C3) of ramifying canals crossing a plate suture (dotted line).
Discussion
Echinocorys as host for bioeroding symbionts.—For better understanding and interpreting the symbiotic association recorded by the new attachment trace fossil Solichnus aestheticus igen. et isp. nov., it is favourable to first elucidate some of the echinoid host’s ecophysiological characters and its role in ecosystem functioning. Members of the holasteroid genus Echinocorys are among the largest and most common epibenthic faunal elements in the Late Cretaceous chalk sea (e.g., Nestler 1965; Müller 1969; Jablonski and Bottjer 1983). Species of Echinocorys possess a large, dome-shaped test covered by short spines, and belong to the epifaunal mobile deposit-feeder guild browsing the sediment surface for nutritious organic matter (e.g., Stephenson 1963; Smith and Wright 2003). Given the soft and monotonous palaeoenvironment of the seafloor of the chalk sea, these large echinoids served as benthic islands (e.g., Kauffmann 1982; Tapanila and Ebbestad 2008; Borszcz et al. 2013) for a wide range of epibenthic organisms and encrusters. This concerns both, encrustation of the dead Echinocorys tests littering the seafloor as well as symbiotic interactions of various commensals and parasites on the living echinoids, in which case the host function (protection, food source, etc.) predominates the merely passive role as a benthic island. However, only those symbionts that bioerode the skeleton or induce alteration thereof, leave their mark in the fossil record, such as is the case for the enigmatic trace maker of Solichnus aestheticus igen. et isp. nov. Hence, the fossil record grossly underestimates the effective number of parasites. For instance, Barel and Kramers (1977) listed 89 parasitic organisms infesting 29 echinoid species in the North Atlantic. Of these, only one recorded parasite (the ascothoracid cirriped Ulophysema oeresundense Brattström, 1936) is capable to alter the skeleton of its host by producing borings in the test (Grygier and Høeg 2005) and thus possessing the potential to leave evidence in the fossil record. All other parasites found were either vagile ectoparasites living on the echinoids’ surface or endoparasites found in their organs or in the coelomic cavity. In any case, the symbiont has to overcome the defensive barrier consisting of spines, pedicellariae, and the epithelium layer covering the skeleton. The echinoderm skeleton (stereom) is a calcareous living tissue that morphs during growth and can react to external disturbances, such as predatory injury (Neumann and Hampe 2018; Wisshak and Neumann 2020), or parasitic bioerosion (Neumann and Wisshak 2006, 2009; Wisshak and Neumann 2006), for instance by means of forming a callus on the inside of the skeleton or by developing bioclaustrations and regeneration textures. It is these regeneration structures that clearly identify a bioerosion structure as syn vivo in nature, providing such evidence also for Solichnus aestheticus, as most of the central depressions exhibit regenerated primary and secondary tubercles (Fig 3A3, A4, B1, C1, C2).
Echinocorys species show a particularly rich spectrum of syn vivo bioerosion traces, including pits formed by unknown trace makers (Oichnus ispp. and others; Müller 1969; Bromley 1981; Donovan and Jagt 2004; Hammond and Donovan 2017), as well as traces presumably formed by eulimid gastropods (Oichnus halo; Neumann and Wisshak 2009), spionid polychaetes (Caulostrepsis isp.; Wisshak and Neumann 2006), and foraminiferans (Kardopomorphos isp.; Neumann and Wisshak 2006). The latter shows close morphological similarity to attachment scars produced by the extant parasitic foraminiferan Hyrrokkin sarcophaga (Cedhagen 1994; Beuck et al. 2008) on coral and bivalve hosts.
A foraminiferal parasite as trace maker of Solichnus aestheticus.—The new attachment trace Solichnus aestheticus igen. et isp. nov. now provides strong evidence for another foraminiferan symbiont. We interpret its central depression as the anchoring site of a foraminiferan test, and the shallow radiating grooves as the work of its long and ramifying pseudopodia.
The formation of bowl-shaped to spiral-shaped attachment etchings in hard substrates, dead and alive, is well known for a variety of extant and fossil foraminiferan species, with the oldest records being of Late Jurassic age (see Vénec-Peyré 1996 and Walker et al. 2017 for reviews). Also, the ability of foraminiferan pseudopodia to dissolve and potentially utilise the hosts’ calcium carbonate skeleton was first studied by Todd (1965) and Poag (1969), and radiating, ramifying, and in some cases anastomosing bioerosion structures likely formed by pseudopodial action have repeatedly been reported. This includes the Jurassic naked foraminiferan Globodendrina monile Plewes, Palmer, and Haynes, 1993, and related extant species that form bioerosion traces of the ichnogenus Nododendrina (Bromley et al. 2007, see Wisshak 2017 for a review of the Dendrinidae). The hitherto unnamed bioerosion trace of similar size and closest morphological affinity to Solichnus aestheticus is produced, however, by the extant Cymbaloporella tabellaeformis (Brady, 1884), as reported by Heron-Allen (1915), Matteucci (1974, 1980), and Smyth (1988). Its trace features a central cavity from which tunnels radiate and ramify into the substrate “obviously for the accommodation of the extruded pseudopodia of the foraminifer” (Heron-Allen 1915: 259). In contrast to S. aestheticus, this bioerosion structure is endolithic, i.e., a chamber formed below the substrate surface and tunnels radiating from it, but in an advanced stage of development, the tunnels do reach and spread along the substrate surface. Matteucci (1980: fig. 5) proposed that in the final stage of trace formation, the roof of the main chamber is dissolved or collapses, allowing the foraminiferan to leave its excavation. It is this final stage that shows the closest morphological similarity to S. aestheticus, even more so if partly abraded. Another extant foraminiferan with a bioerosion trace similar to S. aestheticus is Gypsina vesicularis (Parker and Jones 1860), whose attachment trace is circular in outline and shows short radiating extensions (Wisshak and Rüggeberg 2006; Wisshak et al. 2011). Together, these examples of fossil and recent bioeroding foraminiferans forming bioerosion traces with some affinity to S. aestheticus both support our interpretation and point towards possible taxonomic affinities of its trace maker.
We suggest that the symbiotic relationship was of parasitic nature sensu stricto (a symbiont that lives on or within a host organism, upon which it feeds, causing harm, but usually without killing it; Poulin 2007). According to Walker et al. (2017) a parasitic trophic mode has evolved in at least 9 species of bioeroding foraminiferans and has been suspected in 13 additional ones. In the case of Solichnus aestheticus it appears likely that the trace making foraminiferan parasite fed on the organic tissue of the regenerating epithelium, a trophic mode widespread among echinoderm parasites such as copepods or eulimid gastropods and referred to as epithelium browsing (Jangoux 1987; Ponder et al. 2020). This interpretation is supported by the observation that the presumed pseudopodia stretched out right at the interface of the stereom and epithelium, covering a large area of the host surface and profiting from the regenerative capacity of the epithelium. The morphology of the canals furthermore indicates that the pseudopodia neither penetrated deeply into the skeleton nor reached into the water column, as it would be expected from a filter-feeding or detritus-collecting foraminifer. This explains why the foraminiferan chose a living host, whereas filter- and detritus-feeding works also on dead echinoid tests or other hardground substrates (although lacking the protective role of the living echinoid). The connective tissues and muscles attaching the spines where apparently not appealing to the parasite, as indicated by the grooves circumventing the areole areas of the host spines (Fig. 3B3, C3).
What we don’t know is whether this case of parasitic infestation was harmful for the host, as even small lesions of the echinoid epithelium may become moribund in the case of bacterial infection (Bauer and Young 2000). In the present case, however, the observed skeletal regeneration textures (renewal of spine tubercles on the etched central depression and the channels; Fig. 3A1–A4, B1, B2, C1, C2) indicate that the echinoid survived the infestation of several of these parasites. The corresponding eight traces were most probably formed by eight parasite individuals that colonized the host simultaneously, as can be inferred from the same state of preservation, including host regeneration structures, and from the observation of the pseudopodia channels avoiding interference with those of neighbouring individuals (Fig. 3B1, B2, C1, C2). After all, the echinoid host was not harassed for a long time, considering the longevity of extant echinoids of two to more than 100 years (Ebert and Southon 2003) compared to that of most benthic foraminiferan which only reach a lifespan of several weeks to a year (Boltovskoy and Wright 2013).
The question of rarity.—The phenomenon of rarity of a fossil parasite—or of a trace fossil formed by it—can be explained by a high degree of host specificity, by geographical or habitat (spatial) restrictions, or by stratigraphical (temporal) restrictions (e.g., Donovan 2015; Farrar et al. 2020; De Baets et al. 2021a, b). The peculiar rarity of Solichnus aestheticus igen. et isp. nov. indicated by our unsuccessful attempt to find additional traces with that morphology in thousands of Campanian to Maastrichtian Echinocorys tests, including topotypic material and including hundreds of conspecific Echinocorys jaekeli, could result from any of the above restrictions, or combinations thereof, and it will be interesting to see what further records will be reported to elucidate that question. The peculiar rarity of Solichnus aestheticus might, however, merely suggest that the symbiotic association was erratic and that the foraminiferans have ended up on a false host. This appears unlikely, though, acknowledging that overcoming the echinoid’s defensive barriers and forming such a complex trace requires a high level of specialization, and considering that eight parasite individuals succeeded in infesting the same specimen of the only known host species to date, Echinocorys jaekeli.
Critical reassessment of foraminiferan parasitism on echinoids.—Foraminiferan parasitism on echinoderms, and on echinoid hosts in particular, appears to be very rare, extinct and extant. In their extensive review on parasitic foraminiferans, Walker et al. (2017) list only two out of 22 parasitic and suspected parasitic species considered parasitizing echinoid hosts:
(i) Cibicides antarcticus (= Cibicides refulgens) (Saidova, 1975) (Pliocene to Recent; Alexander and DeLaca 1987; David et al. 2009; Schweizer et al. 2009, 2012). David et al. (2009) figured C. antarcticus (among other foraminifera) attached to the spines of various living cidaroid sea urchins from Antarctic waters. Based on their observation that in some cidaroids the spine cortex has been corroded to some extent by the attaching foraminifer, the authors suggested a parasitic relationship. However, attachment scars formed by various Cibicides species are a very common phenomenon on both, living and dead substrates (e.g., Wisshak and Rüggeberg 2006; Wisshak et al. 2011) and certainly not an indication for parasitism in itself. Although a parasitic behaviour of Cibicides has been documented for several other invertebrate hosts, such as in the Antarctic scallop Adamussium colbecki (Smith, 1902) by Alexander and DeLaca (1987) and Hancock et al. (2015), at present, we see no convincing confirmation of Cibicides antarcticus specifically parasitizing an echinoid host.
(ii) Ramulina globulifera (Brady, 1879) (Late Cretaceous to Recent; Jones and Chapman 1897; Walker et al. 2017). According to Walker et al. (2017) both R. globulifera (Brady, 1879) and the closely related R. parasitica Carter, 1889 are suspected endoparasites and bioerode excavations that match the size of their chambers, or they produce small round holes that penetrate the host skeleton. Jones and Chapman (1897: 344) figured five specimens of fossil R. globulifera attached to various echinoid test fragments from the British Margate Chalk Member (Santonian–Campanian, Upper Cretaceous). Since they neither observed bioerosion nor host growth response, the parasitic nature of the relationship remains doubtful and postmortal epibiosis more likely.
Further, Walker et al. (2017) list an additional species on an unspecified echinoderm host, Cymbaloporella tabellaeformis (Brady, 1884) (Recent; Heron-Allen 1915; Bertram 1936; Matteucci 1974, 1980; Smyth 1988). The bioerosive capacity of this species in various kinds of calcareous substrates (e.g., bivalves, gastropods, corals) is unequivocal and was first described and illustrated in detail by Heron-Allen (1915). Walker’s et al. (2017) reference to an echinoderm host probably refers to the observation of C. tabellaeformis in the test of an echinoid of the genus Clypeaster reported from the Red Sea by Matteucci (1974: pl. 2: 2, 4). However, it is more likely that this particular echinoid specimen was not catched alive but belonged to the shell fragments primarily studied by Matteucci (1974). Hence, the parasitic nature cannot be confirmed and infestation might have just as well occurred post mortem, such as most commonly the case for C. tabellaeformis in bivalves and gastropods.
Conclusions
While all previous records of extinct or extant foraminiferan parasitism on echinoid hosts remain doubtful, two cases of putative foraminiferan attachment traces on the Late Cretaceous holasteroid echinoid Echinocorys currently are the only circumstantial evidence for this parasite-host association. These include Hyrrokkin-like foraminiferan pits formed syn vivo on the oral surface of an Echinocorys perconica, reported from the Maastrichtian of Northern Germany (Neumann and Wisshak 2006), and the new trace fossil Solichnus aestheticus igen. et isp. nov., formed syn vivo on an Echinocorys jaekeli from the Campanian of the Danish Basin. The symbiotic relationship is interpreted as parasitism (sensu stricto), with the foraminiferan feeding on the echinoid’s epithelium and profiting from protection offered by the host’s spines and pedicellariae. The specialisation required to infest the Echinocorys host and to form the complex attachment traces might suggest that the rarity of the trace is not a case of a false host but of host specificity. We hope these records sharpen the eyes for fossil and modern foraminiferans parasitizing on echinoids (or other echinoderms) and stimulate the search for material with the trace makers preserved in situ to verify our interpretation and elucidate the identity of the foraminiferan parasite.
Acknowledgements
We are indebted to the amateur collector Mette Agersnap Grejsen Hofstedt (Hornslet, Denmark) for finding and recognizing this unique fossil. Sten Lennart Jakobsen and Lothar Vallon (both Geomuseum Faxe, Denmark) are thanked for bringing it to our attention. The DLSR photos of the new trace were kindly captured by technician Nicol Mahnken (Senckenberg am Meer, Wilhelmshaven, Germany). Amateur collector Peter Girod (Berlin, Germany) provided access to his substantial fossil collection. Arden Basforth (Natural History Museum of Denmark, Copenhagen, Denmark) and Nils Natorp (Geocenter Møns Klint, Denmark) kindly gave us access to the collections in their care. We gratefully acknowledge reviewers Kenneth De Baets (University of Warsaw, Poland), Alfred Uchman (Jagiellonian University, Kraków, Poland), and Andreas Kroh (Natural History Museum, Vienna, Austria) for their helpful corrections and suggestions.
References
Alexander, S.P. and DeLaca, T.E. 1987. Feeding adaptations of the foraminiferan Cibicides refulgens living epizoically and parasitically on the Antarctic scallop Adamussium colbecki. The Biological Bulletin 173: 136–159. Crossref
Barel, C.D.N. and Kramers, P.G.N. 1977. A survey of the echinoderm associates of the north-east Atlantic area. Zoologische Verhandelingen 156: 1–159.
Bauer, J.C. and Young, C.M. 2000. Epidermal lesions and mortality caused by vibriosis in deep-sea Bahamian echinoids: a laboratory study. Diseases of Aquatic Organisms 39: 193–199. Crossref
Bertram, G.C.L. 1936. Some aspects of the breakdown of coral at Ghardaqa, Red Sea. Proceedings of the Zoological Society of London 106: 1011–1026. Crossref
Beuck, L., López Correa, M., and Freiwald, A. 2008. Biogeographical distribution of Hyrrokkin (Rosalinidae, Foraminifera) and its host-specific morphological and textural trace variability. In: M. Wisshak and L. Tapanila (eds.), Current Developments in Bioerosion, 329–360. Springer, Berlin. Crossref
Boltovskoy, E. and Wright, R.C. (eds.) 2013. Recent Foraminifera. 636 pp. Springer, Berlin.
Borszcz, T., Kuklinski, P., and Zatoń, M. 2013. Encrustation patterns on Late Cretaceous (Turonian) echinoids from southern Poland. Facies 59: 299–318. Crossref
Brady, H.B. 1879. Notes on some of the reticularian Rhizopoda of the “Challenger” Expedition: Part I: On new or little known arenaceous types. Quarterly Journal of Microscopical Science 19: 20–67. Crossref
Brady, H.B. 1884. Report on the Foraminifera dredged by H.M.S. Challenger during the years 1873–1876. Zoology 9 (22): 1–814.
Brattström, H.O. 1936. Ulophysema öresundense n. gen. et sp., eine neue Art der Ordnung Cirripedia Ascothoracica (Vorläufige Mitteilungen). Arkiv fur Zoologi 28A: 1–10.
Breton, G. 1992. Les Goniasteridae (Asteroidea, Echinodermata) Jurassiques et Crétacés de France. Taphonomie, systématique, biostratigraphie, paléobiogéographie, évolution. Bulletin Trimesteriel de la Société Géologique de la Normandie, Supplement 78: 1–590.
Brett, C.E. 1985. Tremichnus: a new ichnogenus of circular-parabolic pits in fossil echinoderms. Journal of Paleontology 59: 625–635.
Bromley, R.G. 1970. Predation and symbiosis in some Upper Cretaceous clionid sponges. Bulletin of the Geological Society of Denmark 19: 398–405.
Bromley, R.G. 1981. Concepts in ichnotaxonomy illustrated by small round holes in shells. Acta Geológica Hispánica 16: 55–64.
Bromley, R.G. and Heinberg, C. 2006. Attachment strategies of organisms on hard substrates: A palaeontological view. Palaeogeography, Palaeoclimatology, Palaeoecology 232: 429–453. Crossref
Bromley, R.G. and Martinell, J. 1991. Centrichnus, new ichnogenus for centrically patterned attachment scars on skeletal substrates. Bulletin of the Geological Society of Denmark 38: 243–252. Crossref
Bromley, R.G., Uchman, A., Kołodziej, B., and Kędzierski, M. 2009. Large chambered sponge borings on a Late Cretaceous abrasion platform at Cracow, Poland. Cretaceous Research 30: 149–160. Crossref
Bromley, R.G., Wisshak, M., Glaub, I., and Botquelen, A. 2007. Ichnotaxonomic review of dendriniform borings attributed to foraminiferans: Semidendrina igen. nov. In: W. Miller III (ed.), Trace Fossils: Concepts, Problems, Prospects, 518–530. Elsevier, Amsterdam. Crossref
Bronn, H.G. 1837. Lethaea Geognostica, Abbildungen und Beschreibungen der für die Gebirgs-Formationen bezeichnendsten Versteinerungen: Erster Band. 544 pp. Schweizerbart, Stuttgart.
Carter, H.J. 1889. Ramulina parasitica, a new species of fossil foraminifera infesting Orbitolites mantelli, var. theobaldi, with comparative observations on the process of reproduction in the mycetozoa, freshwater rhizopoda, and foraminifera. Journal of Natural History 4: 94–101. Crossref
Cedhagen, T. 1994. Taxonomy and biology of Hyrrokkin sarcophaga gen. et sp. n.: a parasitic foraminiferan (Rosalinidae). Sarsia 79: 65–82. Crossref
Clarke, J.M. 1921. Organic dependence and disease, their origin and significance. New York State Museum Bulletin 221–222: 1–113. Crossref
David, B., Stock, S.R., De Carlo, F., Hétérier, V., and De Ridder, C. 2009. Microstructures of Antarctic cidaroid spines: diversity of shapes and ectosymbiont attachments. Marine Biology 156: 1559–1572. Crossref
De Baets, K., Budil, P., Fatka, O., and Geyer, G. 2021a. Trilobites as hosts for parasites: From paleopathologies to etiologies. In: K. De Baets and J.W. Huntley (eds.), The Evolution and Fossil Record of Parasitism, 173–201. Springer, Berlin. Crossref
De Baets, K., Hoffmann, R., and Mironenko, A. 2021b. Evolutionary history of cephalopod pathologies linked with parasitism. In: K. De Baets and J.W. Huntley (eds.), The Evolution and Fossil Record of Parasitism, 203–249. Springer, Berlin. Crossref
Donovan, S.K. 2015. A prejudiced review of ancient parasites and their host echinoderms: CSI Fossil Record or just an excuse for speculation? Advances in Parasitology 90: 291–328. Crossref
Donovan, S.K. and Jagt, J.W. 2004. Site selectivity of pits in the Chalk (Upper Cretaceous) echinoid Echinocorys Leske from France. Bulletin of the Mizunami Fossil Museum 31: 21–24.
Ebert, T.A. and Southon, J.R. 2003. Red sea urchins (Strongylocentrotus franciscanus) can live over 100 years: confirmation with A-bomb 14carbon. Fish B-NOAA 101: 915–922.
Farrar, L., Graves, E., Petsios, E., Portell, R.W., Grun, T.B., Kowalewski, M., and Tyler, C.L. 2020. Characterization of traces of predation and parasitism on fossil echinoids. Palaios 35: 215–227. Crossref
Gibert, J.M. de, Domènech, R., and Martinell, J. 2004. An ethological framework for animal bioerosion trace fossils upon mineral substrates with proposal of a new class, Fixichnia. Lethaia 37: 429–437. Crossref
Grygier, M.J. and Høeg, J.T. 2005. Ascothoracida (ascothoracids). In: K. Rohde (ed.), Marine Parasites, 149–154. CSIRO Publishing, Collingwood.
Hammond, J. and Donovan, S.K. 2017. Shallow traces (pits) in the test of the irregular echinoid Echinocorys scutata Leske from the Chalk (Upper Cretaceous) of the United Kingdom. Ichnos 24: 124–132. Crossref
Hancock, L.G., Walker, S.E., Pérez-Huerta, A., and Bowser, S.S. 2015. Population dynamics and parasite load of a foraminifer on its antarctic scallop host with their carbonate biomass contributions. PLoS ONE 10: article e0132534. Crossref
Heron-Allen, E. 1915. Contributions to the study of the bionomics and reproductive processes of the foraminifera. Philosophical Transactions of the Royal Society of London, Series B 206: 227–279. Crossref
Jablonski, D. and Bottjer, D.J. 1983. Soft-bottom epifaunal suspension-feeding assemblages in the Late Cretaceous. In: M.J.S. Tevesz and P.L. McCall (eds.), Biotic Interactions in Recent and Fossil Benthic Communities, 747–812. Springer, Boston. Crossref
Jangoux, M. 1987. Diseases of Echinodermata: III: Agents metazoans (Annelida to Pisces). Diseases of Aquatic Organisms 3: 59–83. Crossref
Jones, T.R. and Chapman, F. 1897. On the fistulose Polymorphinæ, and on the genus Ramulina. Zoological Journal of the Linnean Society 26: 334–354. Crossref
Kauffman, E.G. 1982. The community structure of “shell islands” on oxygen depleted substrates in Mesozoic dark shales and laminated carbonates. In: G. Einsele and A. Seilacher (eds.), Cyclic and Event Stratification, 502–503. Springer, Berlin. Crossref
Matteucci, R. 1974. Cymbaloporella tabellaeformis (Brady), foraminifero endolitico del Mar Rosso. Geologica Romana 13: 29–43.
Matteucci, R. 1980. Osservazioni sul foraminifero endolitico Cymbaloporella tabellaeformis (Brady) nell’Atollo di Male (north Male), Isole Maldive. Geologica Romana 19: 267–274.
Mikuláš, R. 1992. Early Cretaceous borings from Štramberk (Czechoslovakia). Časopis pro Mineralogii a Geologii 37: 297–312.
Müller, A.H. 1969. Zur Ökologie und Biostratinomie eines Echinocorys (Echinoidea) mit eigentümlichem Naticiden-Befall aus der Oberkreide. Monatsberichte der Deutschen Akademie der Wissenschaften zu Berlin 11: 672–684.
Nestler, H. 1965. Die Rekonstruktion des Lebensraumes der Rügener Schreibkreide-Fauna (Unter-Maastricht) mit Hilfe der Paläoökologie und Paläobiologie. Beihefte zur Zeitschrift Geologie 49: 1–147.
Neumann, C. and Hampe, O. 2018. Eggs for breakfast? Analysis of a probable mosasaur biting trace on the Cretaceous echinoid Echinocorys ovata Leske, 1778. Fossil Record 21: 55–66. Crossref
Neumann, C. and Wisshak, M. 2006. A foraminiferal parasite on the sea urchin Echinocorys: Ichnological evidence from the Late Cretaceous (Lower Maastrichtian, Northern Germany). Ichnos 13: 185–190. Crossref
Neumann, C. and Wisshak, M. 2009. Gastropod parasitism on Late Cretaceous to Early Paleocene holasteroid echinoids—evidence from Oichnus halo isp. n. Palaeogeography, Palaeoclimatology, Palaeoecology 284: 115–119. Crossref
Nietsch, H. 1921 Die irregulären Echiniden der pommerschen Kreide. Abhandlungen aus dem geologisch-palaeontologischen lnstitut der Universität Greifswald 2: 1–69.
Parker, W.K. and Jones, T.R. 1860. On the nomenclature of foraminifera: IV: The species enumerated by Lamarck. Annals and Magazine of Natural History 3: 29–40. Crossref
Plewes, C.R., Palmer, T., and Haynes, J. 1993. A boring foraminiferan from the Upper Jurassic of England and northern France. Journal of Micropalaeontology 12: 83–89. Crossref
Poag, C.W. 1969. Dissolution of molluscan calcite by the attached foraminifer Vasiglobulina, new genus (Vasiglobulininae, new subfamily). Tulane Studies in Geology and Paleontology 7: 45–70.
Ponder, W.F., Lindberg, D.R., and Ponder, J.M. 2020. The evolution of parasitism in Eulimidae. In: W.F. Ponder, D.R. Lindberg, and J.M. Ponder (eds.), Biology and Evolution of the Mollusca: Volume 2, 410–412. CRC Press, Taylor and Francis, Boca Raton. Crossref
Poulin, R. 2007. Evolutionary Ecology of Parasites. 332 pp. Princeton University Press, New Jersey. Crossref
Saidova, K.H.M. 1975. Bentosnye Foraminifery Tihogo Okeana. 875 pp. Institut Okeanologii P.P. Širšova, Akademiâ Nauk SSSR, Moskva.
Santos, A., Mayoral, E., and Bromley, R.G. 2011. Bioerosive structures from Miocene marine mobile‐substrate communities in southern Spain, and description of a new sponge boring. Palaeontology 54: 535–545. Crossref
Schmidt, H. 1992. Mikrobohrspuren ausgewählter Faziesbereiche der tethyalen und germanischen Trias (Beschreibung, Vergleich und bathymetrische Interpretation). Frankfurter Geowissenschaftliche Arbeiten A 12: 1–228.
Schweizer, M., Bowser, S.S., Korsun, S., and Pawlowski, J. 2012. Emendation of Cibicides antarcticus based on molecular, morphological, and ecological data. The Journal of Foraminiferal Research 42: 340–344. Crossref
Schweizer, M., Pawlowski, J., Kouwenhoven, T., and Van Der Zwaan, B. 2009. Molecular phylogeny of common cibicidids and related Rotaliida (Foraminifera) based on small subunit rDNA sequences. The Journal of Foraminiferal Research 39: 300–315.
Smith, E.A. 1902. Mollusca. In: E.R. Lankester (ed.), Report on the Collections of Natural History Made in the Antarctic Regions During the Voyage of the “Southern Cross”, 201–213. British Museum of Natural History, London.
Smith, A.B. and Wright, C.W. 2003. British Cretaceous echinoids: Part 7, Atelostomata: 1, Holasteroida. Monographs of the Palaeontographical Society 156: 440–568. Crossref
Smyth, M.J. 1988. The foraminifer Cymbaloporella tabellaeformis (Brady) bores into gastropod shells. The Journal of Foraminiferal Research 18: 277–285. Crossref
Stephenson, D.G. 1963. The spines and diffuse fascioles of the Cretaceous echinoid Echinocorys scutata Leske. Palaeontology 6: 458–470.
Surlyk, F. 1984. The Maastrichtian Stage in NW Europe, and its brachiopod zonation. Bulletin of the Geological Society of Denmark 33: 217–223. Crossref
Surlyk, F., Rasmussen, S.L., Boussaha, M., Schiøer, P., Schovsbo, N.U., Sheldon, E., Stemmerik, L., and Thibault, N. 2013. Upper Campanian–Maastrichtian holostratigraphy of the eastern Danish Basin. Cretaceous Research 46: 232–256. Crossref
Tapanila, L. and Ebbestad, J.O.R. 2008. Benthic island community on the back of a snail: Silurian, Anticosti Island, Canada. Canadian Journal of Earth Sciences 45: 203–211. Crossref
Todd, R. 1965. A new Rosalina (foraminifera) parasitic on a bivalve. Deep Sea Research and Oceanographic Abstracts 12: 831–837. Crossref
Vénec-Peyré, M.-T. 1996. Bioeroding foraminifera: a review. Marine Micropaleontology 28: 19–30. Crossref
Walker, S.E., Hancock, L.G., and Bowser, S.S. 2017. Diversity, biogeography, body size and fossil record of parasitic and suspected parasitic foraminifera: a review. Journal of Foraminiferal Research 47: 34–55. Crossref
Wisshak, M. 2006. High-latitude Bioerosion: The Kosterfjord Experiment. Lecture Notes in Earth Sciences 109. 202 pp. Springer, Berlin.
Wisshak, M. 2017. Taming an ichnotaxonomical Pandora’s box: Revision of dendritic and rosetted microborings (ichnofamily: Dendrinidae). European Journal of Taxonomy 390: 1–99. Crossref
Wisshak, M. and Neumann, C. 2006. A symbiotic association of a boring polychaete and an echinoid from the Late Cretaceous of Germany. Acta Palaeontologica Polonica 51: 589–597.
Wisshak, M. and Neumann, C. 2020. Dead urchin walking: resilience of an Arctic Strongylocentrotus to severe skeletal damage. Polar Biology 43: 391–396. Crossref
Wisshak, M. and Rüggeberg, A. 2006. Colonisation and bioerosion of experimental substrates by benthic foraminiferans from euphotic to aphotic depths (Kosterfjord, SW Sweden). Facies 52: 1–17. Crossref
Wisshak, M., Alexandrakis, E., and Hoppenrath, M. 2014. The diatom attachment scar Ophthalmichnus lyolithon igen. et isp. n. Ichnos 21: 111–118. Crossref
Wisshak, M., Knaust, D., and Bertling, M. 2019. Bioerosion ichnotaxa: review and annotated list. Facies 65: article 24. Crossref
Wisshak, M., Tribollet, A., Golubic, S., Jakobsen, J., and Freiwald, A. 2011. Temperate bioerosion: ichnodiversity and biodiversity from intertidal to bathyal depths (Azores). Geobiology 9: 492–520. Crossref
Acta Palaeontol. Pol. 68 (1): 13–22, 2023
https://doi.org/10.4202/app.01028.2022