

In vivo and post-mortem bioerosion traces in solitary corals from the upper Pliocene deposits of Tunisia
NADIA GAALOUL, ALFRED UCHMAN, SYRINE BEN ALI, KATARZYNA JANISZEWSKA, JAROSŁAW STOLARSKI, BOGUSŁAW KOŁODZIEJ, and SAMI RIAHI
Gaaloul, N., Uchman, A., Ben Ali, S., Janiszewska, K., Stolarski, J., Kołodziej, B., and Riahi, S. 2023. In vivo and post-mortem bioerosion traces in solitary corals from the upper Pliocene deposits of Tunisia. Acta Palaeontologica Polonica 68 (4): 659–681.
The polychaete borings Caulostrepsis taeniola, Caulostrepsis cretacea, Caulostrepsis avipes, Caulostrepsis penicillus isp. nov., Maeandropolydora elegans, Maeandropolydora sulcans, Sulcichnus sigillum, the bryozoan boring Pinaceocladichnus onubensis and the phoronid boring Talpina cf. hackberryensis occur in coralla of the solitary scleractinian coral Ceratotrochus (Edwardsotrochus) duodecimcostatus in the upper Pliocene middle/lower neritic to upper bathyal fine-grained deposits of NE Tunisia. This very rich assemblage of borings is produced in vivo as suggested by (i) their occurrence close to the surface and mostly in the upper part of coralla (Caulostrepsis ispp., M. elegans), even if they are known to penetrate deeply in the substrate, or (ii) evidence of corallum deformation in response to the boring action (Sulcichnus sulcans). The remaining borings were probably produced post mortem; they penetrate deeply into the corallum (M. sulcans) or always occur shallowly in the substrate (Talpina) and, in addition, cross cut other borings (Pinaceocladichnus). The polychaete borings are dominant. The abundance of the borings is probably caused by ecological pressure from shallower zones in subtropical waters. This resulted in the colonization of hard, small-sized substrates located in relatively deep (offshore) waters. The interpretation of age and palaeoenvironment was elucidated by the analysis of benthic and planktonic foraminifers.
Key words: Anthozoa, palaeoecology, ichnotaxonomy; commensalism, Pliocene, Mediterranean basin.
Nadia Gaaloul [nadia.gaaloul@fst.utm.tn; ORCID: https://orcid.org/0009-0009-0693-0501 ], Syrine Ben Ali [syrine.benali@etudiant-fst.utm.tn; ORCID: https://orcid.org/0009-0005-6627-5343 ], and Sami Riahi [sami.riahi@fst.utm.tn; ORCID: https://orcid.org/0009-0005-4248-4423 ], Faculty of Science, University of Tunis El-Manar, LR18ES07, 2092 Tunis, Tunisia.
Alfred Uchman [alfred.uchman@uj.edu.pl; ORCID: https://orcid.org/0000-0002-0591-777X ] (corresponding author) and Bogusław Kołodziej [boguslaw.kolodziej@uj.edu.pl; ORCID: https://orcid.org/0000-0002-9838-3411 ], Institute of Geological Sciences, Jagiellonian University, Gronostajowa 3a, 30-387 Kraków, Poland.
Katarzyna Janiszewska [ k.janiszewska@twarda.pan.pl; ORCID: https://orcid.org/0000-0002-7094-1821 ] and Jarosław Stolarski [stolacy@twarda.pan.pl; ORCID: https://orcid.org/0000-0003-0994-6823 ], Institute of Paleobiology, Polish Academy of Sciences, Twarda 51/55, 00-818 Warsaw, Poland.
Received 28 July 2023, accepted 23 October 2023, available online 15 December 2023.
Copyright © 2023 N. Gaaloul et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The study of bioerosion is a valuable tool to recognize ecological relationships between organisms, such as predation, parasitism, competition, or symbiosis (e.g., Kelley and Hansen 2003; Gibert et al. 2004) in Recent environments and in the geological past. Among the plethora of recent and fossil skeletons of organisms, scleractinian solitary corals are an object of in vivo and post-mortem macrobioerosion, but the literature on this subject is still scarce (but see, e.g., Savazzi 1982; Bromley and D’Alessandro 1990; Fodor 2001; Martinell and Domènech 2009; Vescogni et al. 2018). It is not clear if this is because of the rarity of such phenomena or an insufficient survey. Traces of their bioerosion provide insight into the ecology of fossil corals and/or the sedimentary environment of the sediments in which they occur (e.g., Perry 2000; Scoffin and Bradshaw 2000; Tribollet 2008; Wisshak et al. 2012). In contrast, microendoliths (boring algae, bacteria, and fungi) in vivo associated with modern corals are ubiquitous. These microendoliths represent the skeletal microbiome and are important for coral health and coral reef conditions (Ricci et al. 2019). The skeletal microbiome of fossil scleractinians deciphered from microborings was only recently recognized in corals as old as the Jurassic (Salamon and Kołodziej 2022; Salamon et al. 2022). In this paper, a rich material of borings in solitary corals from fine-grained deposits of the Pliocene in north-eastern Tunisia (Cap Bon Peninsula) is presented for the first time. For better recognition of their stratigraphic and palaeoecological context, planktonic and benthic foraminifers have been studied in addition.
The Pliocene sedimentary deposits of the north-eastern coast of Tunisia are well known for their richness in well-preserved fossils, which have been studied for decades. Among the many groups represented, corals are the least studied. Cuif (1968) described Ceratotrochus (Edwardsotrochus) duodecimcostatus (Goldfuss, 1826) from the Pliocene of the Djebel Hammamet in Tunisia and Liguria in Italy, including some specimens showing the bioerosion trace Sulcichnus isp. (Martinell and Domènech 2009), but provided no information on whether the bioeroded specimens are from Tunisia or not. Zibrowius et al. (1975) mentioned the same coral species with borings of Lumbrineris (now Sulcichnus Martinell and Domènech, 2009) in the collection of the British Museum of Natural History, London, which specimens derive partially from Tunisia but without a closer location. Fekih (1970) and later Derbel-Damak and Zaghbib-Turki (2002) reported the presence of madreporaries with the species Edwardsotrochus duodecimcostatus but without providing any description or illustration.
Institutional abbreviations.—FSTDG, Faculty of Science, University of Tunis El-Manar, Tunisia; INGUJ, Institute of Geological Sciences, Jagiellonian University (collection in the Nature Education Centre of the Jagiellonian University [CEP]–Museum of Geology in Kraków, Poland).
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: urn:lsid:zoobank.org:pub:5763EFEE-14 D2-4B02-8706-ECA 3A601B402.
Geological setting
The Pliocene deposits of Tunisia crop up in several basins along the Mediterranean coastline from the north-east to the south-east (Fig. 1). Their depositional environments were individualized during the Pliocene Mediterranean transgression, which followed the Messinian salinity crisis (Derbel-Damak 1992; Moisette et al. 2010). The sedimentary succession mainly consists of marly and sandy siliciclastic deposits. In the Ghar El Melah-Bizerte region, northern Tunisia (Fig. 2) and in the offshore domain, it is subdivided into two units (Burollet 1951): (i) the Raf Raf Formation (lower Pliocene), which unconformably overlies Messinian deposits and consists of a thick fossiliferous greenish clay, and (ii) the Porto Farina Formation (upper Pliocene), which consists of yellow calcareous beds. In the Cap Bon Peninsula, north-eastern Tunisia, Colleuil (1976) defined four sedimentary units (Fig. 2): (i) Argiles des Potiers Formation, which consists of marine blue clays with macro- and microfaunal fossils; (ii) Sables de Nabeul Formation, which are yellow continental sands; (iii) Les argiles de Sidi Barka Formation, which is composed of open marine grey clays with rich bivalve shells and sandy intercalations in the upper part, and (iv) Sables et Grès de Hammamet Formation, which embraces marine sand and sandstones rich in macrofauna. The first two units are ascribed to Zanclean (lower Pliocene). The latest unit was initially attributed to the “Astien” (Fekih 1970), then to the upper Pliocene (Colleuil 1976; Derbel-Damak and Zaghbib Turki 2002), and more recently to the Gelasian (Lower Pleistocene; Temani et al. 2016; Bejaoui et al. 2017).
The present work concerns a sedimentary succession exposed at a tributary of the El Melah stream, a few kilometres from the tourist town of Hammamet, on the south-eastern coast of the Cap Bon Peninsula (Fig. 1B). The Pliocene deposits in this region are also available in a network of ditches dug out for hydrographic regulations.
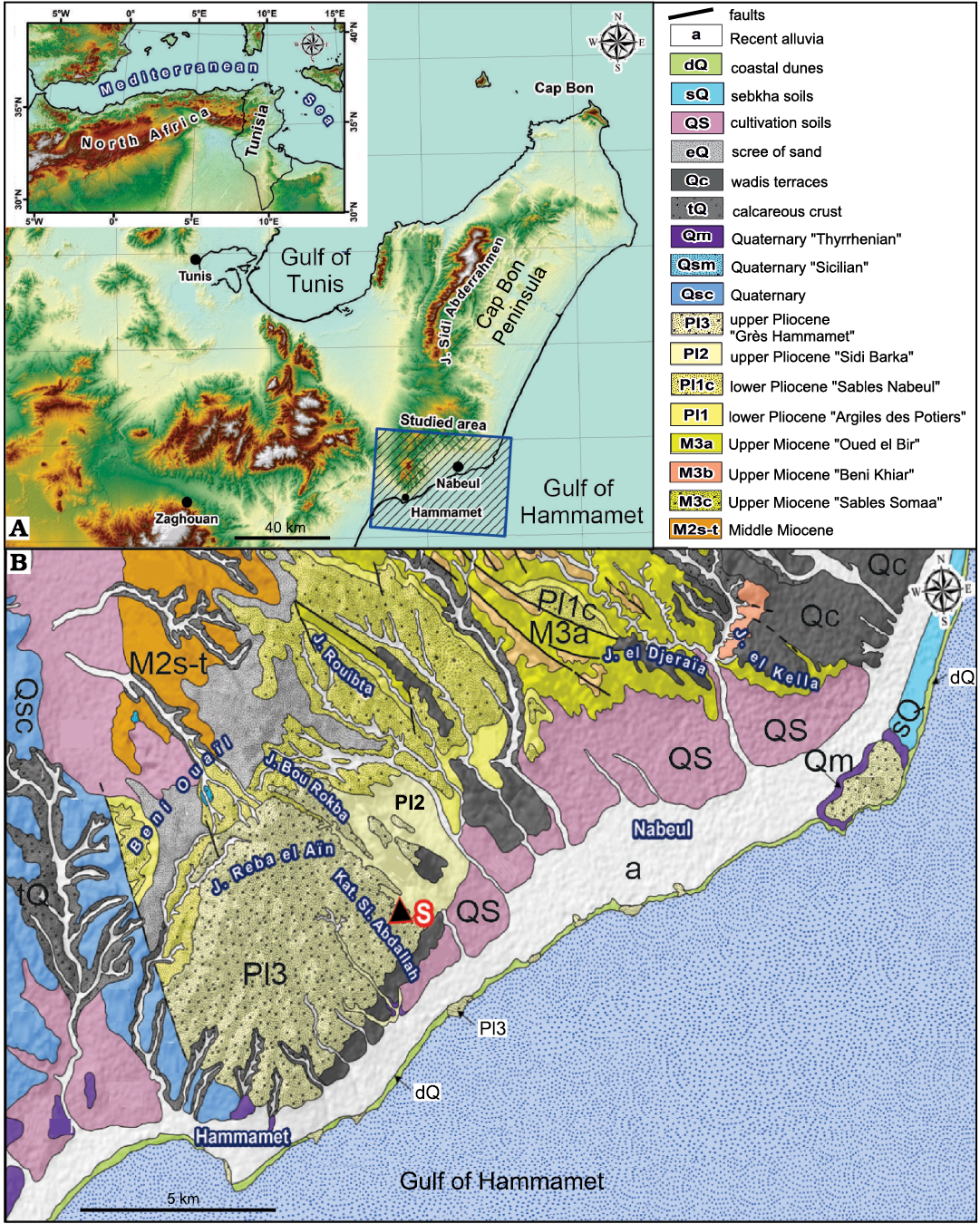
Fig. 1. A. Location of the studied area in the north-eastern Tunisia and north Africa (inset). (Ben Ali and Gaaloul 2021). B. Detailed geological map of northern and north-eastern Tunisia (1:50000; National Mining Office of Tunisia) showing Pliocene marine deposits of the Hammamet area. S, study section.
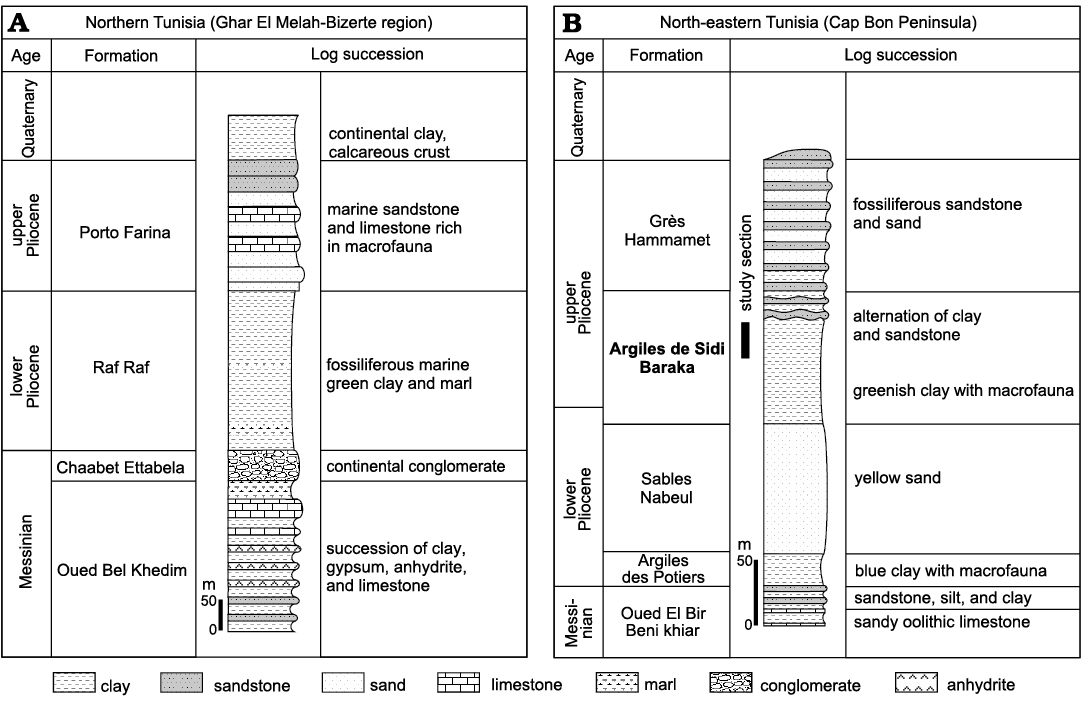
Fig. 2. Lithostratigraphy and subdivision of the Neogene and Quaternary series of northern (A) and north-eastern Tunisia (B) (Burollet 1951; Colleuil 1976).
The investigated section, 13 m thick, was logged in the upper part of the Argiles de Sidi Barka Formation, below the first siliciclastic beds of the overlying Sables et Grès de Hammamet unit (Fig. 3). The lower part of the section consists of 1.5 m of greenish clay with bivalves and scaphopods. At their top, these clays become nodular and are rich in the shells of bivalve Meretrix sp. These are succeeded by about 10 m of clay and silty clay rich in faunal micro- and macrofossils, including planktonic and benthic foraminifera, ostracods, bryozoans, pectinids, oysters, scaphopods, echinids, and corals. Three levels of solitary corals, level I, level II, and level III, are distinguished (Figs. 3, 4A1, C1, D). Corals are well preserved (Fig. 4A2, C2) and many of them are bioeroded. The presence of several articulated bivalve shells (Fig. 3: sample H8) in association with corals, suggests that they are probably not transported.
The middle to upper part of the section is composed of fine-grained sand and silt alternating with brown clay. These deposits are devoid of corals, rich in fragile chalky shells of molluscs, and have numerous benthic and fewer planktonic foraminifera. The top of the studied section is marked by thick bioclastic beds that rest on an erosive unconformity and mark the base of the Grès de Hammamet Formation.

Fig. 3. Lithological column of the Oued El Melah section with lithostratigraphic divisions, indications of samples, and field photographs of some fossiliferous levels.

Fig. 4. Exposures of the El Melah stream the section, upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia. A. Coral level III. General view (A1), close view of the sampling area (A2). B. The coral level II/coral level III boundary. C. Coral level II. General view (C1), close view of the sampling area (C2). D. General view of coral level I. Quadrangles indicate sampling areas.
Material and methods
The study is based on 33 sediment samples (H1–33) and 183 coral specimens (INGUJ265P1–139, 150–193) collected from three coral levels in the lower part of the section. The material was collected in March 2018 and April 2022 from an outcrop section located in a tributary valley of the El Melah stream, north-east of Hammamet town (Fig. 1). Twin 250 g sediment samples were collected and stored in polyethylene bags. The first was used for the analysis of the microfauna, and the second for further studies. In the laboratory, after washing through 63, 125, 250, and 500 µm sieves, all specimens of foraminifers were observed under a binocular microscope, picked out, counted, and transferred into cells. Their generic taxonomical determination was based on Loeblich and Tappan (1987) and the latest list of the online word register of marine species (WORMS) data base. The palaeoecological interpretation of the investigated foraminifers is based on Murray (1991, 2006), Bresler and Yanko-Himbash (2000), Debenay et al. (1996, 2000), Den Georgescu (2013) and Gaaloul et al. (2022). The foraminifers were photographed by the scanning electron microscope (SEM) of the ETAP (Tunisian Company of Petroleum Activities). All foraminifera specimens are archived in the Laboratory of Sedimentary Basins and Petroleum Geology of the Faculty of Sciences, University of Tunis El-Manar (specimens FSTDG22H).
The corals and bioerosion structures in them were examined at the Institute of Geological Sciences of the Jagiellonian University in Kraków, Poland, using a binocular microscope. Some thin sections have also been prepared. Micro-CT images of a few specimens were made at the Institute of Paleobiology of the Polish Academy of Sciences in Warsaw.
MicroCT data were collected with a Zeiss Xradia Micro XCT-200 imaging system equipped with a 90 kV/8W tungsten X-ray source in the Laboratory of Microtomography, Institute of Paleobiology, Polish Academy of Sciences, Warsaw. The scans were performed using the following parameters: voltage: 80 kV, power: 8W, exposure time: 3 s (INGUJ 265P152_92 and INGUJ265P153_86) or 4 s (INGUJ265P150), voxel size: 26.78 µm (INGUJ265P153_86 and INGUJ265P150) or 20.45 µm (INGUJ265P152_92). Radial projections were reconstructed with the XMReconstructor software provided with the Xradia system. The 3D images of specimens, micro-CT sections, and animations were obtained with Avizo 7.1 Fire Edition software.
Results
Scleractinian coral specimens.—The bioeroded solitary scleractinian corals are herein attributed to Ceratotrochus (Edwardsotrochus) duodecimcostatus (Goldfuss, 1826) (Fig. 5; see Vertino et al. 2014). Considering major reshuffling in scleractinian coral phylogenetic relationships resulting from in-depth molecular and following microstructural analyses of the skeleton (e.g., Kitahara et al. 2016; Seiblitz et al. 2022), traditional family (Caryophylliidae) and generic (Ceratotrochus) attribution of this taxon is tentative. Although the calices of the majority of specimens are either broken or at least partly eroded, the morphological features typical of the species can be discerned. Coralla are trochoid to ceratoid (Fig. 5E2, G). The largest specimens are ca. 45 mm tall, with calice diameter of 22 × 16 mm. Calices are elliptical. Calicular fossa deep. Typically, septa develop in five cycles according to the formula: 10 (S1 = S2) > 20 S3 ≥ (variable number of S4 ≥ S5). Specimens with a full fifth cycle are rarely observed. The septa of the first two cycles (S1 and S2) are identical, reaching the columella. S3 thinner also reaches the columella. S4 merge at the columella at the bottom of the calice. S5, adjacent to the dominant S1 and S2 septa, is fused with S4 septa. The so-called mid-septal zone (transversely sectioned Rapid Accretion Deposits sensu Stolarski 2003) has zig-zag pattern in S1, S2 septa, is slightly undulated in S3 septa, and is rather straight in S4, S5 septa (Fig. 5C). Numerous tabular dissepiments (Fig. 5D2, E2). Columella is composed of anastomosed lobes (chicoraceous columella) (Fig. 5A2, B, D1, E1, F, G). In sectioned upper parts of coralla, the costate wall is trabeculothecal or septothecal (see also Stolarski 1995).
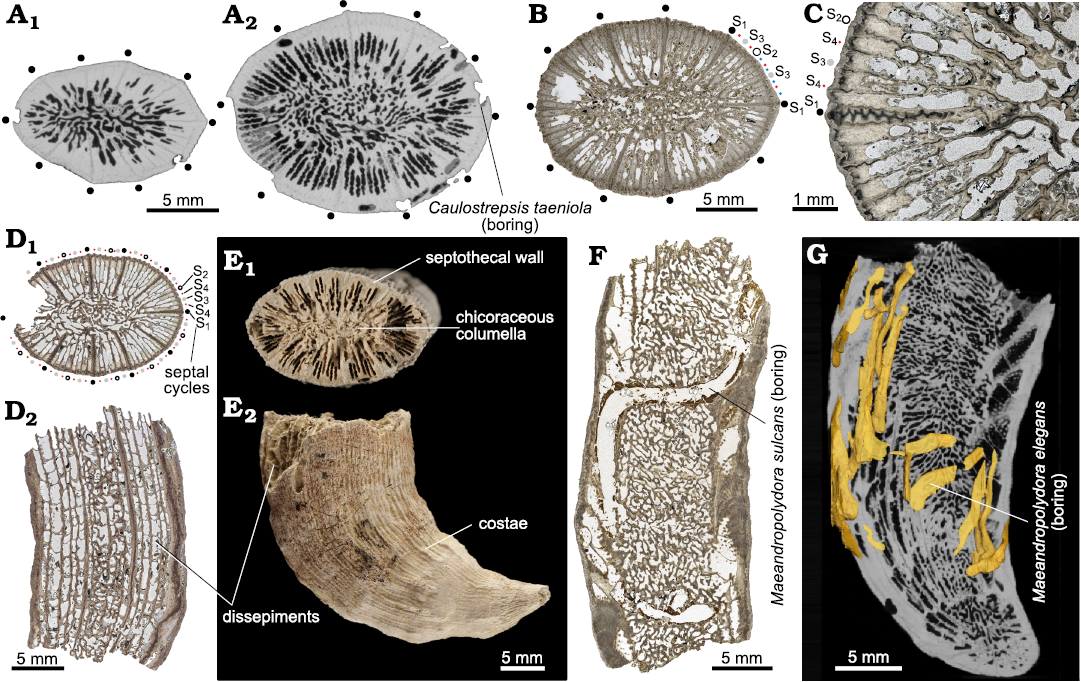
Fig. 5. Structural features of the caryophylliid coral Ceratotrochus (Edwardsotrochus) duodecimcostatus (Goldfuss, 1826) coralla from upper Pliocene deposits in a tributary valley of the El Melah stream, north-east of Hammamet town, Tunisia. A. Virtual sections of INGUJ265P153 in lower (A1) and in more distal part (A2) of corallum. B. Thin-section 3/76 of INGUJ265P139 that shows nearly completely developed 5 cycles of septa. Positions of 10 S1 septa marked with black dots. C. Thin section 2/118 of INGUJ265P138, enlarged region of transversely sectioned corallum to show diverse patterns of mid-septal zone development (from zig-zag in S1, S2 septa to straight in S4 septa). D. Thin sections of INGUJ265P137, transverse (D1, thin section T1/8) and longitudinal (D2, thin section T1/9) sections to show some variability of septal cycle development (D1), and development of tabular dissepiments (D2). E. INGUJ265P188, distal (E1) and lateral (E2) views of specimen with major structural features indicated. F. Longitudinal thin section 5/29 of INGUJ265P136 with the boring Maeandropolydora sulcans Voigt, 1965. G. Virtual longitudinal section of INGUJ265P188 with the boring Maeandropolydora elegans Bromley and D’Alessandro, 1983.
Foraminifera.—The microfaunistic samples from the investigated section contain planktonic (Fig. 6) and benthic (Fig. 7) foraminifers, ostracods, bryozoans and molluscs. The planktonic foraminifera include Globorotalia (Globoconella) puncticulata (d’Orbigny in Deshayes, 1832) in samples H2–8, Globigerinoides ruber (d’Orbigny, 1839b) (Fig. 6B), Globigerinoides (Trilobatus) sacculifer (Brady, 1877), Globigerina bulloides d’Orbigny, 1826 (Fig. 6A), Orbulina universa (d’Orbigny, 1839a) (Fig. 6D), Globigerinoides (Trilobatus) trilobus (Reuss, 1850), and Globorotalia (Truncorotalia) crassaformis (Galloway and Wissler, 1927) in sample H30 (Fig. 6E).
The benthic foraminifera content includes Uvigerina peregrina Cushman, 1923 (Fig. 7A), Melonis affinis (barleeanum) (Reuss, 1851) (Fig. 7B), Nonion commune (d’Orbigny, 1846) (Fig. 7C), Textularia (Sahulia) conica d’Orbigny, 1839b (Fig. 7D), Bulimina costata (striata) d’Orbigny, 1852 (Fig. 7L), Elphidium crispum (Linnaeus, 1758) (Fig. 7E), Neoconorbina terquemi (Rzehak, 1888) (Fig. 7G), Uvigerina mediterranea Hofker, 1932 (Fig. 7H), Bolivina dilatata Reuss, 1850 (Fig. 7I), Amphicoryna scalaris (Batsch, 1791) (Fig. 7J), Ammonia beccarii (Linnaeus, 1758) (Fig. 7F), Planulina ariminensis d’Orbigny, 1826 (Fig. 7K), Spiroloculina sp. (Fig. 7M), Bolivina punctata d’Orbigny, 1839c (Fig. 7N), Lobatula lobatula (Walker and Jacob in Kanmacher, 1798) and Cibicoides sp.
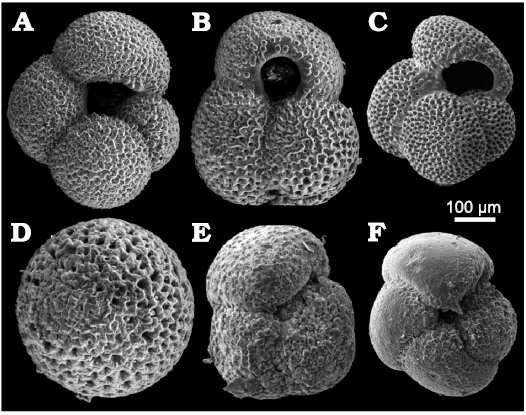
Fig. 6. Planktonic foraminifera foraminifera from upper Pliocene deposits in a tributary valley of the El Melah stream, north-east of Hammamet town, Tunisia. A. Globigerina bulloides d’Orbigny, 1826, (FSTDG22H31, sample H3). B. Globigerinoides ruber (d’Orbigny, 1839b) (FSTDG22H21, sample H2). C. Globigerinoides extremus Bolli and Bermudez, 1965, (FSTDG22H71, sample H7). D. Orbulina universa (d’Orbigny, 1839a) (FSTDG22H181, sample H18). E. Globorotalia crassaformis (Galloway and Wissler, 1927) (FSTDG22H30, sample H30). F. Globorotalia puncticulata (Deshayes, 1832) (FSTDG22H17, sample H17).
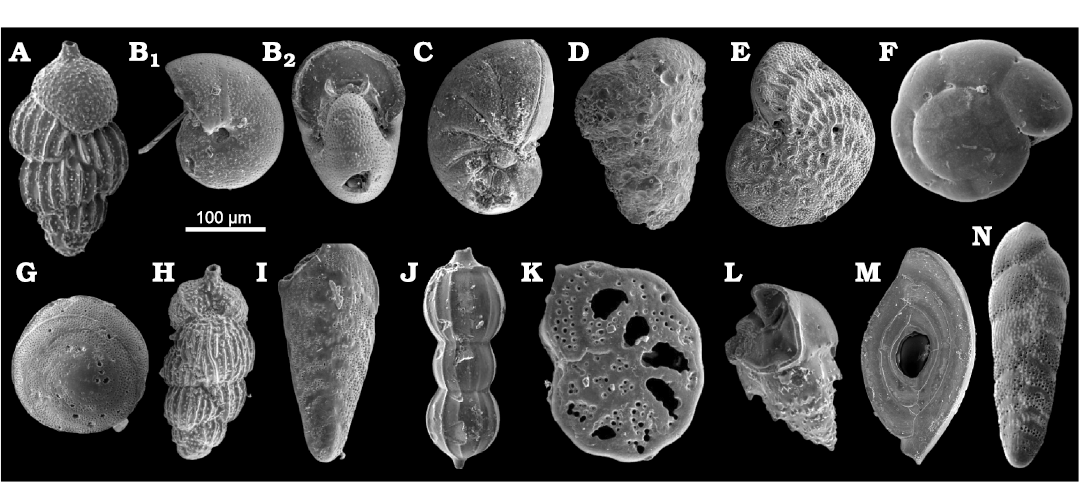
Fig. 7. Benthic foraminifera from upper Pliocene deposits in a tributary valley of the El Melah stream, north-east of Hammamet town, Tunisia. A. Uvigerina peregrina Cushman, 1923, (FSTDG22H2, sample H2). B. Melonis affinis (Reuss, 1851) (FSTDG22H11, sample H1), side (B1) and apertural (B2) views. C. Nonion commune (d’Orbigny, 1846) (FSTDG22H10, sample H10). D. Textularia conica d’Orbigny, 1839b, (FSTDG22H12, sample H1). E. Elphidium crispum (Linnaeus, 1758) (FSTDG22H25, sample H25). F. Ammonia beccarii (Linnaeus, 1758) (FSTDG22H201, sample H20). G. Neoconorbina terquemi (Rzehak, 1888) (FSTDG22H21, sample H21). H. Uvigerina mediterranean Hofker, 1932, (FSTDG22H17, sample H17). I. Bolivina dilatata Reuss, 1850, (FSTDG22H22, sample H2). J. Amphycoryna scalaris (Batsch, 1791) (FSTDG22H1, sample H17). K. Planulina ariminensis d’Orbigny, 1826, (FSTDG22H24, sample H2). L. Bulimina costata d’Orbigny, 1852, (FSTDG22H9, sample H9). M. Spiroloculina sp. (FSTDG22H72, sample H7). N. Bolivina punctata d’Orbigny, 1839c, (FSTDG22H241, sample H24).
Systematic palaeoichnology
Ichnogenus Caulostrepsis Clarke, 1908
Type ichnospecies: Caulostrepsis taeniola Clarke, 1908, Siegener Schichten Formation (Devonian: Pragian–Emsian), Seifen, Germany.
Emended diagnosis.—Borings with one entrance or embedment structure, pouch-shaped, created by a U-shaped gallery. More complex structures can result from multiple lobes of similar structure. The individual limbs of the gallery can be clearly visible along their total length. They can be connected by the vane, or they can be fused in an oval or flattened pouch shape without the vane. The distal end has at least double the width of the apertural end. The cross section is variably flat oval, elliptical, constricted, or dumbbell-shaped. Symmetrical, radially organized grooves or deep pits can be developed near the aperture in some cases. The aperture may have a similar form to the proximal cross section, or it may be modified by the development of superficial branches, grooves, and/or holes (modified after Bromley and D’Alessandro 1983, with some modifications by Pokorný and Štofik 2017).
Remarks.—Information about the number of grooves branching from the aperture “(normally 2 to 4 in number)” is removed from the diagnosis of Bromley and D’Alessandro (1983: 286) because the new ichnospecies of Caulostrepsis described below has much more grooves. Caulostrepsis is a boring produced mostly by species of the spionid polychaete Polydora Bosc, 1802 (Boekschoten 1966), foremost P. ciliata (Johnston, 1838) (Radwański, 1969), and the eunicid polychaete Lysidice ninetta Audouin and Milne-Edwards, 1833 (Bromley 1978, 2004). Caulostrepsis ranges from the Devonian to the present (Clarke 1908; Bromley 2004). It occurs mainly in the infralittoral Plio-Pleistocene and the recent Mediterranean coasts (Bromley and D’Alessandro 1990), but mostly in the lower intertidal and subtidal zones, rarely deeper, and very rarely on the continental slope and the abyssal zone (Ekdale et al. 1984: 127).
Caulostrepsis taeniola Clarke, 1908
Figs. 8B, D, 9A4, SOM 1.
Diagnosis.—Gallery cylindrical, bent in a narrow U-form which is sometimes enlarged in the shape of a tongue. The inward facing margins of the limbs are always interconnected by a distinct vane. Limbs closer or partially fused towards the apertural extremity. Transverse section dumbbell-shaped, aperture 8-shaped (cited from Bromley and D’Alessandro 1983).
Material.—INGUJ265P153, 154, 161, 162, 180; U-shaped, tongue depressions on the surface of coralla; El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia.
Description.—A narrow, straight, curved, or slightly winding tongue depression running along the surface of coralla within the corallum wall, usually along their axis. The depression widens slightly distally. It is bordered by semi-circular cylindrical marginal gutter (limbs). The distal part of some depressions has a thin roof built of the corallum wall. The vane (the area between the limbs) is equal to or wider than the limbs. The boring is up to 21 mm long and up to 1–1.5 mm wide. It is concentrated in the corallum wall (SOM 1 available at http://app.pan.pl/SOM/app68-Gaaloul_etal_SOM.pdf).
Remarks.—The presence of the roof at the end of some depressions suggests that the roof was more extensive but was broken or collapsed. Caulostrepsis taeniola is the most common ichnospecies of Caulostrepsis. It is produced mainly by the spionid polychaete Polydora ciliata (Johnston, 1838), which is common on mid-latitude Atlantic coasts (Radwański 1969, and references therein), mostly up to a depth of 25 m (Boekschoten 1966). Polydora lives in different substrates and conditions; it can also live in polar regions (Hanken et al. 2012) and in brackish waters (D’Andrea et al. 1996; Murina 1997).
Stratigraphic and geographic range.—Lower Devonian–Recent, worldwide, Cenzoic occurrences mostly in the Atlantic and the Mediterranean regions.
Caulostrepsis cretacea (Voigt, 1971)
Figs. 8A, 12C2.
Diagnosis.—Galleries bent in a long, narrow U-form with the inward-facing walls of the limbs fused by complete removal; the original position of the median wall is sometimes indicated by a very shallow axial depression along the structure. Vane absent. Transverse section always flattened-elliptical but showing gradual decrease in width toward the aperture. Shape of aperture flattened-oval (cited from Bromley and D’Alessandro 1983).
Material.—INGUJ265P151, 156, 166; U-shaped, tongue depressions on the surface of coralla; El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia.
Description.—Straight to slightly winding depression/gallery, which is distinctly gradually widened towards the distal semicircular termination, from 0.25 mm up to 1 mm, up to 10.5 m long, without vane. In the illustrated case, two groves converge in the proximal part and diverge at an angle of 40°. About 1 mm before the aperture, the galleries diverge again. It is not clear whether this is an effect of the intersection of two galleries or the divergence of one fused gallery. The borings are concentrated within the corallum wall. Some depressions have a roof in the most distal part.
Remarks.—Bromley and D’Alessandro (1983) observed broader specimens, which are at least 2 mm wide and never show interpenetration. However, the characteristic distal widening is diagnostic of this ichnospecies. Originally, this ichnospecies was described by Voigt (1971) as Dodecaceria cretacea. Later, it was transferred to Trypanites by Bromley (1972) and finally to Caulostrepsis by Bromley and D’Alessandro (1983). Voigt (1971) and Bromley and D’Alessandro (1983) regarded it as having been produced by the cirratulid polychaete Dodecacería concharum Örsted, 1843, but Gibson (2016) who examined the material studied by the aforementioned author and compared it with recent borings of this polychaete, questioned this view and suggested that Caulostrepsis cretacea is similar to the borings of Polydora.
Stratigraphic and geographic range.—Santonian (possibly Cenomanian) (Voigt 1970)–Recent. However, poorly documented specimens are known since the Cenomanian (El-Hedeny 2007) to the Recent (Voigt 1970); the Atlantic and the Mediterranean regions.
Caulostrepsis avipes Bromley and D’Alessandro, 1983
Figs. 8C, 9A8.
Emended diagnosis.—Caulostrepsis with or without a vane, dumbbell-shaped to flattened oval in cross section, characterized by the possession of two to four grooves branched out from the aperture. The grooves are shorter than the triple width of the boring. In some cases, the branching occurs beneath the substrate surface, so that each diverging branch bears its own aperture.
Material.—INGUJ265P153, 176; U-shaped, tongue depressions, preserved on the surface of coralla; El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia.
Description.—Surface or shallow subsurface, straight, curved, or slightly winding tongue-like depression/gallery with or rarely without vane, up to 9–13 mm long, up to 0.8–1.5 mm wide. The groves branching from the aperture are slightly divergent and bifurcating at the end. The borings are concentrated within the corallum wall.

Fig. 8. Caulostrepsis, a domichnion produced by polychates, in the skeleton of caryophylliid coral Ceratotrochus (Edwardsotrochus) duodecimcostatus (Goldfuss, 1826), from the El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia. A. Caulostrepsis cretacea (Voigt, 1971), INGUJ265P156. B. Caulostrepsis taeniola Clarke, 1908 and Pinaceocladichnus onubensis Mayoral, 1988 (Pn), INGUJ265P154. C. Caulostrepsis avipes Bromley and D’Alessandro, 1983, INGUJ265P176. D. A cluster of Caulostrepsis taeniola Clarke, 1908, INGUJ265P162. E–G. Caulostrepsis penicillus isp. nov. E. INGUJ265P158 holotype (a), and paratype (b). F. INGUJ265P174 paratype. G. INGUJ265P172, partial views (G1, G2).
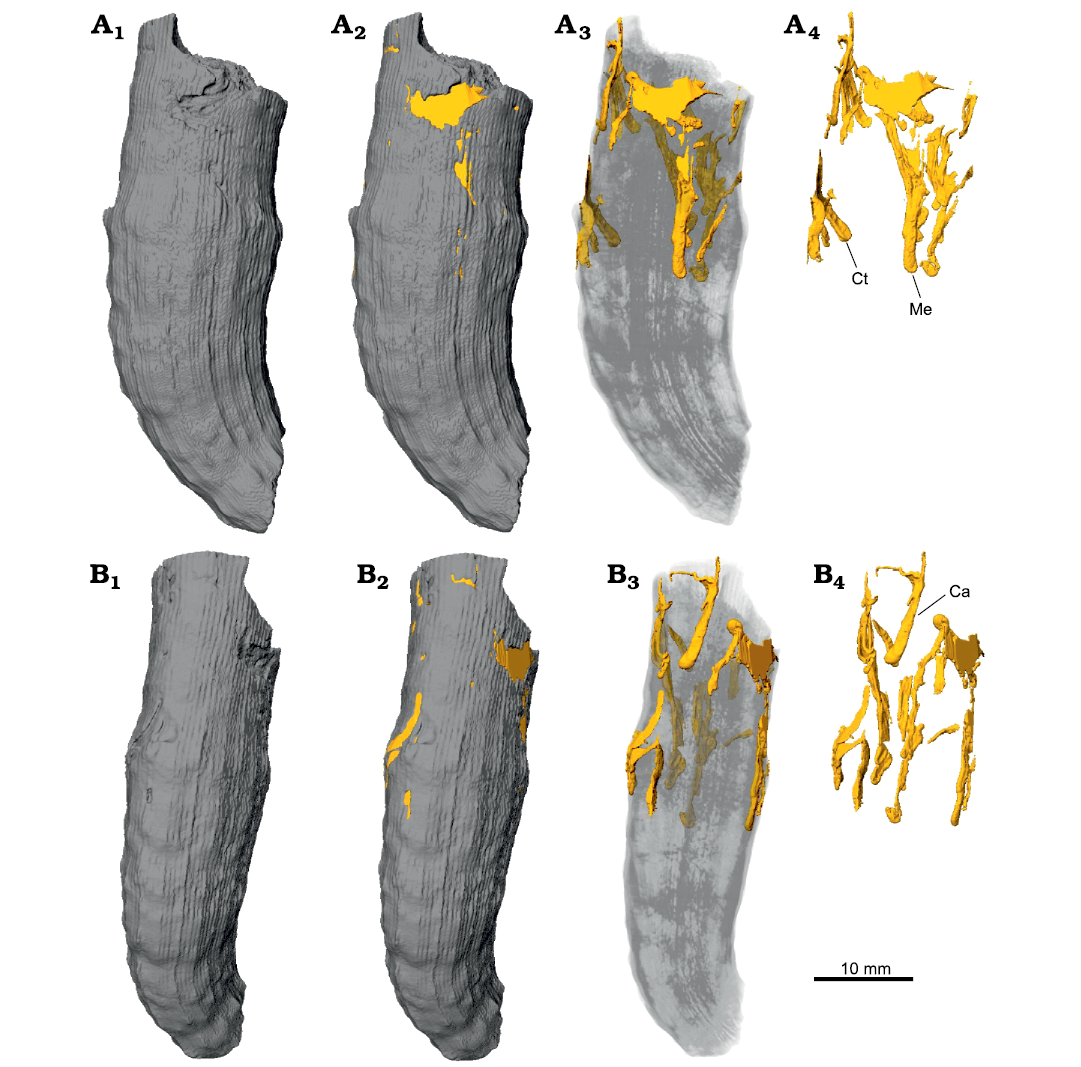
Fig. 9. Snapshots from micro-CT images of Caulostrepsis in the skeleton of caryophylliid coral Ceratotrochus (Edwardsotrochus) duodecimcostatus (Goldfuss, 1826) (INGUJ265P153), from the El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia. A. The surface with indications of bioerosion traces, with partial transparency, and without corallum, A1–A4, respectively; Ct, Caulostrepsis taeniola Clarke, 1908; Me, Maeandropolydora elegans Bromley and D’Alessandro, 1983. B. View from the other side, B1–B4, respectively; Ca, Caulostrepsis avipes Bromley and D’Alessandro, 1983.
Remarks.—Most probably, the surface depressions resulted from erosion/collapse of the roof of subsurface galleries. The roof is still present in the distal part of some galleries. Information that the grooves branching out from the apertures are much shorter than the remaining part of the boring is added to the diagnosis by Bromley and D’Alessandro (1983) in order to describe the distinction between Caulostrepsis avipes and the new ichnospecies described in the following. The grooves were probably produced by the tentacles of spionid polychaetes. It is the first appearance of this ichnospecies beyond the Upper Cretaceous, where it is documented by Reis (1921) but without assignation to any ichnotaxon, and by Hillmer and Schulz (1973) under Ramosulcichnus biforans Gripp, 1967, which was transferred to Caulostrepsis and split into Caulostrepsis as C. biforans and C. avipes (see Bromley and D’Alessandro 1983).
Stratigraphic and geographic range.—Santonian–Maastrichtian of Germany, Denmark, England, and Ukraine, upper Pliocene of Tunisia.
Caulostrepsis penicillus isp. nov.
Fig. 8E–G.
Zoobank LSID: urn:lsid:zoobank.org:act:5763EFEE-14D2-4B02-8706-ECA3A601B402.
Etymology: From Latin penicillus, brush, in reference to the overall shape.
Type material: Holotype INGUJ265P158 (Fig. 8E: a) well preserved, straight, U-shaped surface structure with several grooves branching out fom the apertures (Fig. 8E: a). Paratypes: INGUJ265P158 (Fig. 8E: b) well preserved, curved, U-shaped surface structure with several grooves branching out fom the apertures (Fig. 8E: b), INGUJ265P174 incomplete, straight, U-shaped surface structure with several grooves branching out fom the apertures; marginal tunnel almost invisible (Fig. 8F) from the type locality and horizon.
Type locality: El Melah stream, north-east of Hammamet town, Tunisia.
Type horizon: Coral level II, Argiles de Sidi Barka Formation (upper Pliocene).
Material.—Type material and INGUJ265P172 (Fig. 8G), NGUJ265P185, surface, slightly lobate depression, with several grooves in the apertural part, from the type locality and horizon.
Diagnosis.—Caulostrepsis with a vane, characterized by the possession of several commonly overlapping grooves branched out from the aperture, forming a fan-like structure. The grooves are generally longer than the tripled width of the boring.
Description.—Surface depressions or shallow subsurface galleries, straight, curved, or slightly winding tongue-like depression with a vane, up to 4.5–8 mm long, up to 0.8–1.8 mm wide. It can be slightly wider in the distal part. The surface depressions can transit into a roofed gallery in the distal part. Several slightly curved or slightly winding groves run out from the proximal part (aperture). They overlap and form a symmetric or an asymmetric fan-like structure, which is up to 7 mm wide and up to 7 mm long. With the remaining part of the boring, it looks like a brush. The groves, about 0.1 mm wide, can bifurcate at the end under low angles, become gradually shallower, emerge, and disappear.
Remarks.—Similarly to the other described ichnospecies of Caulostrepsis, it is interpreted that C. penicillus isp. nov. was originally a subsurface gallery, whose roof was eroded or collapsed. It is most similar to C. avipes, but its grooves branch out from the aperture, and they are much more numerous and distinctly longer. The grooves were probably produced by the tentacles of spionid, cirratulid, or similar bioeroding polychaetes, but the tracemaker presumably had them much more than the tracemaker of C. avipes. There were no transitions between C. penicillus isp. nov. and C. avipes observed in the material studied.
Stratigraphic and geographic range.—Upper Pliocene, Tunisia.
Ichnogenus Maeandropolydora Voigt, 1965
Type ichnospecies: Maeandropolydora decipiens Voigt, 1965, Kunrader Member of the Maastricht Formation, upper Maastrichtian, Kunrade, the Netherlands.
Diagnosis.—Long cylindrical galleries having two or more apertures, running through the substrate sinuously or in irregular contortions. Galleries may run parallel in contact with each other in pairs, with or without fusion. Loose or tight loops may occur; the limbs of these may be connected by a vane or form a pouch (cited from Bromley and D’Alessandro 1983).
Remarks.—Maeandropolydora Voigt, 1965, is a boring of suspension-feeding spionid polychaete (e.g., Bromley and D’Alessandro 1983). It occurs since the Triassic and Palaeozoic occurrences are uncertain (Bromley 2004).
Maeandropolydora elegans Bromley and D’Alessandro, 1983
Figs. 5G, 9A4, 10, 11A, 10B, 13B2, SOM 2.
Material.—INGUJ265P150, 151, 153–155, 159, 163, 170, 171, 184, 188, 192; branched, flat galeries on the surface of coralla; the El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia.
Diagnosis.—System composed of cylindrical galleries of constant diameter, irregularly sinuous, tending to run in paired fashion, the limbs touching but normally not fused. Numerous apertures (cited from Bromley and D’Alessandro 1983).
Description.—Surface groves or subsurface branched galleries running in pairs, mostly parallel, within the corallum wall. Some stretches of the subsurface galleries capped by a thin roof commonly transit into surface grooves (Fig. 10D). Most of the surface grooves show overhanging side margins. These pairs are 0.5–1 mm wide, with a very narrow, usually indistinct, vane between the galleries/grooves or without a vane. The observed length may exceed 10 mm. Branches at an acute or normal angle. The plane of the borings is usually more or less parallel to the corallum, but some segments are perpendicular. The boring is concentrated in the corallum wall (SOM 2).

Fig. 10. Maeandropolydora elegans Bromley and D’Alessandro, 1983, a dominichnion produced by polychates, in the skeleton of caryophylliid coral Ceratotrochus (Edwardsotrochus) duodecimcostatus (Goldfuss, 1826), from the El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia. A. INGUJ265P171. B. INGUJ265P155. C. INGUJ265P159. D. INGUJ265P163.
Remarks.—The surface galleries are usually a part of subsurface galleries, where the roof was broken or collapsed. So far, Maeandropolydora elegans is known from bivalve and brachiopod shells.
Stratigraphic and geographic range.—Middle Triassic (Knaust 2007)–Recent; the Atlantic and the Mediterranean regions.
Maeandropolydora sulcans Voigt, 1965
Figs. 5F, 11B, SOM 3.
Material.—INGUJ265P152, 166, 169, 181, and thin section 5/29 from INGUJ265P136; cylindrical, contorted tunnels in coralla; El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia.
Diagnosis.—Cylindrical gallery having at least two apertures, irregularly contorted, commonly bent to loops, never showing fusion where walls are in mutual contact; vane absent (cited from Bromley and D’Alessandro 1983).
Description.—Loosely contorted cylindrical tunnels spreading through the whole volume of the corallum, 1.3–2 mm in diameter (SOM 3). They show a few oval openings that are oblique or perpendicular to the surface of the corallum.
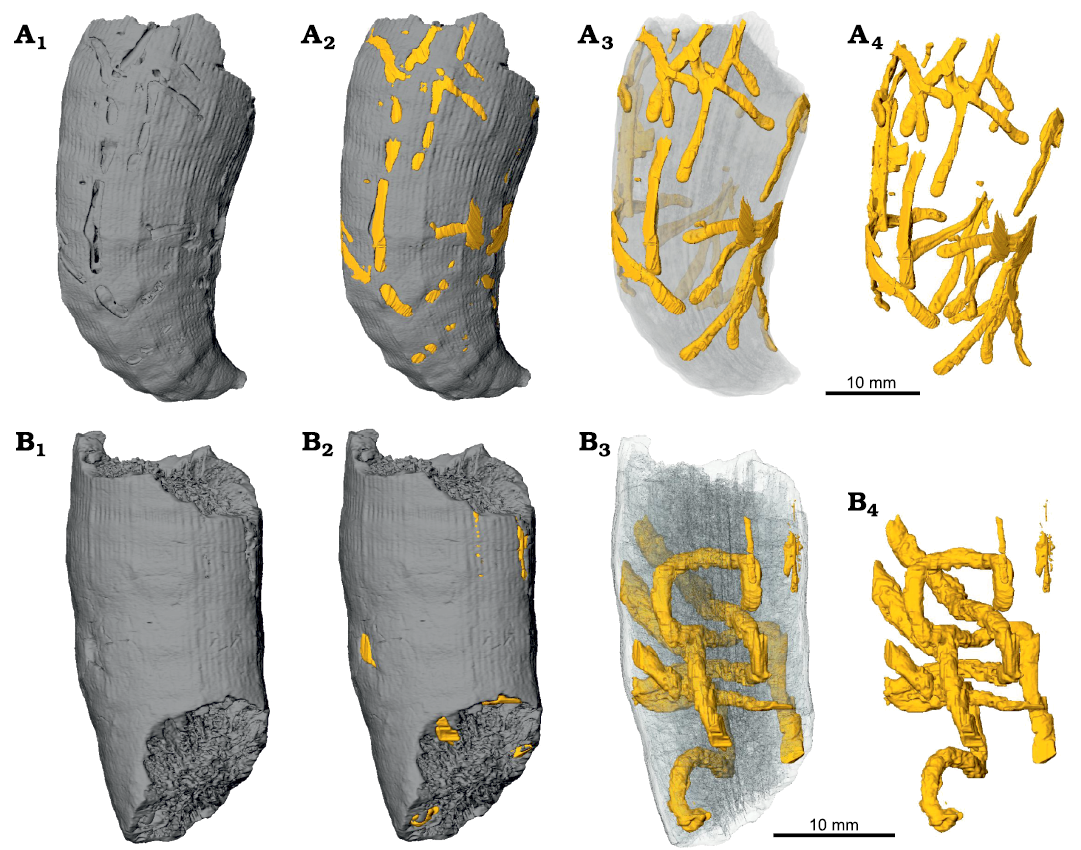
Fig. 11. Micro-CT images of the skeletons of caryophylliid coral Ceratotrochus (Edwardsotrochus) duodecimcostatus (Goldfuss, 1826) with domichnia Maeandropolydora elegans Bromley and D’Alessandro, 1983, INGUJ265P150 (A) and Maeandropolydora sulcans Voigt, 1965, INGUJ265P152 (B) from the El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia; surface view (A1, B1), surface view with indication of bioerosion structures (A2, B2), partial transparency (A3, B3), and without corallum (A4, B4).
Remarks.—Maeandropolydora sulcans is usually described from diverse skeletal elements, mostly shells, and rarely the rocky substrates (e.g., Łaska et al. 2021).
Stratigraphic and geographic range.—Common in the Cretaceous and the Cenozoic, foremost in the Atlantic and the Mediterranean regions; the Paleozoic occurrences are rare and problematic (Bromley 2004).
Ichnogenus Pinaceocladichnus Mayoral, 1988
Type ichnospecies: Pinaceocladichnus onubensis Mayoral, 1988, Arenas de Huelva Formetion (lower Pliocene), Moguer, Huelva Province, Spain.
Diagnosis.—Boring pattern constituted by a regular network of fine tunnels, slightly curved and laterally opposite (cited from Botquelen and Mayoral 2005).
Remarks.—This boring is a network of stolon tunnels with apertures produced by ctenostome bryozoans similar to those produced by the genus Immergentia Silén, 1946, or Terebripora d’Orbigny, 1847 (Mayoral 1988). This trace fossil occurs in marine environments from the littoral to neritic zones on calcareous substrates. Pinaceocladichnus occurs since the Ordovician (Mayoral 1991; Toom et al. 2019).
Pinaceocladichnus onubensis Mayoral, 1988
Figs. 8B, 12A.
Material.—INGUJ265P151, 152, 154, 189; cylindrical, contorted tunnels in coralla; El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia.
Diagnosis.—Pinaceocladichnus with very regular bifurcation systems. They present sub-oval or fusiform openings located very close to the corresponding tunnels that connect with elongated, cylindrical or sac-shaped chambers. They are arranged parallel to the previous tunnels (translated from Mayoral 1988).
Description.—A system of furrows, which are ca. 0.1–0.15 mm wide, composed of straight, slightly curved, or slightly winding master furrows, and shorter straight to slightly curved furrows that branch from the same point of the master furrow (pinnate branching) under the angle 35–65°. Very small, elongated widenings are situated along or very close to the furrows.
Stratigraphic and geographic range.—Ordovician (Estonia; Toom et al. 2019) to the Recent (worlwide).
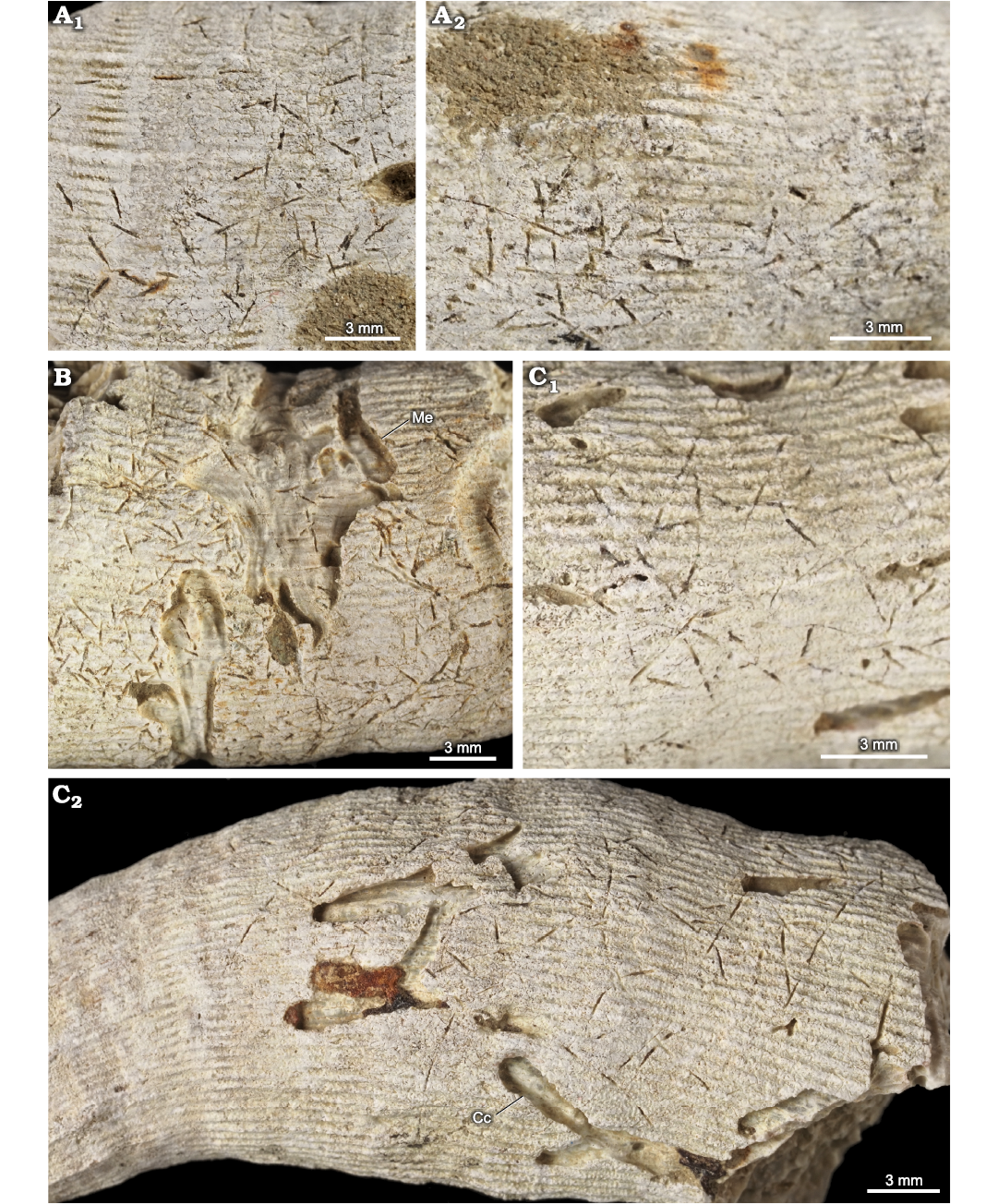
Fig. 12. Pinaceocladichnus onubensis Mayoral, 1988, (a domichnion produced by ctenostome bryozoans) and associated trace fossils in the skeleton of caryophylliid coral Ceratotrochus (Edwardsotrochus) duodecimcostatus (Goldfuss, 1826), from the El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia. A. INGUJ265P152, different places of the same corallum (A1, A2). B. INGUJ265P154, also Maeandropolydora elegans Bromley and D’Alessandro, 1983 (Me). C. INGUJ265P151, C1, C2 different places of the same corallum, also Caulostrepsis cretacea (Voigt, 1971) (Cc).
Ichnogenus Sulcichnus Martinell and Domènech, 2009
Type ichnospecies: Sulcichnus maeandriformis Martinell and Domènech, 2009, upper Zanclean (Pliocene) silt, Velerín, Estepona Basin, E Andalusia, Spain.
Diagnosis.—Long grooves, sometimes branched, running along the surface substrate sinuously or in a contorted fashion. Grooves never run in parallel, and loose or tight loops may occur (cited from Martinell and Domènech 2009).
Remarks.—Sulcichnus is a fixichnion produced by commensal polychaetes, recently by Helmutneris flabellicola (Fage, 1936), formerly Lumbrineris flabellicola Fage, 1936, on deep-sea solitary corals, but the fossil examples are known mostly from the shallow-marine Pliocene of the Western Mediterranean (Martinell and Domènech 2009). Sulcichnus occurs since the Ordovician (Knaust and Dronov 2013).
Sulcichnus sigillum Martinell and Domènech, 2009
Fig. 13.
Material.—INGUJ265P167, 170, 173, 175, 179, 190, 191; semicircular grooves, perpendicular to the axis of coralla; El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia.
Diagnosis.—Shallow groove parallel to the columella and bending 90° close to the calyx, to form a deeper, ring-shaped groove (cited from Martinell and Domènech 2009).
Description.—Sulcichnus sigillum Martinell and Domènech, 2009, is a semi-circular furrow, 1.5 mm wide, running around three-quarters of the perimeter of corallum, perpendicularly to its axis, with an abrupt turn up.
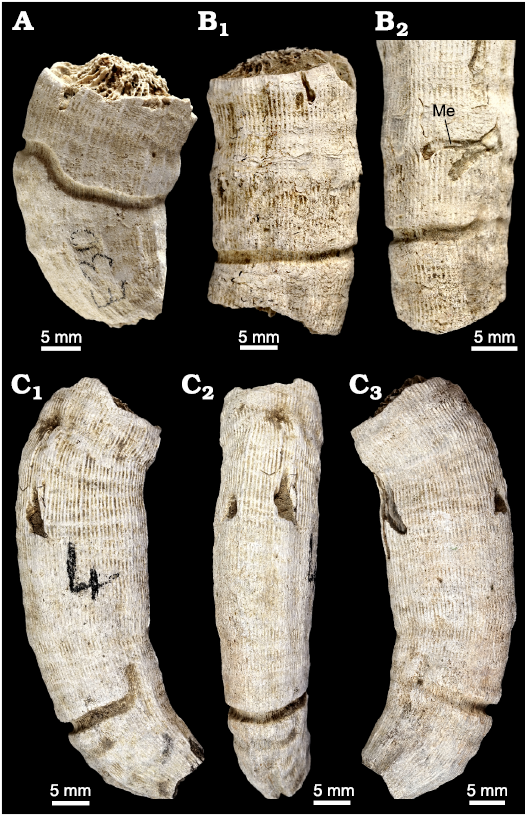
Fig. 13. Sulcichnus sigillum Martinell and Domènech, 2009, a fixichnion produced by commensal polychaetes in the skeleton of caryophylliid coral Ceratotrochus (Edwardsotrochus) duodecimcostatus (Goldfuss, 1826), from the El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia. A. INGUJ265P173. B. INGUJ265P170 in different side views (B1, B2). C. INGUJ265P175 in different side views (C1, C2). Abbreviation: Me, Maeandropolydora elegans.
Remarks.—The specimens studied show the typical morphology of Sulcichnus sigillum.
Stratigraphic and geographic range.—Since the early Pliocene worldwide (Martinell and Domènech 2009; Spadini 2019).
Ichnogenus Talpina Hagenow, 1840
Type ichnospecies: Talpina ramosa Hagenow, 1840, lower Maastrichtain chalk, Rügen, Germany.
Emended diagnosis.—Narrow, tubular borings in lithic or hard skeletal substrates, forming multi-branching tunnel systems with apertures towards the exterior. Tunnels with circular to oval cross-section, straight to strongly curved; short side branches towards apertures often developed near branching points of the main tunnel system. Tunnels may anstomose.
Remarks.—Conchotrema Teichert, 1945, was subjectively synonymized with Talpina Hagenow, 1840, by Wisshak et al. (2019). Their possible synonymization has been announced since longer (Voigt 1972; Bromley 2004), but Bromley and D’Alessandro (1987) suggested that Conchotrema is smaller, less regular, and shows an anastomosing course of tunnels (see also Stiller 2005). Nevertheless, the differences seem to be not principal at the ichnogenus level. Both ichnogenera fit well to the diagnosis of the ichnofamily Talpinidae Wisshak, Knaust, and Bertling, 2019, typified by Talpina Hagenow, 1840, which reads “Branched cylindrical borings that may an anstomose” (Wisshak et al. 2019: 24). The most recent diagnosis of Talpina by Stiller (2005) reads: “Narrow, tubular borings in hard substrates with overall morphology changing astogenetically from short, simple borings to extensive, multi-branching tunnel systems with numerous apertures towards exterior (borings of pseudocolonies); completely buried in the substrate. Tunnels with circular to oval cross-section, straight to strongly curved; short side branches towards apertures often developed near branching points of the main tunnel system.” This diagnosis contains a lot of interpretative elements. Therefore, it is emended. However, a better understanding of all the differences between Talpina and other members of the ichnofamily Talpinidae, which is beyond the scope of this paper, may necessitate of a new emendation. Talpina and its synonym Conchotrema are interpreted as borings produced by pseudocolonies of phoronids (Voigt 1972, 1975). It is known since the Early Devonian (Voigt 1967; Stiller 2005).
Talpina cf. hackberryensis (Thomas, 1911)
Fig. 14.
Material.—INGUJ265P164, 168; curved, branched grooves on the surface of coralla; El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia.
Description.—Straight, slightly or strongly curved, dichotomously or irregularly branched smooth furrows on the surface of corallum, 0.1–0.15 mm wide. Some of the furrows pass into very shallow subsurface tunnels of the same size, which are filled with fine clastic material. The furrows/tunnels run mostly along or obliquely to the corallum, some of them anastomosing or missing on slightly different levels. The distance between the braches ranges from 0.5 to 4 mm. Close to the furrows/tunnels, some circular/oval pits of the same size are dispersed.
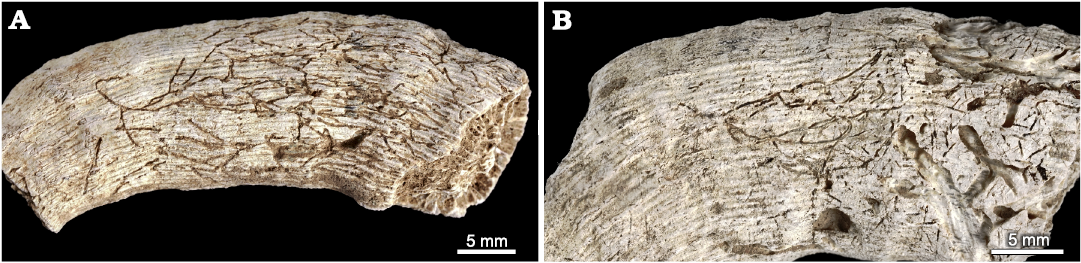
Fig. 14. Talpina cf. hackberryensis (Thomas, 1911), a domichnion produced by phoronids in the skeleton of caryophylliid coral Ceratotrochus (Edwardsotrochus) duodecimcostatus (Goldfuss, 1826), from the El Melah stream section, the upper part of the Argiles de Sidi Barka Formation (upper Pliocene) of Tunisia. A. INGUJ265P168. B. INGUJ265P164.
Remarks.—Bromley and D’Alessandro (1987) synonymized Clionolithes canna Price, 1916, and Conchotrema tubulosa Teichert, 1945, under Conchotrema Teichert, 1945. Further, Wisshak (2017) followed an unpublished PhD thesis by Plewes from 1996, who synonymized Clionolithes canna Price, 1916, and Cliona hackberryensis Thomas, 1911, under the ichnogenus Talpina Hagenow, 1840. This taxonomic treatment is followed here. However, the distances between branches are larger than in the diagnosis of C. canna by Bromley and D’Alessandro (1987), and the apertures (visible as circular/oval pits) are numerous. On the other hand, they are very similar to Talpina tubulosa (Teichert, 1945) visible in the Permian Spirifer valves from Western Australia (Teichert 1945: pl. 2: 1, 2). As ichnospecies of Talpina are pending a revision, the determination is left in the open nomenclature without a diagnosis.
Discussion
Biostratigraphy.—The Oued El Melah sedimentary succession was deposited during the Piacenzian (late Pliocene). This age is attested by the occurrence of Globorotalia puncticulata in the lower and middle parts of the section (Fig. 15), which is the biostratigraphic marker of the sub-zones MPL4a and MPL4b of the Mediterranean Pliocene Zone (MPL) scheme according to Violanti (2012). The occurrence of Globorotalia crassaformis in the uppermost part of the sedimentary succession suggests the MPL5 Zone. The dating is in accordance with the previous stratigraphic attribution advanced by Derbel-Damak and Zaghbib Turki (2002) and Bejaoui et al. (2017).
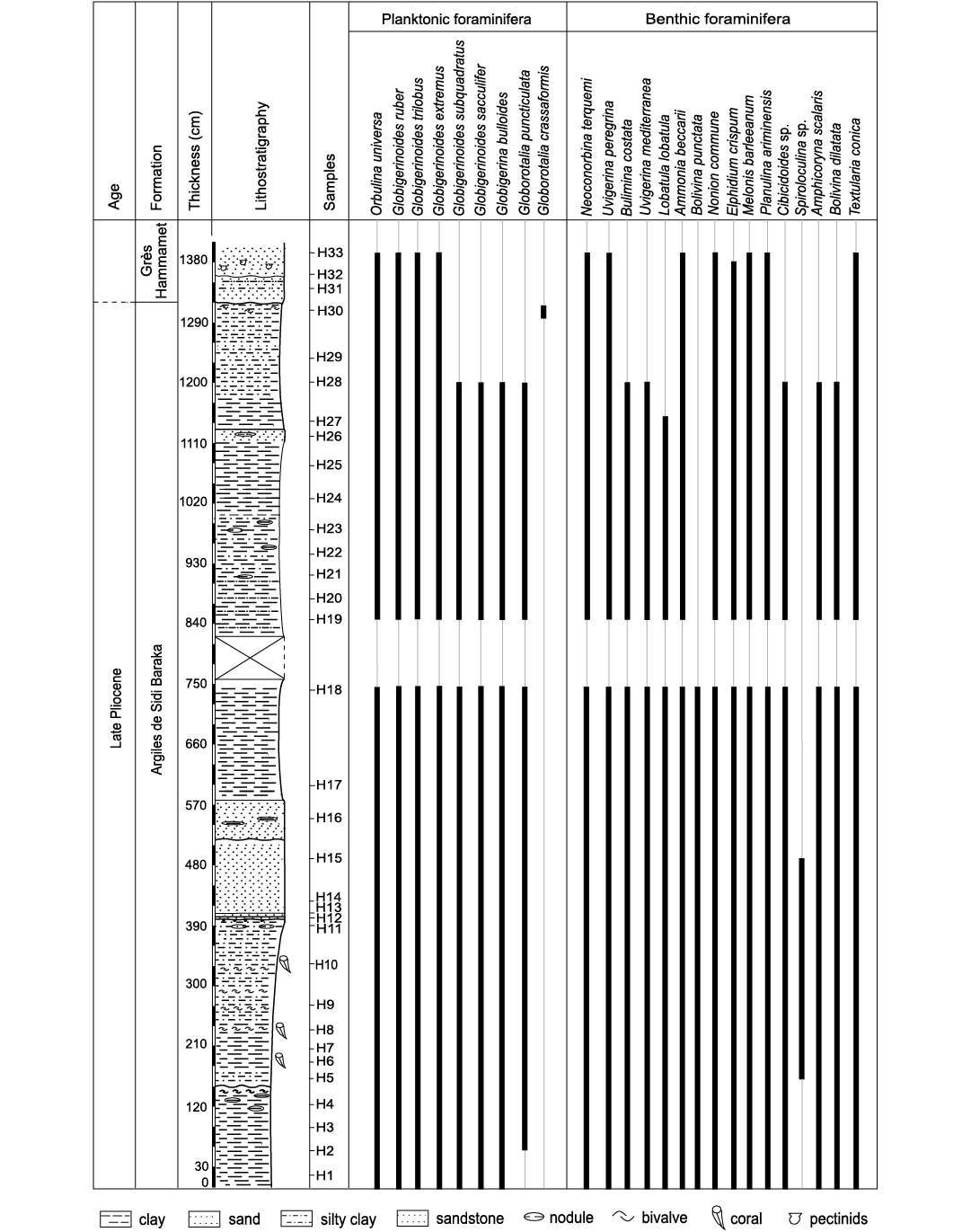
Fig. 15. Distribution of foraminifera in the Oued El Melah section.
Palaeoenvironment.—Foraminifera are excellent indicators of the environmental conditions that prevailed during their life (Bergh et al. 2018). Based on the distribution and ecological preferences of benthic foraminiferal taxa, two assemblages, FA1 and FA2, were distinguished throughout the study section.
The assemblage FA1 (samples H1–11) points to a palaeobathymetry ranging from the outer neritic zone to the epibathyal zone. This is attested by the simultaneous abundance of the deep-marine species represented by Planulina ariminensis, Uvigerina peregrina, Melonis barleeanum, and the shallower water species Ammonia beccarii and Neoconorbina terquemi. It is worth noting that U. peregrina has been recorded from a water depth of 300 m in the North Atlantic to 2000 m in the Guinea Basin (Tim 1992). It was also reported in Recent sediments of the Mediterranean Sea, Celtic Sea (Lei and Li 2016) and the Gulf of Mexico, where it shows its shallowest bathymetric range (Pflum and Frerichs 1976). Frontalini et al. (2010) described U. peregrina as a shallow infaunal species. P. ariminensis was documented in marine environments extending from the circalittoral to bathyal stages (Van Morkhoven et al. 1986). Violanti (2012) linked the presence of P. ariminensis from the Neogene sediments of north-western Italy to the epibathyal zone. Ammonia beccarii and Neoconorbina terquemi are mostly considered shallow-water benthic forms. In parallel, planktonic foraminifera are common within the microfaunistic content, where a relative abundance of Orbulina universa and Globigerinoides trilobus is noted. G. trilobus is considered an outer neritic to upper bathyal species (Murray and Alve 1999; Wilson 2003), while O. universa was found in the Red Sea at depths ranging from 50 to 100 m (Haenel 1987). Moreover, the value of the P/B ratio is usually associated with distance from shore (Van der Zwaan et al. 1990; Murray 2006) and consequently with the palaeobathymetry (Sen-Gupta and Machain-Castillo 1993; Drinia et al. 2008). In the investigated section, the ratio of planktonic/benthic taxa (30%) suggests a middle neritic environment (middle shelf). Based on the FA1 foraminiferal assemblage analysis, it could be concluded that the lower part of the studied section reflects marine offshore conditions.
The investigated solitary corals in the lower part of the sedimentary succession together with the foraminifers Orbulina universa and Globigerinoides trilobus. Orbulina universa is known to be a warm water indicator (Bé et al. 1973; Zachariasse et al. 1997; Gallagher et al. 2001; Kucera 2007; Drinia et al. 2008; Schiebel and Hemleben 2017). This species is abundant in the central water bodies of the sub-tropical province of the Indian Ocean (Bé et al. 1973) and at a temperature of 21.7 ± 2.9°C in the Red Sea (Haenel 1987). Globigerinoides trilobus lives in warm to temperate climates (Van der Zwaan et al. 1990; Stephanelli 2003) with temperatures ranging from 21–29°C (Bé 1977; Lombard et al. 2009; Bé and Toderlund 1971; Tolderlund and Bé 1971; Bijma et al. 1990). The distribution of modern azooxanthellate solitary corals is controlled primarily by the availability of nutrients and water temperature, which typically ranges between a few up to 20°C, rarely higher (Roberts et al. 2009). This suggests that although surface sea-water temperatures interpreted from planktic foraminifera were probably about 20–25°C, the investigated corals were dwelling in colder waters.
The foraminiferal assemblage FA2 contains abundant shallow marine taxa, which indicate a progressively shallowing of the basin in the upper part of the section (samples H12–32). It is to be noted that the faunal content is devoid of any coral specimens. In comparison to the association FA1, Ammonia beccarii, Neoconorbina terquemi, Lobatula lobatula, and miliolids are more abundant. This points to the outer/inner neritic zone. In the eastern Mediterranean Sea and Europe, A. beccarii is encountered in the most of estuarine systems (Debenay et al. 1996). Parker (1953) proposed for this species a water depth ranging 20–179 m. Baggley (2000) considered A. beccarii a stenobathymetric shelf species. Neoconorbina terquemi and L. lobatula are reported as a common species of inner neritic coarse sediments and as epiphytic shallow water taxa, respectively (Violanti 2012). The shallow marine environments interpreted for the upper part of the sedimentary section were influenced by high terrigenous supply episodes. This is marked by an increase in the silty fraction and the appearance of sandy intercalations. The abundance of Eocene larger benthic foraminifera and some Cretaceous foraminifera (Heterohelix sp.) in sample H32 confirms fluvial supply from older sediments. Meanwhile, coral-bearing deposits are totally absent. The increasing supply of clastic material has probably accelerated the degradation of coral life, leading to its disappearance.
Borings in coralla.—One of the basic questions is whether the borings were produced in vivo or post mortem. The criteria for borings produced in vivo can be as follows: (i) borings influencing the growth of corallum were produced during the life of the coral, Sulcichnus sigillum, belongs to them; (ii) borings that are distributed very close to the surface of the corallum but are known from deep penetration into the substrate in other cases, are also probably produced during the coral’s life. A vast majority of the polychaete borings presented in this paper (Caulostrepsis taeniola, C. cretacea, C. avipes, C. penicillus isp. nov., Maeandropolydora elegans) occur in the shallow subsurface, with partial exposition on the surface of coralla (so they are, in fact, half-borings). In some cases, the borings have a very thin roof, which can be easily broken during collection or cleaning. Most probably, all the borings ascribed to Caulostrepsis and Maeandropolydora preserved as depression/grooves were originally produced under the surface. However, the septothecal wall of the coralla can be more attractive for some borers than the porous internal part. This counterargument is weakened by the fact that Caulostrepsis occurs in coralline algae, which are porous (Checconi et al. 2010; Wisshak et al. 2021). A considerable number of borings are located in the upper part of coralla. Some of them start at the upper edge. This can be an additional criterion that suggests that the boring organisms colonized the corals in vivo.
Borings that penetrate deeply into the corallum and those that are always close to the surface, irrespective of the substrate, can be regarded as produced post mortem. Among the studied borings, only Maeandropolydora sulcans penetrates deeply within coralla. The bryozoan boring Pinaceocladichnus onubensis is always a close-surface boring. In the studied coralla, it cross cuts other borings and is not preferentially distributed on the corallum. The same concerns Talpina cf. hackberryensis (Thomas, 1911). This suggests that these borings record post mortem colonization.
The ichnospecies of Caulostrepsis and Maeandropolydora are smaller than their usual representatives described in the literature. It seems that their tracemakers were influenced by the Lilliput effect. It is not excluded that this is caused by some effort of the corals to limit their activity and by limited space of the coralla. However, the limited space is also the case for mollusc shells, where these ichnotaxa attain the usual size in many formations.
Most of the borings observed are in the upper half of coralla. This is in agreement with Fodor (2001) who observed such concentrations in the upper one third of uppermost Oligocene–lowermost Miocene (Egerian) solitary corals in Hungary. The borings belong to Caulostrepsis taeniola, C. biforans, C. cretacea, Maeandropolydora decipiens, M. sulcans, M. elegans, Trypanites solitarius, and T. weisei. All of them are produced by spionid polychaetes. We are not aware of recent in vivo infestations of solitary corals by this group of polychaetes. It is worthy of notice that the Pliocene and Recent deep-sea coral species of Desmophyllum, Madrepora, and Corallium from the Mediterranean Sea contain abundant sponge borings Entobia ispp. (Bromley and D’Alessandro 1990), but this boring was not found in the analyzed coralla from Tunisia. In virtual sections across coralla (Fig. 5A) we noticed that most of the borings belonging to Caulostrepsis ispp. and Maeandropolydora elegans are developed in corallum wall region composed of Rapid Accretion Deposits; these regions are structurally weaker due to enrichment in organics and thus are more prone to bioeroding activity.
Among the 177 coralla analyzed, 32.23% do not contain any visible macrobioerosion. 57.5% of the bored coralla contain Caulostrepsis, 18.33% Maeandropolydora elegans, 17.5% Pinacocladichnus, and 9.16% Sulcichnus. 34.17% of bored coralla contain other borings, mostly undeterminable forms, and Maeandropolydora sulcans. The number of the latest ichnotaxon is difficult to determine as it is poorly manifested on the surface. The data show that most of the borings are produced in vivo by polychaetes. Tracemakers of the in vivo borings were probably commensals, which benefited from the hard substrate of the coralla sticking above the fine-grained bottom sediment, protection used by corals to defend themselves, and from the possible weak currents bringing food and creating eddies around the coralla.
The diversity and abundance of borings decrease with depth, except for Talpina, and Caulostrepsis is a typical shallow water ichnogenus (Bromley and D’Alessandro 1990). The boring assemblage studied, and their substrate (coralla) were produced in outer/middle neritic to upper bathyal depths and under the subtropical climate, as suggested by foraminifers (former chapter). Abundant Pliocene borings in rocky substrates and bioclasts are known from several rocky shores in the Mediterranean region (e.g., Bromley and D’Alessandro 1990; Bromley and Asgaard 1993; Gibert et al. 1998; Łaska et al. 2021). It is possible that there was some ecological pressure that forced the borers to use the unusual substrates even in deeper zones. This can be caused by competition for space under conditions of proliferation. In such a situation, even solitary corals living on fine-grained substrates were suitable habitats.
Conclusions
Coralla of the solitary scleractinian coral Ceratotrochus (Edwardsotrochus) duodecimcostatus from the upper Pliocene middle/lower neritic to upper bathyal fine-grained deposits of NE Tunisia contain abundant in vivo and post-mortem borings. The in vivo borings are shallow and concentrated within the corallum wall in the upper part of the coralla or on the surface. They are produced mostly by polychaetes, among which Caulostrepsis taeniola, C. cretacea, C. avipes, C. penicillus isp. nov. and Maeandropolydora elegans are unknown to play such a role. Sulcichnus sigillum is a typical in vivo surface polychaete boring. The post-mortem borings are obligatory shallow structures (the phoronid boring Talpina cf. hackberryensis, the bryozoan boring Pinaceocladichnus onubensis) or deeply penetrating structures (Maeandropolydora sulcans). The possible ecological pressure in the shallow water caused that even the small, hard substrate of coralla in deeper water was colonized by boring organisms.
Acknowledgements
Max Wisshak (Senckenberg am Meer, Wilhelmshaven, Germany) and Árpád Dávid (University of Debrecen, Hungary) provided constructive reviews, which helped to improve the paper. Laboratory investigations on the borings were supported by a grant from the Faculty of Geography and Geology under the Strategic Programme Excellence Initiative at Jagiellonian University. Field work and SEM analysis of foraminifera were funded by LR18ES07 Laboratory (Bassins Sédimentaires et Géologie du pétrole), University of Tunis El Manar, Tunisia.
References
Audouin, J.V. and Milne Edwards, H. 1833. Classification des Annélides et description de celles qui habitent les côtes de la France. Annales des Sciences Naturelles, Paris 1 28: 187–247. Crossref
Baggley, K.A. 2000. The late Tortonian–early Messinian foraminiferal record of the Abad Member (Turre Formation), Sorbas Basin, Almeria, south-east Spain. Palaeontology 43: 1069–1112. Crossref
Batsch, A.J.G.K. 1791. Testaceorum arenulae marinae Tabulae sex prioves ad opus, Testacea minutiora huc usque nota, vel nondum in schiptis divulgata . secundum naturam delineatae et aeri incisae. 15 pp. Akademische Buchhandlung, Jena.
Bé, A.W.H. 1977. An ecological, zoogeographic and taxonomic review of recent planktonic foraminifera. In: A.T.S. Ramsey (ed.), Oceanic Micropaleontology, 1–101. Academic Press, New York.
Bé, A.W.H. and Harrison, S.M., and Lott, L. 1973. Orbulina universa d’Orbigny in the Indian Ocean. Micropaleontology 19: 150–192. Crossref
Bé, A.W.H. and Toderland, D.S. 1971. Distribution and ecology of living planktonic Foraminifer in surface waters of the Atlantic and Indian oceans. In: B.M. Funnel and W.R. Riedl (eds.), The Micropaleontology of the Oceans. Cambridge University Press, London 105–149.
Ben Ali, S. and Gaaloul, N. 2021. Early Pliocene benthic foraminifera of the south-eastern coast of Cap Bon Peninsula (northern Tunisia). In: Mediterranean Geosciences Union. Annual Meeting, 523–523. Springer ASTI Series, Istanbul.
Bejaoui, S., Sciuto, F., Karoui-Yaakoub, N., and Bel Haj Ali, N. 2017. Les ostracodes plio-pléistocènes de la côte orientale de la Péninsule du cap Bon (Tunisie). Annales de Paléontologie 104: 71–80. Crossref
Bergh, E.W., Compton, J.S., and Frenzel, P. 2018. Late Neogene foraminifera from the northern Namibian continental shelf and the transition to the Benguela Upwelling System. Journal of African Earth Sciences 141: 33–48. Crossref
Bijma, J., Walter, W., Fabe, Jr., and Hemleben, C. 1990. Temperature and salinity limits for growth and survival of some planktic foraminifers in laboratory cultures. Journal of Foraminiferal Research 20: 95–116. Crossref
Boekschoten, G.J. 1966. Palaeoecology of some mollusca from the Tielrode sands (Pliocene, Belgium). Paleogeography, Paleoclimatology, Paleoecology 3: 311–362. Crossref
Bolli, H.M. and Bermúdez, P J. 1965. Zonation based on planktonic foraminifera of middle Miocene to Pliocene warm-water sediments. Boletin Informativo Asociación Venezolana de Geología Minería y Petróleo 8: 121‒149.
Bosc, L.A.G. 1802. Histoire Naturelle des Vers: contenant leur description et leurs moeurs, avec figures dessinées d’après nature. 324 pp. Guilleminet, Paris. Crossref
Botquelen, A. and Mayoral, E. 2005. Early Devonian bioerosion in the Rade de Brest, Armorican Massif, France. Palaeontology 48: 1057–1064. Crossref
Brady, H.B. 1877. Supplementary note on the foraminifera of the Chalk(?) of the New Britain group. Geological Magazine, New Series 4: 534‒536. Crossref
Bresler, V.M. and Yanko-Hombach, V.V. 2000. Chemical ecology of foraminifera parameters of health environmental pathology, and assessment of environmental quality. In: E.M. Roland (ed.), Environmental Micropaleontology. Topics in Geobiology 15: 516–525. Crossref
Bromley, R.G. 1972. On some ichnotaxa in hard substrates, with a redefinition of Trypanites Mägdefrau. Paläontologische Zeitschrift 46: 93–98. Crossref
Bromley, R.G. 1978. Bioerosion of Bermuda reefs. Paleogeography, Paleoclimatology, Paleoecology 23: 169‒197. Crossref
Bromley, R.G. 2004. A stratigraphy of marine bioerosion. In: D. McIlroy (ed.), The Application of Ichnology to Palaeoenvironmental and Stratigraphic Analysis. Geological Society, London, Special Publication 228: 455‒481. Crossref
Bromley, R.G. and Asgaard, U. 1993. Endolithic community replacement on a Pliocene rocky coast. Ichnos 2: 96–116. Crossref
Bromley, R.G. and D’Alessandro, A. 1983. Bioerosion in the Pleistocene of southern Italy: Ichnogenera Caulostrepsis and Maeandropolydora. Rivista Italiana di Paleontologia e Stratigrafia 89: 283‒309.
Bromley, R.G. and D’Alessandro, A. 1987. Bioerosion of the Plio-Pleistocene transgression of southern Italy. Rivista Italiana di Paleontologia e Stratigrafia 93: 379–422. Crossref
Bromley, R.G. and D’Alessandro, A. 1990. Comparative analysis of bioerosion in deep and shallow water, Pliocene to recent, Mediterranean Sea. Ichnos 1: 43–49. Crossref
Burollet, P.F. 1951. Etude géologique des bassins mio-pliocènes du Nord-Est de la Tunisie: Région entre Mateur, Ferryville et Porto-Farina. Annales des mines et de la géologie 7: 1–91.
Checconi, A., Bassi, D., Carannante, G., and Monaco, P. 2010. Re-deposited rhodoliths in the Middle Miocene hemipelagic deposits of Vitulano (Southern Apennines, Italy): Coralline assemblage characterization and related trace fossils. Sedimentary Geology 225: 50–66. Crossref
Clarke, J.M. 1908. The beginnings of dependent life. Bulletin of New York State Museum 121: 146–196.
Colleuil, B. 1976. Etude stratigraphique et néotectonique des formations néogènes et quaternaire de région de Nabeul-Hammamet (Cap Bon Tunisie). 94 pp. Thèse inédite, Université de Nice, Nice.
Cuif, J.P. 1968. Etude ontogenique de quelques Madreporaires Caryophyllidae actuels et fossiles. Mémoires du Muséum national d‘Histoire naturelle de Paris, n. s. 16 (3): 101–156.
Cushman, J.A. 1923. The Foraminifera of the Atlantic Ocean. Part 4: Lagenidae. Bulletin of the United States National Museum 104: 1‒228. Crossref
d’Orbigny, A. 1826. Tableau méthodique de la classe des Céphalopodes. Annales des Sciences Naturelles 7: 96‒169, 245‒314.
d’Orbigny, A. 1839a. Foraminifères des îles Canaries. Histoire naturelle des Iles Canaries 2: 120‒146.
d’Orbigny, A. 1839b. Foraminifères, in de la Sagra R. Histoire physique, politique et naturelle de l’ile de Cuba. 224 pp. A. Bertrand, Paris.
d’Orbigny, A. 1839c. Voyage dans l’Amérique Méridionale. Foraminifères, t. 5 pt. 5. 86 pp. P. Bertrand, Paris.
d’Orbigny, A. 1841–1847. Voyage dans l’Amérique Méridionale (le Brésil, la république orientale de l’Uruguay, la République argentine, la Patagonie, la république du Chili, la république de Bolivia, la république du Pérou), exécuté pendant les années 1826, 1827, 1828, 1829, 1830, 1831, 1832, et 1833. Volume 5 (4). Zoophytes, 17‒28. P. Bertrand, Paris. Crossref
d’Orbigny, A. 1846. Foraminifères fossiles du bassin tertiaire de Vienne (Autriche). 312 pp. Gide et Compe, Paris. Crossref
d’Orbigny, A. 1852. Prodrome de paléontologie stratigraphique universelle des animaux mollusques et rayonnés, faisant suite au cours élémentaire de paléontologie et de géologie stratigraphiques, Volume 3. Table alphabétique et synonymique des genres et des espèces; addenda, errata, Table of Contents. 196 pp. Victor Masson, Paris.
D’Andrea, A.F., Craig, N.I., and Lopez, G.R. 1996. Benthic macrofauna and depth of bioturbation in Eckernförde Bay, southwestern Baltic Sea. Geo-Marine Letters 16: 155–159. Crossref
Debenay, J.P., Guillou, J.J., Redois, F., and Geslin, E. 2000. Distribution trends of foraminiferal assemblages in paralic environments. A base for using foraminifera as bioindicators. In: R.E. Martin (ed.), Environmental Micropaleontology: The Application of Microfossils to Environmental Geology, 39–67. Kluwer Academic Publishers, New York. Crossref
Debenay, J.P., Pawlowski, J., and Decrouz, D. 1996. Les foraminifères actuels. 329 pp. Masson, Paris.
Den Georgescu, M. 2013. Foraminifera: Aspects of Classification, Stratigraphy, Ecology and Evolution. 326 pp. Nova Science Publishers, New York.
Derbel-Damak, F. 1992. Biostratigraphie, sédimentologie et paléoenvironnement du Pliocène de la région de Nabeul-Hammamet (Cap-Bon, Tunisie). 151 pp. Thèse de 3ème Cycle, Faculté des Sciences de Tunis, Université Tunis El Manar, Tunis.
Derbel-Damak, F. and Zaghbib-Turki, D. 2002. Identification des zones biostratigraphiques méditerranéennes dans le Pliocène du Cap-Bon (Tunisie). Geobios 35: 253–264. Crossref
Deshayes, G.P. 1832. Encyclopédie méthodique ou par ordre de matières. Histoire naturelle des Vers et Mollusques 3: 595‒1152.
Drinia, H., Antonarakou, A., and Kontakiotis, G. 2008. On the occurrence of Early Pliocene marine deposits in the Ierapetra Basin, Eastern Crete, Greece. Bulletin of Geosciences 83: 63–77. Crossref
Ekdale, A.A., Bromley, R.G., and Pemberton, G.S. 1984. Ichnology—the use of trace fossils in sedimentology and stratigraphy. The Society of Economic Geologists and Mineralogists, Short Course 15: 1–317. Crossref
El-Hedeny, M.M. 2007. Ichnology of the Upper Cretaceous (Cenomanian–Campanian) sequence of western Sinai, Egypt. Egyptian Journal of Paleontology 7: 121–132.
Fage, L. 1936. Sur l’association d’un annélide polychète “Lumbriconereis flabellicola” n. sp. et d’un Madrépore “Flabellum pavonium distinctum” E. et H. In: A.R. Jorge (ed.), Comptes Rendus XII Congrès International de Zoologie, 941–945. Arquivos do Museu Bocoage, Lisbon.
Fekih, M. 1970. Paléoécologie du Pliocène marin au Nord de la Tunisie. 362 pp. Thèse Dr. Sc., Faculté des Sciences de Paris, Paris VI.
Fodor, R. 2001. Polychaeta életnyomok vizsgálata egerien magános korallokon (Wind-féle téglagyár, Eger). Folia Historico Naturalia Musei Matraensis 25: 5–24.
Frontalini, F., Semprucci, F., Coccicioni, R., and Balsamo, M. 2010. On the quantitative distribution and community structure of the meio and macrofaunal communities in the coastal area of the Central Adriatic Sea (Italy). Environmental Monitoring and Assessment 180: 325–344. Crossref
Gaaloul, N., Amrouni, O., Heggy, E., Douss, N., Hzami, A., Khélifi, N., Bejaoui, B., and Sánchez, A. 2022. Impacts of water stress on lagoonal ecosystem degradation in semi-arid coastal areas. Marine Pollution Bulletin 179: 113445. Crossref
Gallagher, S.J., Smith, A.J., Jonasson, K., Wallace, M.W., Holdgate, G.R., Daniels, J., and Taylor D. 2001. The Miocene palaeoenvironmental and palaeoceanographic evolution of the Gippsland Basin, Southeast Australia: a record of Southern Ocean change. Palaeogeography, Palaeoclimatology, Palaeoecology 172: 53–80. Crossref
Galloway, J.J. and Wissler, S.G. 1927. Pleistocene Foraminifera from the Lomita Quarry Palos Verdes Hills, California. Journal of Paleontology 1: 35–87.
Gibert, J.M. de, Martinell, J., and Domènech, R. 1998. Entobia ichnofacies in fossil rocky shores, Lower Pliocene, northwestern Mediterranean. Palaios 13: 476–487. Crossref
Gibert, J.M. de, Domènech, R., and Martinell, J. 2004. An ethological framework for animal bioerosion trace fossils upon mineral substrates with proposal of a new class, fixichnia. Lethaia 37: 429–437. Crossref
Gibson., P.H. 2016. A search for trace fossils of the burrowing cirratulid polychaetes Dodecaceria fimbriata and D. concharum. Historical Biology 24: 1–8. Crossref
Goldfuss, A. 1826. Abbildungen und Beschreibungen der Petrefacten Deutschlands und der angrenzenden. Petrefecta Germaniae 1: 1–70. Crossref
Gripp, K. 1967. Polydora biforans n. sp. ein in Belemniten-Rostren bohrender Wurm der Kreide-Zeit. Meyniana 17: 9–10.
Haenel, P. 1987. Interêt paleoocéanographique d’Orbulina universa d’Orbigny (Foraminifère). Oceanologica Acta 10: 15–25.
Hagenow, F., von. 1840. Monographie der Rügen’schen Kreideversteinerungen, II. Abtheilung: Radiarien und Annulaten nebst Nachträgen zur ersten Abtheilung. Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde 7: 630–672.
Hanken, N., Uchman, A., and Jakobsen, S.L. 2012. Late Pleistocene–early Holocene polychaete borings in NE Spitsbergen and their palaeoecological and climatic implications: An example from the Basissletta area. Boreas 41: 42–55. Crossref
Hillmer, G. and Schulz, M.G. 1973. Ableitung der Biologie and Ökologie eines Polychaten der Bohrgänges Romosulcichnus bioforans (Gripp) nov. Ichnogene. Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg 42: 5–24.
Hofker, J. 1932. Notizen über die Foraminiferen des Golfes von Neapel, III. Die Foraminiferenfauna der Ammontatura. Pubblicazioni Stazione Zoologica di Napoli 12: 61–144.
Johnston, G. 1838. Miscellanea Zoologica. III. The British Ariciadae. Magazine of Zoology and Botany, Edinburgh 2: 63–73.
Kanmacher, F. 1798. Essays on the Microscope. The Second Edition, with Considerable Additions and Improvements. 724 pp. Dillon & Keating, London.
Kelley, P.H. and Hansen, T.A. 2003. The fossil record of drilling predation on bivalves and gastropods. In: P.H. Kelley, M. Kowalewski, and T.A. Hansen (eds.), Predator-Prey Interactions in the Fossil Record. Topics in Geobiology 20: 113–139. Crossref
Kitahara, M., Fukami, H., Benzoni, F., and Huang, D. 2016. The new systematics of Scleractinia: Integrating molecular and morphological evidence. In: S. Goffredo and Z. Dubinsky (eds.), The Cnidaria, Past, Present and Future: The World of Medusa and her Sisters, 41–59. Springer International Publishing, Dordrecht. Crossref
Knaust, D. 2007. Invertebrate trace fossils and ichnodiversity in shallow-marine carbonates of the German Middle Triassic (Muschelkalk). In: R.G. Bromley, L.A. Buatois, M.G. Mángano, J.F. Genise, and R.N. Melchor (eds.), Sediment–Organism Interactions: A Multifaceted Ichnology. SEPM Special Publication 88: 223–240. Crossref
Knaust, D. and Dronov, A. 2013. Balanoglossites ichnofabrics from the Middle Ordovician Volkhov formation (St. Petersburg Region, Russia). Stratigraphy and Geological Correlation 21: 265–279. Crossref
Kucera, M. 2007. Planktonic foraminifera as tracers of past oceanic environments. Developments in Marine Geology 1: 6. Crossref
Łaska, W., Rodríguez-Tovar, F.J., and Uchman, A. 2021. Bioerosion structures from the Pliocene of the Agua Amarga sub-basin (Almería, SE Spain): palaeoecological and depositional environment implications. Palaeogeography, Palaeoclimatology, Palaeoecology 562: 110071. Crossref
Lei, Y. and Li, T. 2016. Atlas of Benthic Foraminifera from China Seas: The Bohai Sea and the Yellow Sea. 399 pp. Science Press, Beijing. Crossref
Linnaeus, C. 1758. Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata. 824 pp. Typis Ioannis Thomae, Vindobonae. Crossref
Loeblich, A.R. and Tappan, H. 1987. Foraminifera Genera and their Classification. 970 pp. Van Nostrand Reinhold, New York. Crossref
Lombard, F., Labeyrie, L., Michel, E., Spero, H.J., and Lea, D.W. 2009. Modelling the temperature dependent growth rates of planktic foraminifera. Marine Micropaleontology 70: 1–7. Crossref
Martinell, J. and Domènech, R. 2009. Commensalism in the fossil record: Eunicid polychaete bioerosion on Pliocene solitary corals. Acta Palaeontologica Polonica 54: 143–154. Crossref
Mayoral, E. 1988. Pennatichnus nov. icnogen,; Pinaceocladichnus nov. icnogen., e Iramena. Huellas de bioerosión debidas a Bryozoa perfo rantes (Ctenostomata, Plioceno inferior) en la Cuenca del Bajo Guadalquvir. Revista Española de Paleontología 3: 13–22. Crossref
Mayoral, E. 1991. Actividad bioerosiva de Briozoos Ctenostomados en el Ordovícico superior de la zona Cantábrica del Mazico Hespérico (Cabo Vidrias, Oviedo). Revista Española de Paleontología 6: 27–37. Crossref
Moisette, P., Cornée, J.J., Mannaï, T.B., Rabhi, M.C., André, J.P., Koskeridou, E., and Méon, H. 2010. The western edge of the Mediterranean Pelagian Platform: a Messinian mixed siliciclastic-carbonate ramp in northern Tunisia. Paleogeography, Palaeoclimatology, Palaeoecology 285: 85–103. Crossref
Murina, V. 1997. Pelagic larvae of Black Sea polychaeta. Bulletin of Marine Sciences 60: 427–432.
Murray, J.W. 1991. Ecology and Palaeoecology of Benthic Foraminifera. 397 pp. Logman Scientific & Technical, London.
Murray, J.W. 2006. Ecology and Application of Benthic Foraminifera. 440 pp. Cambridge University Press, New York. Crossref
Murray, J.W. and Alve, E. 1999. Natural dissolution of modern shallow water benthic foraminifera: taphonomic effects on the palaeoecological record. Paleogeography, Palaeoclimatology, Palaeoecology 146: 195–209. Crossref
Örsted, A.S. 1843. Annulatorum danicorum conspectus. Fasc. I. Maricolæ. 52 pp. Sumtibus Librariæ Wahlianæ, Hafniæ. Crossref
Parker, F.L. 1953. Ecology of Foraminifera from San Antonio Bay and Environs, Southwest Texas. Cushman Foundation for Foraminiferal Research, Special Publication 2: 1–75.
Perry, C. 2000. Macroboring of Pleistocene coral communities, Falmouth Formation, Jamaica. Palaios 15: 483–491. Crossref
Pflum, C.E. and Freichs, W.E. 1976. Gulf of Mexico deep water foraminifers. Cushman Foundation for Foraminiferal Research, Special Publication 14: 1–123.
Plewes, C.R. 1996. Ichnotaxonomic Studies of Jurassic Endoliths. 313 pp. Unpublished Ph.D. Thesis, University of Wales, Aberystwyth.
Pokorný, R. and Štofik, M. 2017. Evidence of bioerosive structures in Quaternary glaciomarine sediments from southwestern Iceland. Ichnos 24: 204–221. Crossref
Price, W.A. 1916. Notes on the Paleontology of Raleigh, Wyoming, McDowell, and Adjacent Counties, 663–734. West Virginia Geological Survey.
Radwański, A. 1969. Transgresja dolnego tortonu na południowych stokach Gór Świętokrzyskich (strefa zatok i ich przedpola). Acta Geologica Polonica 19: 1–164.
Reis, O.M. 1921. Über Bohrröhren in fossilen Schalen und Spongeliomorpha. Zeitschrift der Deutschen Geologischen Gesellschaft 73: 224–236.
Reuss, A.E. 1850. Neue Foraminiferen aus den Schichten des österreichischen Tertiärbeckens. Denkschriften der Kaiserlichen Akademie der Wissenschaften 1: 365–390.
Reuss, A.E. 1851. Ueber die fossilen Foraminiferen und Entomostraceen der Septarienthone der Umgegend von Berlin. Zeitschrift der Deutschen Geologischen Gesellschaft 3: 49–92.
Ricci, F., Marcelino, V.R., Blackall, L.L., Kühl. M., Medina, M., and Verbruggen, H. 2019. Beneath the surface: community assembly and functions of the coral skeleton microbiome. Microbiome 7: 159. Crossref
Roberts, J.M., Wheeler, A., Freiwald, A., and Cairns, S. 2009. Cold-Water Corals: The Biology and Geology of Deep-Sea Coral Habitats. 334 pp. Cambridge University Press, Cambridge. Crossref
Rzehak, A. 1888. Die Foraminiferen der Nummuliten-schichten des Waschberges und Michelsberges bei Stockerau in Nieder-Osterreich. Verhandlungen der Geologischen Bundesanstalt 1888: 226‒229.
Salamon, K. and Kołodziej, B. 2022. Unravelling the microbiome of fossil corals: a message from microborings. Historical Biology 34: 1228–1239. Crossref
Salamon, K., Kołodziej, B., Radtke, G., Schnick, H.H., and Golubic, S. 2022. Microborings in Jurassic scleractinians: a glimpse into the ancient coral skeleton microbiome. Coral Reefs 41: 863–867. Crossref
Savazzi, E. 1982. Commensalism between a boring mytilid bivalve and a soft bottom coral in the Upper Eocene of northern Italy. Paläontologische Zeitschrift 56: 165–175. Crossref
Schiebel, R. and Hemleben, C. 2017. Planktonic Foraminifers in the Modern Ocean. 358 pp. Springer-Verlag, Berlin. Crossref
Scoffin, T.P. and Bradshaw, C. 2000. The taphonomic significance of endoliths in dead-versus live-coral skeletons. Palaios 15: 248–254. Crossref
Seiblitz, I.G.L., Vaga, C.F., Capel, K.C.C., Cairns, S.D., Stolarski, J., Quattrini, A.M., and Kitahara, M.V. 2022. Caryophylliids (Anthozoa, Scleractinia) and mitochondrial gene: Insights from mitochondrial and nuclear phylogenomics. Molecular Phylogenetics and Evolution 175: 107565. Crossref
Sen-Gupta, B.K. and Machain-Castillo, M.L. 1993. Benthic foraminifera in oxygen-poor habitats. Marine Micropaleontology 20: 183–201. Crossref
Silén, L. 1946. On two groups of Bryozoa living in shells of molluscs. Arkiv för Zoologi. Stockholm 38B (1): 1–7.
Spadini, V. 2019. Pliocene scleractinians from Estepona (Malaga, Spain). Atti della Società Toscana di Scienze Naturali, Serie A 126: 75–94.
Stephanelli, S. 2003. Cyclic changes in oxygenation based on Foraminifera microhabitats: Early–Middle Pleistocene, Lucania Basin (Southern Italy). Journal of Micropaleontology 23: 81–95. Crossref
Stiller, F. 2005. An Early Jurassic Talpina-dominated assemblage of borings in bivalve shells from southern Hunan, China, with remarks on the ichnogenus Talpina Hagenow, 1840. Acta Palaeontologica Sinica 44: 396–411.
Stolarski, J. 1995. Ontogenetic development of the thecal structures in caryophylliine scleractinian corals. Acta Palaeontologica Polonica 40: 19–44.
Stolarski, J. 2003. 3-Dimensional micro- and nanostructural characteristics of the scleractinian corals skeleton: a biocalcification proxy. Acta Palaeontologica Polonica 48: 497–530.
Teichert, C. 1945. Parasitic worms in Permian brachiopod and pelecypod shells in western Australia. American Journal of Science 243: 197–209. Crossref
Temani, R., Nachite, D., Sciuto, F., Razgallah, S., Bekkali, R., Hayet, K., and Gaaloul, N. 2016. Les Ostracodes Plio-Pléistocènes des séries sédimentaires de la bordure orientale du cap Bon (coupe de l’Oued Lebna, Tunisie orientale). Carnets de Géologie 16: 431–447. Crossref
Thomas, A.O. 1911. A fossil burrowing sponge from the Iowa Devonian. Bulletin from the Laboratories of Natural History of the State University of Iowa 6: 165–169.
Tim, S. 1992. Rezente Tiefsee-Benthosforaminiferen aus Oberflächensedimenten des Golfes von Guinea (Westafrika)—Taxonomie, Verbreitung, Ökologie und Korngrößenfraktionen. Geologisch-Paläontologisches Institut der Universität Kiel, Berichte − Reports 59: 1–155.
Tolderlund, D.S. and Bé, A.W.H. 1971. Seasonal distribution of planktonic foraminifera in the western North Atlantic. Micropaleontology 17: 297–329. Crossref
Toom, U., Vinn, O., and Hints, O. 2019. Ordovician and Silurian ichnofossils from carbonate facies in Estonia: A collection-based review. Palaeoworld 28: 123–144. Crossref
Tribollet, A. 2008. The boring microflora in modern coral reef ecosystems: a review of its roles, 67–94. In: M. Wisshak and L. Tapanila (eds.), Current Developments in Bioerosion, 67–94. Springer, Berlin. Crossref
Van der Zwaan, G.J., Jorissen, F.J., and De Stigter, H.C. 1990. The depth dependency of planktonic/ benthic foraminiferal ratios: Constraints and applications. Marine Geology 95: 1–16. Crossref
Van Morkhoven, F.P.C.M., Berggren, W.A., and Edwards, A.S. 1986. Cenozoic cosmopolitan deep-water benthic foraminifera. Bulletin des Centres de Recherches Exploration-Production Elf-Aquitaine Mémoire 11: 1–421.
Vertino, A., Stolarski, J., Bosellini, F., and Taviani, M. 2014. Mediterranean corals through time: from Miocene to present. In: S. Goffredo and Z. Dubinsky (eds.), The Mediterranean Sea: Its History and Present Challenges, 257–274. Springer, Dordrecht.
Vescogni, A., Vertino, A., Bosellini, F.R., Harzhauser, M., and Mandic, O. 2018. New paleoenvironmental insights on the Miocene condensed phosphatic layer of Salento (southern Italy) unlocked by the coral-mollusc fossil archive. Facies 64: 21. Crossref
Violanti, D. 2012. Pliocene Mediterranean foraminiferal biostratigraphy: A Synthesis and application to the paleoenvironmental evolution of northwestern Italy. In: Ö. Elitok (ed.), Stratigraphic Analysis of Layered Deposits, 124–160. InTech, Rijeka. Crossref
Voigt, E. 1965. Über parasitische Polychaeten in Kreide-Austern sowie einige andere in Muschelschalen bohrende Würmer. Paläontologische Zeitschrift 39: 193‒211. Crossref
Voigt, E. 1970. Endolithische Wurm-Tunnelbauten (Lapispecus cuniculus n. g. n. sp. und Dodecaceria[?] sp.) in Brandungsgeröllen der oberen Kreide im nördlichen Harzvorlande. Geologische Rundschau 60: 355–380. Crossref
Voigt, E. 1971. Fremdskulpturen an Steinkernen von Polychaeten-Bohrgängen aus der Maastrichter Tuffkreide. Paläontologische Zeitschrift 45: 144–153. Crossref
Voigt, E. 1972. Über Talpina ramosa v. Hagenow 1840, ein wahrscheinlich zu den Phoronidea gehöriger Bohrorganismus aus der Oberen Kreide, nebst Bemerkungen zu den übrigen bisher beschriebenen kretazischen „Talpina”-Arten. Nachrichten der Akademie der Wissenschaften zu Göttingen (II: Mathematisch-physikalische Klasse) 7: 93–126.
Voigt, E. 1975. Tunnelbaue rezenter und fossiler Phoronidea. Paläontologische Zeitschrift 49: 135–167. Crossref
Wilson, B. 2003. Foraminifera and paleodepths in a section of the Early to Middle Miocene Brasso Formation, Central Trinidad. Caribbean Journal of Science 39: 209–214.
Wisshak, M. 2017. Taming an ichnotaxonomical Pandora’s box: revision of dendritic and rosetted microborings (ichnofamily: Dendrinidae). European Journal of Taxonomy 390: 1–99. Crossref
Wisshak, M., Knaust, D., and Bertling, M. 2019. Bioerosion ichnotaxa: review and annotated list. Facies 65: 24. Crossref
Wisshak, M., Meyer, N., Kuklinski, P., Rüggeberg, A., and Freiwald, A. 2021. “Ten Years After”—a long-term settlement and bioerosion experiment in an Arctic rhodolith bed (Mosselbukta, Svalbard). Geobiology 20: 112–136. Crossref
Wisshak, M., Schönberg, C., Form, A., and Freiwald, A. 2012. Ocean acidification accelerates reef bioerosion. PLOS One 7: 3–10. Crossref
Zachariasse, W.J., Jorissen, F.J., Perissoratis, C., Robling, E.D., and Tsapralis, V. 1997. Late Quaternary foraminiferal changes and the nature pf of sapropels in Skopelos Basin. In: Proceedings of 5th Hellenic Symposium of Oceanography and Fisheries. Kavala, Greece, Volume 1, 391–394. National Centre for Marine Research.
Zibrowius, H., Southward, E.C., and Day, J.H. 1975. New observations on a little-known species of Lumbrineris (Polychaeta) living on various cnidarians, with notes on its Recent and fossil scleractinian hosts. Journal of Marine Biological Association of the United Kingdom 55: 83–108. Crossref
Acta Palaeontol. Pol. 68 (4): 659–681, 2023
https://doi.org/10.4202/app.01095.2023