

Muscle attachment scars in helcionelloids from Denmark cast light on mollusc evolution in the Cambrian
JOHN S. PEEL and VIVIANNE BERG-MADSEN
Peel, J.S. and Berg-Madsen,V. 2023. Muscle attachment scars in helcionelloids from Denmark cast light on mollusc evolution in the Cambrian. Acta Palaeontologica Polonica 68 (4): 625–638.
Multiple, small (diameter <20 µm) swellings on the apex of internal moulds of the laterally compressed helcionelloids Eotebenna viviannae and the new species Eotebenna danica from the middle Cambrian (Miaolingian) of Bornholm, Denmark, are interpreted as a muscle attachment scars. The scar pattern is unique amongst currently known helcionelloids both in the abundance of attachment sites and in crossing the median plane of symmetry on the supra-apical (dorsal) surface. Sites of typically two pairs of dorsal muscle scars in other helcionelloids are distributed symmetrically on the dorso-lateral areas. The recognition of four groups of muscle scar patterns in helcionelloids suggests a degree of anatomical diversity within the group that is obscured by the morphological simplicity of the enclosing cap-shaped shells, although evolutionary links to mollusc crown groups are unresolved. In addition to the muscle scars, traces of shell micro-structure are described.
Key words: Mollusca, Helcionelloida, muscle scars, Miaolingian, Cambrian, Denmark.
John S. Peel [john.peel@pal.uu.se; ORCID: https://orcid.org/0000-0002-1774-7931 ], Department of Earth Sciences (Palaeobiology), Uppsala University, Villavägen 16, SE-75236, Uppsala, Sweden.
Vivianne Berg-Madsen [vivianne.berg-madsen@em.uu.se; ORCID: https://orcid.org/0009-0000-2404-7848 ], Museum of Evolution, Uppsala University, Norbyvägen 16, SE-75236 Uppsala, Sweden.
Received 10 August 2023, accepted 15 October 2023, available online 27 November 2023.
Copyright © 2023 J.S. Peel and V. Berg-Madsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The recognition of muscle attachment scars on the interior of the shell of various lower Palaeozoic univalve molluscs has proved significant in elucidating their relationships to modern molluscan groups. Typically, the scars are found as low swellings on internal moulds that represent muscle attachment sites depressed into the inner shell surface on the shell interior, and are exposed by exfoliation of the calcareous shells. Less frequently, muscle scars are visible on the interior of the shell itself, etched free from surrounding sediment (Lindström 1880, 1884; Peel 1993). In some cases, the muscle scar patterns offer decisive information concerning affinity. None more so than in the case of the familiar Silurian species Tryblidium reticulatum Lindström, 1880, and Tryblidium unguis Lindström, 1880 (= Pilina Koken and Perner, 1925) where muscle scar patterns indicate identity with the tryblidiid tergomyan (“monoplacophoran”) Neopilina Lemche, 1957, first described from modern seas almost eight decades later (Wingstrand 1985; Lindberg 2009). Similarly, the presence of a pair of retractor muscle scars in helically coiled Ordovician gastropods (Peel 2019) supports interpretation of bilaterally symmetrical shells such as Bellerophon Montfort, 1808, and Carinaropsis Hall, 1847, as gastropods. Often, however, the interpretation of muscle scar patterns is ambiguous, as demonstrated by uncertainties concerning the early record of limpet-like shells interpreted as patellogastropods, the most basal gastropod stock (Yochelson 1988; Horný 1963; Peel and Horný 1999; Lindberg 2009; Peel 2020a, b; Ponder et al. 2020).
Helcionelloids are the dominant univalve molluscs in the early and middle Cambrian. Despite their abundance and diversity only a few records exist of preserved muscle scars (Parkhaev 2001, 2002, 2014a, b; Vendrasco et al. 2010; Li et al. 2021; Peel 2023). These studies were summarized by Peel (2023) in an overview of the variation in known helcionelloid muscle scar patterns. Unlike univalves of younger age, recognition of helcionelloid muscle scars in specimens from the early–middle Cambrian is mainly based on phosphatized internal moulds from Small Shelly Fossil assemblages recovered from carbonate rock samples by digestion in weak acids. The diagenetic phosphatization often replicates in great detail the inner surface of the shell, preserving details of fine shell structure (Runnegar 1985; Kouchinsky 1999, 2000a, b; Vendrasco et al. 2010, 2011a, b, 2015; Li et al. 2019), some of which have been interpreted as polygonal epithelial or myostracal impressions (Ushatinskaya and Parkhaev 2005, but see Vendrasco et al. 2010; Li et al. 2023a, b). Given the excellence of such preservation, it is perhaps surprising that muscle attachment scars have not been more widely recognized, a feature noted by Missarzhevsky (1989) who coined the unofficial name Eomonoplacophora for the group on account of its apparent lack of muscle scars. The small size (1–2 mm) of individual specimens, in many cases juveniles, may be a factor since muscle scars in other lower Palaeozoic univalves are generally described from much larger, originally thicker shelled specimens. However, many other Cambrian helcionelloids of similar size display ontogenetic changes, such as the morphological differentiation of the protoconch from later growth stages (e.g., Gubanov et al. 2004: fig. 6A; Skovsted 2004: fig. 5; Parkhaev 2014a) that strongly suggest that they are not juveniles, despite their small size. In many cases, the co-occurrence of supposed juveniles and miniature adults likely reflects preservational constraints.
The present paper describes multiple small swellings on the apical area of internal moulds of the laterally compressed helcionelloids Eotebenna viviannae Peel, 1991a, and Eotebenna danica sp. nov. from the middle Cambrian (Miaolingian) of Bornholm, Denmark (Fig. 1). The swellings are interpreted as muscle attachment sites rooted in the inner surface of the univalve shell and differ from other records of helcionelloid muscle scars on account of their small size, abundance and location astride the median dorsal plane of symmetry. Variation in described muscle scar patterns suggests that anatomical diversity in helcionelloids is obscured by the simple morphology of the cap-shaped shells, promoting enquiry into the relationship of these groups to their crown group mollusc descendants.
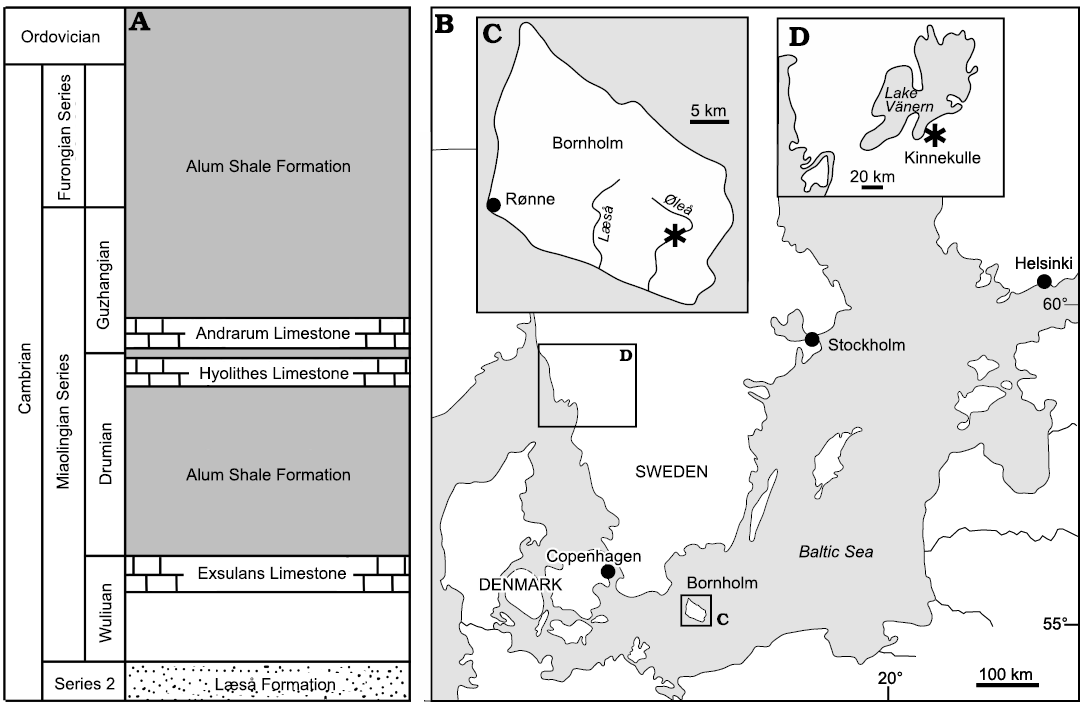
Fig. 1. Geological and geographical background. A. Cambrian stratigraphy of southern Bornholm, Denmark (based on Nielsen and Schovsbo 2007). B. Map of the Baltic area showing location of Bornholm, with location of studied locality (asterisk) on the rivulet Øleå (C), and the Lake Vänern area in southern Sweden (D), with collection locality on the western slopes of the hill Kinnekulle (asterisk).
Institutional abbreviations.—GGU, Grønlands Geologiske Undersøgelse (Geological Survey of Greenland), now a part of the Geological Survey of Denmark and Greenland (GEUS), Copenhagen, Denmark; MGUH, Natural History Museum of Denmark, Copenhagen, Denmark; PMU, palaeontological collections of the Museum of Evolution, Uppsala University, Sweden.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: urn:lsid:zoobank.org.pub:A16E2206-D792-464A-8E16-7790460B57BE
Material and methods
Limestone samples from Bornholm, Denmark, were collected by VB-M from the Andrarum Limestone exposed in the rivulet Øleå in southern Bornholm (Fig. 1A–C) as a prelude to Berg-Madsen (1985). Samples derive from the Solenopleura brachymetopa Biozone of Westergård (1946); Guzhangian, Miaolingian, Cambrian. This biozone was included within the overlying Lejopyge laevigata Biozone by Axheimer et al. (2006), Weidner and Nielsen (2009) and Weidner et al. (2014). However, Geyer (2019) recognized an Erratojincella brachymetopa Biozone that he considered to be of latest Drumian age.
Specimens from southern Sweden were collected by VB-M during 1997 in a small ditch in Storängen (“the large meadow”) on the western slope of the hill Kinnekulle, between the lakeside road and the shore of Lake Vänern (58.6°N, 13.3°E; Fig. 1D). They derive from the Acidiscus atavus Biozone (early Drumian age) and thus are slightly older than the Bornholm specimens.
The samples from Bornholm were treated with 10% acetic acid to liberate insoluble residues containing microfossils. The residues were wet sieved and hand-picked, and phosphatized specimens were selected for later study using scanning electron microscopy. While some of the rare specimens from Kinnekulle are internal moulds, shell is retained as a thin translucent calcite layer in others, so samples were left in their rock matrix and not subjected to acetic acid treatment. SEM imaging was carried out at the universities of Copenhagen and Uppsala (JSP) and illustrations assembled using Adobe Photoshop.
Systematic palaeontology
Phylum Mollusca Cuvier, 1797
Class Helcionelloida Peel, 1991a
Order Helcionellida Geyer, 1994
Family Yochelcionellidae Runnegar and Jell, 1976
Genus Eotebenna Runnegar and Jell, 1976
Type species: Eotebenna pontifex Runnegar and Jell, 1976, from the Currant Bush Limestone (Miaolingian, Cambrian), Thorntonia area, Queensland, Australia.
Eotebenna viviannae Peel, 1991a
Figs. 2, 3.
1985 rostroconch sp. I; Berg-Madsen 1985: fig. 5D.
1991 Eotebenna viviannae sp. nov.; Peel 1991a: 173, fig. 19.
1991 Eotebenna viviannae; Peel 1991b: fig. 31.
1997 Eotebenna cf. viviannae; Hinz-Schallreuter 1997: pl. 2: 4.
2004 Eotebenna viviannae; Gubanov et al. 2004: 10, fig. 8.
2004 Eotebenna viviannae; Peel 2004: fig. 1C.
2006 Eotebenna viviannae; Peel 2006: text-fig. 2H.
2021 Eotebenna viviannae; Peel 2021c: fig. 1H.
Type material: Holotype MGUH 19564, well preserved shell internal mould (Fig. 2). Paratypes: MGUH 19565 and three additional internal moulds (MGUH 19566–19568), that were described by Peel (1991a: fig. 19H–N) but are not figured herein. All from type locality and horizon.
Type locality: The rivulet Øleå, Bornholm, Denmark.
Type horizon: Andrarum Limestone, Guzhangian, Miaolingian, middle Cambrian.
Material.—MGUH 34273–34276, internal moulds from type locality and horizon.
Description.—Apical attachment scars and fibrous shell microstructure are added to the description of Peel (1991a).
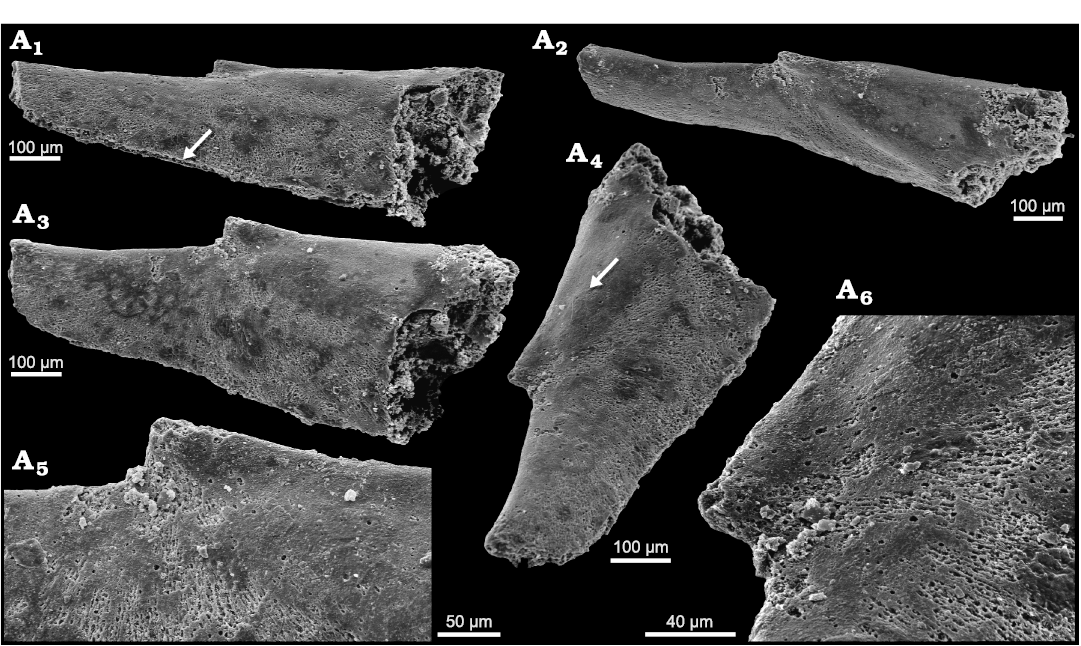
Fig. 2. Helcionelloid mollusc Eotebenna viviannae Peel, 1991a, MGUH 19564, holotype, internal mould, Andrarum Limestone, Bornholm, Denmark, Guzhangian, Miaolingian, middle Cambrian. A1. Oblique lateral view showing margin of shell (arrow) along the narrow slit joining the sub-apical and supra-apical apertures. A2. Oblique apico-lateral view. A3. Lateral view. A4. Oblique view showing inverted teardrop-shaped sub-apical aperture and irregular area (arrow) of possible muscle scar. A5. Lateral view of apex. A6. Oblique lateral view of apex showing radial fibrous structure beneath smooth outer layer.

Fig. 3. Helcionelloid mollusc Eotebenna viviannae Peel, 1991a,
internal moulds, Andrarum Limestone, Bornholm, Denmark, Guzhangian,
Miaolingian, middle Cambrian. A.
MGUH 19565, paratype, lateral view (A1)
with detail of apex (A2).
B. MGUH 34273, lateral (B1) and apico-lateral
views showing impression of comarginal ornamentation and cylindrical
form of median sub-apical area (B2).
C. Specimen lost, lateral
view (C1)
with detail of apex (C2),
arrow locates detail of shell structure (C3).
D. MGUH 34274, lateral view
with detail of radial fibrous structure and overlying imbricated
lamellae (D2),
and patch of ornamented outer shell (D1,
arrow). E. MGUH 34275,
lateral view with detail of apex (E2)
with muscle scars; arrows locate possible muscle scar. F. MGUH 34276, lateral view (F1) with detail of
possible muscle scar (F2)
located by arrows.
Remarks.—Eotebenna viviannae was described by Peel (1991a) on the basis of exfoliated specimens and internal moulds without details of external ornamentation from the Andrarum Limestone of Bornholm, Denmark. The shell is characteristically elongate and laterally compressed (Figs. 2, 3). Its overall shape is similar to that of the Ordovician rostroconch Pinnocaris lapworthi Etheridge, 1878, but the growth vectors are dissimilar (Peel 2004). A well preserved specimen clearly showing the comarginal growth lines on the elongate shell was illustrated by Hinz-Schallreuter (1997) and similar ornamentation is retained in a partially exfoliated specimen (MGUH 34273) illustrated here (Fig. 3B). In addition to material from Bornholm, ten silicified specimens of Eotebenna viviannae were described by Gubanov et al. (2004) from the Kuonamka Formation (Drumian, Miaolingian) of northern Siberia, Russia.
In terms of its elongate shape, Eotebenna viviannae is similar to the type species Eotebenna pontifex Runnegar and Jell, 1976, from the Currant Bush Limestone (Miaolingian) of Queensland, Australia but the latter is distinguished by its massive, circular snorkel at the sub-apical margin when compared to the inverted teardrop-shaped sub-apical opening in Eotebenna viviannae (Fig. 2A4).
Stratigraphic and geographic range.—Drumian of Siberia, Russia and Guzhangian of Denmark (both middle Cambrian).
Eotebenna danica sp. nov.
Figs. 4, 5.
1985 rostroconch sp. II; Berg-Madsen 1985: fig. 5E.
Zoobank LSID: urn:lsid:zoobank.org:act:32FA3A63-AF30-4131-8364- 42866C61F75E
Etymology: From the occurrence of the type suite on the Danish island of Bornholm (Fig. 1).
Type material: Holotype: MGUH 34280 (Fig. 4D). Paratypes: MGUH 34277–34279, 34281, 34282. All phosphatized internal moulds from the type locality and horizon.
Type locality: The rivulet Øleå, southern Bornholm, Denmark (Fig. 1C).
Type horizon: Andrarum Limestone, Guzhangian, Miaolingian, middle Cambrian.
Material.—In addition to the type suite from southern Bornholm, rare specimens were collected from the lower Drumian (Miaolingian, middle Cambrian), on the western slope of Kinnekulle in southern Sweden (Fig. 1D), one of which is figured (Fig. 5A; MGUH 34283).
Diagnosis.—Laterally compressed species of the univalve Eotebenna (Fig. 4D2) in which height (as oriented in Fig. 4D1) is about two thirds of length. Apex overhanging the concave sub-apical surface and slightly closer to the sub-apical margin. Aperture oval, joined to the narrow, inverted teardrop-shaped opening (snorkel) at the sub-apical margin by a narrow slit. Ornamentation of growth lines crossed by fine radial lines. Multiple small swellings on the internal mould, interpreted as muscle scars on the apex, occur principally on the supra-apical (dorsal) surface.
Description.—In lateral view (Fig. 4D1), in which the upright orientation follows Peel (1991b: fig. 30) and Parkhaev (2001: fig. 2), the height of the shell is about two thirds of its overall length. The overturned apex forms the tip of an isosceles triangle, representing the main part of the body cavity, the sides of which diverge at about 50° to the shallowly convex, basal, apertural margin. The supra-apical surface is slightly sigmoidal, initially shallowly convex but becoming shallowly concave; the dorsum is uniformly rounded. The sub-apical surface is concave, ultimately rising to a higher level than the apex as it forms the upper margin of a sail-like extension of the main part of the body cavity (left side of Fig. 4D1), the junction with which may be marked by a radial fold. The supra-apical surface terminates at the inverted teardrop-shaped aperture of the snorkel (Fig. 4D2), which is connected to the ovoid aperture by a thin slit around the margin. The entire margin, from tip of the snorkel to the supra-apical margin, is almost semicircular. Ornamentation consists of comarginal growth lines, which may be rugose near the aperture, that cross closely spaced fine growth lines. The apex of the internal mould retains an irregular pattern of small (up to 20 µm in diameter) swellings, mainly on the supra-apical surface, interpreted as muscle attachment scars (see below).
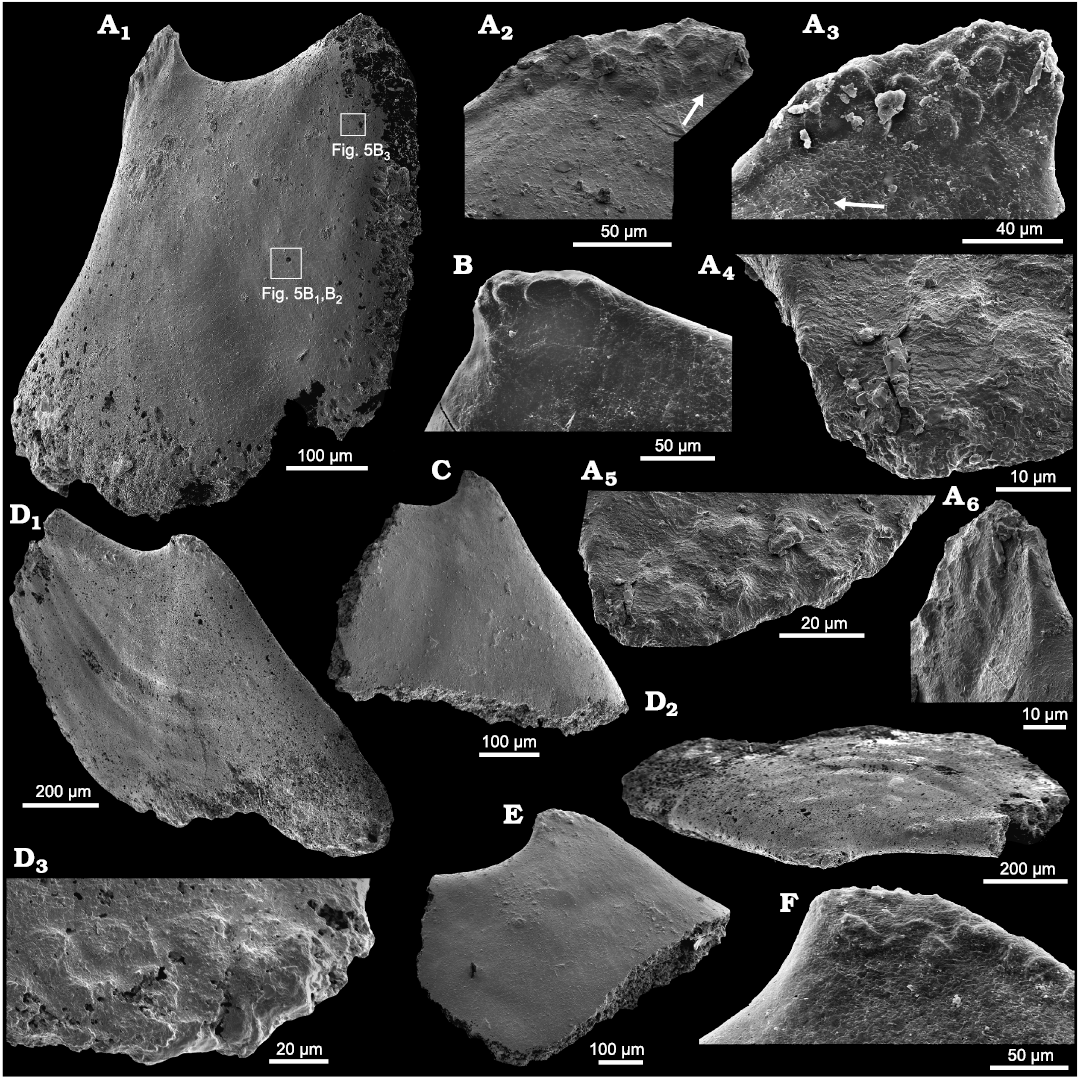
Fig. 4. Helcionelloid mollusc Eotebenna danica sp. nov., internal moulds, Andrarum Limestone, Bornholm, Denmark, Guzhangian, Miaolingian, middle Cambrian. A. MGUH 34277, lateral view (A1) with rectangles indicating location of Fig. 5B1, B2, and B3. Muscle scars at apex illustrated in different orientations (A2–A6), with arrow in A2 indicating slight diagenetic compression or deformation along edge of muscle field. Arrow in A3 indicating imbricated lamellar structure shown in detail in A4. B. MGUH 34278, apex in lateral view. C. MGUH 34279, lateral view. D. MGUH 34280, holotype, lateral (D1) and apico-lateral (D2) views, the latter showing the laterally compressed shell form, with detail of apical muscle scars (D3). E. MGUH 34281, lateral view. F. MGUH 34282, apex in lateral view.
Remarks.—Eotebenna danica sp. nov. differs from Eotebenna papilio Runnegar and Jell, 1976, described from a single specimen from the Coonigan Formation of New South Wales, Australia, in which height and length are equal, in terms of its more elongate form and the lack of widely spaced, prominent comarginal rugae. More closely spaced, weak comarginal rugae are present on the internal moulds of the Scandinavian materal in the later growth stages (Fig. 4D1, D2). In addition, the apex in Eotebenna danica slightly overhangs the sub-apical surface. Eotebenna papilio has widely spaced radial ribs on the shell exterior but radial ornamentation is not seen in the type suite of Eotebenna danica from Bornholm, which may reflect their preservation as internal moulds. Eotebenna papilio is a silica replica of the shell with well-preserved details of the shell exterior (Runnegar and Jell 1976: 10–14, fig. 11B). Ornamentation of closely spaced growth lines is seen in specimens assigned to Eotebenna danica from the middle Cambrian (Miaolingian) of Kinnekulle in southern Sweden (Fig. 5A, arrow).
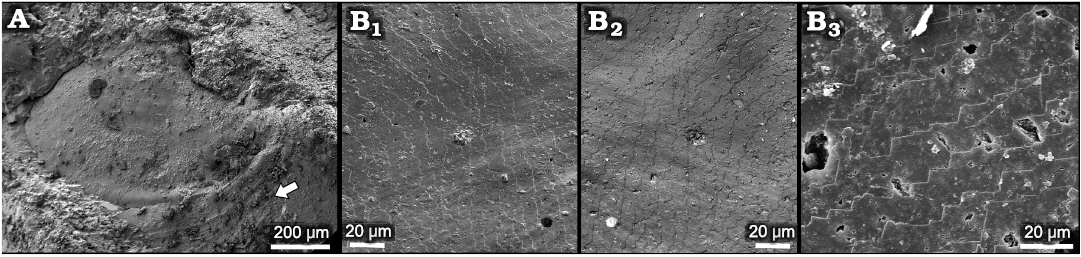
Fig. 5. Helcionelloid mollusc Eotebenna danica sp. nov. from Miaolingian, middle Cambrian A. MGUH 34283, internal mould with traces of comarginal ornamentation and rugae (arrow), western slopes of Kinnekulle, southern Sweden, Drumian. B. MGUH 34277, details of shell structure, Andrarum Limestone, Bornholm, Denmark, Guzhangian (general view of the specimen in Fig. 4A). Surface of internal mould (B1) digitally inverted and mirrored here (B2) to depict shell structure on the interior surface of the shell. Detail of imbricate lamellae on internal mould (B3).
Eotebenna viviannae differs from the co-occurring Eotebenna danica by its profoundly extended shell form when viewed in lateral aspect (Figs. 2A1–A3, 3A1, B1). Specimens of the former (Figs. 2, 3) are illustrated with the sub-apical and supra-apical surfaces horizontal but the long axis is sub-vertical in reconstructions by Peel (1991b, 2006). A radial fold separates the main part of the body cavity from the sub-apical sail and snorkel (Fig. 2A4), as in Eotebenna danica, but is often inconspicuous. The type species of Eotebenna, Eotebenna pontifex Runnegar and Jell, 1976, from the Currant Bush Limestone (Miaolingian) of Queensland, Australia, is also much more elongate than Eotebenna danica but the massive snorkel is circular in cross-section compared to the inverted teardrop-shape in the two Bornholm species.
Eotebenna arctica Peel, 1989, from the Henson Gletscher Formation (uppermost Series 2, Stage 4, lower Cambrian) of southern Freuchen Land, North Greenland is more strongly coiled than Eotebenna danica, with a convex supra-apical surface, in lateral view, and the apex strongly overhanging the sub-apical surface (Peel 1989, 1991b).
Stratigraphic and geographic range.—Drumian of Sweden and Guzhangian of Denmark (both middle Cambrian).
Discussion
Shell structure in Eotebenna.—The holotype of Eotebenna viviannae (Fig. 2) displays a prominent, open, fibrous texture radiating from the apex. Individual fibres are about 1.5 µm in width, with a fine, irregular acanthose texture, and are spaced at 1–2 µm (Fig. 2A5, A6). Slightly wider, more closely spaced and less regular fibres have been described in hyoliths by Kouchinsky (2000b). However, in some areas of the internal moulds, the fibrous layer in Eotebenna viviannae appears to be a preservational variant of an imbricate lamellose structure in which the strike of the inclined lamellae corresponds to the length of the fibres (Fig. 3D). If so, the apparent fibres would probably represent the edges of lamellae, or packets of lamellae, separated from each other by differential preservation. The fibrous layer and its equivalent lamellae are overlain on the internal mould by thin discontinuous shell patches displaying traces of imbricate lamellae forming a step-like pattern, with about 10 µm wide steps, that strikes perpendicular to the underlying fibrous texture (Fig. 2A6), see also (Fig. 3D). The latter specimen (Fig. 3D) appears to preserve a composite replacement of shell and the internal mould, showing comarginal growth lines and fine radial lines (arrow in Fig. 3D1) on an outer shell surface that overlies the imbricate layer on the internal mould. The apical muscle scars are thus draped by overlying shell layers and are obscured.
An internal mould of Eotebenna viviannae with clearly delimited multiple muscle scars (Fig. 3C) preserves imbricate lamellae near the apex that strike oblique to the supra-apical surface (Fig. 3C2). Individual lamellae slope away from the apex with striations also downslope, perpendicular to the lamellar margins (Fig. 3C3).
Collectively, the specimens might suggest a shell with lamellar and fibrous layers. However, interpretation of the fibrous layer as a preservational variant of imbricate lamellae, promotes a simpler explanation, with two lamellar layers showing different orientations. Runnegar (1985) and Vendrasco et al. (2010) noted an inner laminar layer (foliated calcite) in Eotebenna pontifex from Australia, but other layers were not recognized.
One illustrated specimen of Eotebenna danica shows distinct multiple muscle scars and a fine pattern of imbricate lamellae (Figs. 4A, 5B). Due to the clarity of the structures, it is considered to be a direct impression of the inner surface of the shell. There is no evidence of the fibrous layer and outer ornamented surface seen in specimens of Eotebenna viviannae. The individual lamellae form steps about 10–15 µm wide that are laterally continuous across the lateral areas of the shell. Individual lamellae are comarginal from the supra-apical margin (bottom in Fig. 4A1) but lose their curvature as they pass into the sail beneath the sub-apical surface, such that they meet the sub-apical margin, just below the snorkel (top right in Fig. 4A1) at an angle of about 45º. Individual lamellae slope towards the apertural margin, stepping down towards the apex (Figs. 4A1, 5B1, B2). At the apex, the imbricate lamellae become more closely spaced and persist into the elevated muscle scars (Fig. 4A2, A3), where individual lamellae have a thickness of about 0.5 µm (Fig. 4A4).
Similar imbricated lamellae composed of foliated calcite or calcite semi-nacre were described by Vendrasco et al. (2010, 2011b) and Vendrasco and Checa (2015) in helcionelloids. Continuous bands of imbricated lamellae interpreted as aragonite blades, with a finely digitate edge to the lamellae, as distinct to the sharply angular edges seen here (Fig. 5B), were described by Vendrasco et al. (2011a) in the bivalve Pojetaia Jell, 1980. A full discussion of crystallography was given by Vendrasco and Checa (2015) and Vendrasco et al. (2015), while Vendrasco et al. (2019) described similar structures from Late Ordovician molluscs.
Muscle scars in Eotebenna.—Most available internal moulds of Eotebenna danica show a concentration of closely spaced, sub-circular swellings at the apex, although most of these are located on the supra-apical surface (Fig. 4A, D). About 15 small swellings are visible in paratype MGUH 34277 in lateral view (Fig. 4A), suggesting that about 25 in total are distributed around the apex. The swellings lie together within a single field that crosses the median plane of symmetry but they are not symmetrically disposed around that plane. In this specimen they are arranged in three rather irregular bands parallel to the dorsum on the visible lateral surface (Fig. 4A3) but this banding is less regular in other specimens. The swellings vary from irregularly circular to oval, with a maximum dimension of about 20 µm. They are flattened on the upper surface, with distinct lateral margins. A similar pattern is seen in the holotype of Eotebenna danica (MGUH 34280) but the individual swellings are fewer and less sharply delimited (Fig. 4D). The pattern and number of individual swellings is less clear in other figured specimens (Fig. 4C, E, F). In internal moulds of Eotebenna viviannae, the apex varies from acute (Fig. 2) to bluntly rounded, with the latter sometimes showing fewer, more weakly expressed apical swellings comparable to those in Eotebenna danica (Fig. 3C).
Cellular, polygonal, textures occur on the internal moulds of a number of Cambrian helcionelloids (Runnegar 1985; Bengtson et al. 1990; Kouchinsky 2000a; Skovsted 2004; Ushatinskaya and Parkhaev 2005; Vendrasco et al. 2010) and may take the form of a framework of raised margins around central depressions or depressed margins around generally convex central swellings (Fig. 6). The textures have been considered to be of epithelial origin or a reflection of microcrystalline shell structure (Runnegar 1985; Ushatinskaya and Parkhaev 2005; Winrow and Sutton 2012; Vendrasco et al. 2010), while studies by Li et al. (2019, 2023a, b) suggested that they probably have multiple origins. Polygonal cellular imprints were described in Cambrian and present day brachiopods and bivalves by Williams and Wright (1970), Winrow and Sutton (2012), Parkhaev (2014b) and Li et al. (2019), respectively, while Dong et al. (2022) noted that the prismatic pattern formed by aragonite in the myostracal layer is widespread in present day molluscan classes. Li et al. (2023a) demonstrated the formation of polygonal texture by the prismatic organic matrix rather than as an imprint of mineral prisms, and that the matrix can form both concave and convex polygonal textures on internal moulds. Correspondingly the margins of the polygons may be raised or depressed.
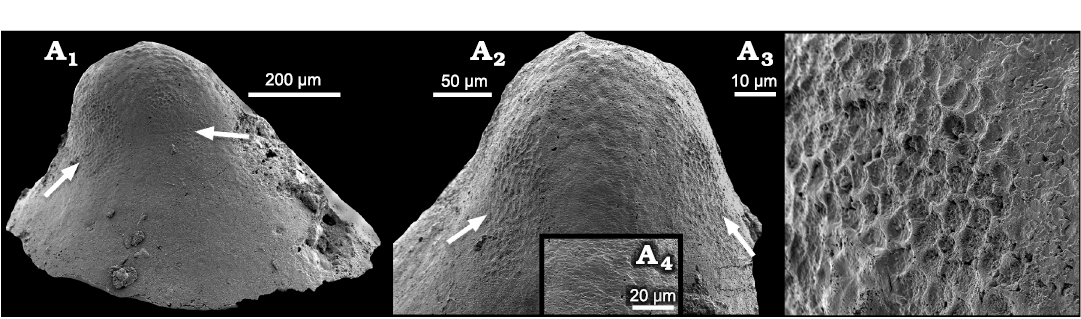
Fig. 6. Surface textures on internal mould of a helcionelloid mollusc Vendrascospira frykmani Peel and Kouchinsky, 2022, PMU 39208 from GGU sample 271492, Henson Gletscher Formation, Løndal, Peary Land, North Greenland, Miaolingian, middle Cambrian. A1. Lateral view with one muscle attachment scar from each of the two pairs of muscle scars (arrows). A2. Dorsal view showing pair of symmetrically placed muscle scars (arrows) on the supra-apical surface (right side of A1). The muscle scars lie on each side of the median area with botryoidal surface texture. A3. Detail of muscle scar (left scar in A1). A4. Detail of finely imbricate shell structure from the median area of A2.
An internal mould of Vendrascospira frykmani Peel and Kouchinsky, 2022, from the Miaolingian Henson Gletscher Formation of North Greenland preserves two different polygonal textures (Peel 2023; Fig. 6). Polygonal structures interpreted as muscle attachment scars have well-defined ridges surrounding concave depressions that are about 5 µm in diameter (Fig. 6A3). The ridges thus correspond to grooves in the internal surface of the shell. Two pairs of equidimensional scars are present on the lateral areas, with one of each pair displayed in lateral view (Fig. 6A1) and with both scars of one pair on each side of the median plane of symmetry on the sub-apical surface (Fig. 6A2). Muscle scars showing the same morphology were also described by Peel (2023) in Hensoniconus siku (Peel and Kouchinsky, 2022) from the Miaolingian Henson Gletscher Formation in North Greenland. Ushatinskaya and Parkhaev (2005) interpreted a similar texture located on the sub-apical surface in Securiconus sp. from the Tommotian (early Cambrian) of the Siberian platform as myostracum.
The polygonal texture on the central apical area of Vendrascospira frykmani has low, rounded elevations on the internal mould bordered by indistinct depressed margins that represent low ridges on the shell interior (Fig. 6A2). This was referred to as botryoidal pattern by Peel (2023) and is seen to lie between the arrowed muscle scars in Fig. 6A2. This texture is opposite in relief to that of the adjacent muscle scars (Fig. 6A3) and with individual polygons that are two or three times larger (Fig. 6A2). Ushatinskaya and Parkhaev (2005) described a comparable texture in Oelandiella korobkovi Vostokova, 1962, from the Tommotian of Siberia. Kouchinsky (1999) and Li et al. (2019) have illustrated similar structures in Anabarella from Siberia and China. Vendrasco et al. (2015) gave an overview of shell microstructures, composition and preservation. Most of the surface of internal moulds of Anhuiconus microtuberus Zhou and Xiao, 1984, illustrated by Li et al. (2021) from the Xinji Formation show superficially similar, but more strongly expressed, tubercles. In detail, the botryoidal surface of Vendrascospira frykmani forms the upper surface on the internal mould of a layer of finely imbricate lamellae (Fig. 6A4), the surfaces of which slope towards the aperture (down in Fig. 6A2) and step down towards the apex (up in Fig. 6A2). Thus, this specific botryoidal surface does not represent the actual surface of contact between the mantle and the inner surface of the shell but is likely an organic layer within the innermost shell layer. The polygons of the muscle scars in Vendrascospira frykmani appear to overlie this botryoidal texture on the internal mould and were therefore more deeply embedded into the inner surface of the shell.
In terms of its generally sharp delimitation, the pattern of apical swellings in Eotebenna danica compares closely with the muscle attachment scars in Vendrascospira frykmani and Hensoniconus siku described by Peel (2023; Fig 6A3). Therefore, the agglomeration of individual swellings in Eotebenna danica is also interpreted as the site of muscle attachment, although the individual swellings are up to twice the diameter of the cells in the muscle attachment scars described by Peel (2023). The greater size of the swellings and their more irregular form and spacing than the individual polygonal cells in Vendrascospira frykmani and Hensoniconus siku suggests that the agglomeration represents numerous closely spaced muscles or bundles of muscle fibres rather than individual prismatic elements within a single muscle attachment scar. Support for this interpretation is found by comparison with the abundance and distribution of pedal muscle attachment scars in some burrowing Palaeozoic bivalves (Fig. 7). In contrast to the three or four pairs of pedal retractor muscles present in most bivalves (Driscoll 1964), the Middle Ordovician Neofordilla Krasilova, 1977, shows a tight grouping of numerous pedal muscle scars on the umbo (Krasilova 1977: fig. 1). The familiar Early Ordovician Babinka Barrande, 1881 (McAlester 1965; Soot-Ryen 1969; Babin 1977; Polechova 2013; Fig. 7A), the Middle Ordovician Coxiconcha britannica (Roualt, 1851) as illustrated by Křiz (1995) and Polechova (2013) and Palaeoneilo musculosa (Knod, 1908) from the Devonian of Bolivia (Fig. 7B–D) preserve numerous closely spaced pedal (accessory) muscle scars close to the umbones (Babin and Farjat 1994; Křiz 1995) in a similar arrangement to the agglomeration of scars in Eotebenna danica sp. nov. (Fig. 4). A comparable distribution of umbonal muscles was described by Skawina and Dzik (2011) in Silesunio parvus Skawina and Dzik, 2011, from the Triassic of Poland. Furthermore, the number and location of pedal scars in Palaeoneilo musculosa varies between individuals and the left and right valves in the same individual (Fig. 7C, D), reflecting the variation seen in Eotebenna danica. It is noteworthy that the early–middle Cambrian ancestral bivalves Fordilla Barrande, 1881, and Pojetaia Jell, 1980, do not show this multiplicity of pedal muscle scars at the umbo (Pojeta 1975, 2000; Runnegar and Bentley 1983; Elicki and Gürsu 2009).
Comparable swellings have not been observed in other areas of the shells of species of Eotebenna. The location of irregular raised patches at similar locations below the apex in some specimens of Eotebenna viviannae (indicated by arrows in Figs. 2A1, A4, 3E, F) may indicate attachment of tissue, but the evidence is inconclusive; they probably result just from exfoliation of the shell interior.
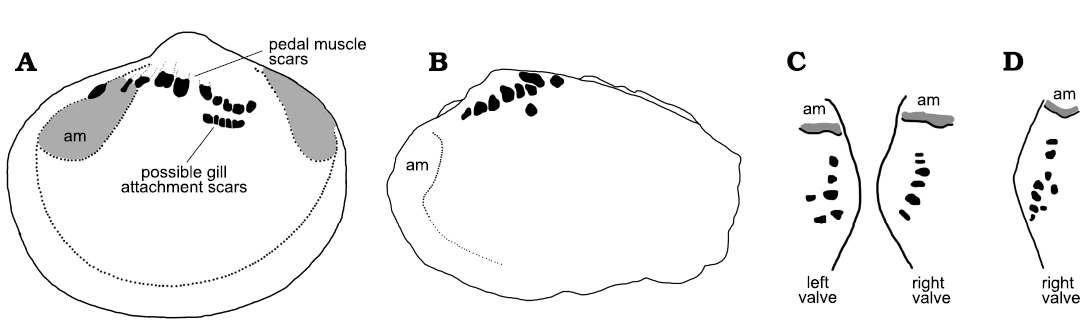
Fig. 7. Muscle scars on internal moulds of Palaeozoic bivalves. A. Left valve of Babinka Barrande, 1881, from the Lower Ordovician of Öland, Sweden (after Soot-Ryen 1969, length of specimen about 20 mm). B. Left valve of Palaeoneilo musculosa (Knod, 1908) from the Devonian of Bolivia (after Babin and Farjat 1994, length of specimens about 20 mm). C, D. Sketches in apical view showing asymmetry between pedal muscle scars (black) on internal molds of Palaeoneilo musculosa between left and right valves, and variation in pattern of pedal muscle between specimens (after Babin and Farjat 1994). Abbreviation: am, anterior adductor muscle scar.
Muscle scars in helcionelloids.—Muscle scars are well known in the early–middle Cambrian bivalves Fordilla and Pojetaia (Pojeta 1975, 2000; Runnegar and Bentley 1983) and have also been described in the problematic helically coiled univalves Aldanella Vostokova, 1962 (Gubanov and Peel 2000; Parkhaev 2006; Isakar and Peel 2007; Parkhaev and Karlova 2011; Dzik and Mazurek 2013) and Pelagiella (Runnegar 1981; Thomas et al. 2020; Landing et al. 2021; Peel and Kouchinsky 2022). Despite the abundance and diversity of helcionelloids, only a few records exist of preserved muscle scars from the early–middle Cambrian (Parkhaev 2001, 2002, 2014b; Ushatinskaya and Parkhaev 2005; Vendrasco et al. 2010; Li et al. 2019; Peel 2023; Fig. 8). Two different patterns of muscle scars in helcionelloids were recognized by Peel (2023), but the muscle scars described here in Eotebenna are unique on account of both their location and form. Given the small size of specimens, the question arises if the muscle scar patterns reflect musculature in the protoconch or the patterns seen in miniature adult forms. Studies of present day gastropods have demonstrated that different patterns occur during development and that there is not always continuity between the musculature of the protoconch and the teleoconch (Page 1997; Kristof et al. 2015). In the absence of precise information from the early fossil record, input is mainly restricted to shell morphology.
Smooth internal moulds of Bemella communis Parkhaev, 2001, from Cambrian Series 2 (lower Cambrian) in Australia preserve three pairs of muscle scars according to Parkhaev (2002, 2014b), with two pairs distributed as bands along the supra-apical (dorsal) surface (Fig. 8D). Li et al. (2021) described two pairs of muscle scars on internal moulds of Figurina figurina Parkhaev, 2001, from the Xinji Formation (Cambrian Series 2, Stages 3–4) of North China that were similar to those in Bemella communis, but considered that just a single pair of scars extended along the supra-apical surface of Bemella communis.
In Anhuiconus microtuberus, from Cambrian Series 2 (lower Cambrian) in Australia, Kouchinsky (2000a) and Parkhaev (2002) illustrated a single muscle scar on the sub-apical surface of the tightly coiled shell that passed beneath the apex from one lateral surface to the other (Fig. 8B) and a similar scar seems to be present in Securiconus sp. from Siberia (Ushatinskaya and Parkhaev 2005).
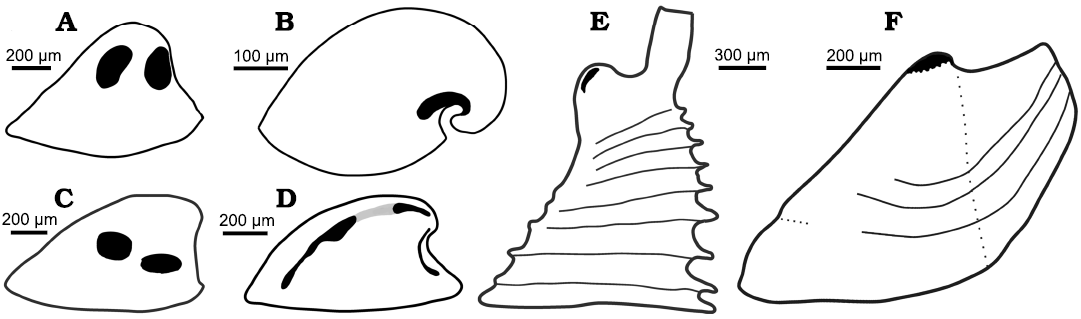
Fig. 8. Muscle scars on internal moulds of helcionelloids. All sketches oriented in lateral view with the apex to the right. A. Vendrascospira frykmani Peel and Kouchinsky, 2022 (after Peel 2023). B. Anhuiconus microtuberus Zhou and Xiao, 1984 (after Parkhaev 2002). C. Hensoniconus siku (Peel and Kouchinsky, 2022) (after Peel 2023). D. Bemella communis Parkhaev, 2001 showing three pairs of muscle scars (black, after Parkhaev 2014b); Li et al. (2021) considered the two pairs of scars on the supra-apical surface (joined by grey) to be traces of a single pair of scars. E. Yochelcionella (based on outline of Yochelcionella ostentata Runnegar and Jell, 1974) showing pair of apical muscle scars described by Vendrasco et al. (2010) in Yochelcionella snorkorum Vendrasco, Porter, Kouchinsky, Li, and Fernandez, 2010. F. Eotebenna danica sp. nov., with multiple scars forming a muscle attachment area at the apex.
Peel (2023) described two pairs of relatively large muscle attachment scars on the lateral surfaces of internal moulds of Vendrascospira frykmani and Hensoniconus siku from the Henson Gletscher Formation (Wuliuan, Miaolingian, Cambrian) of North Greenland (Fig. 8A, C). The muscle scars of the Greenland specimens display a myostracal texture of raised ridges around central depressions that are about 5 µm in diameter on the internal mould, but a similar polygonal pattern is not known in Eotebenna. The apical area of the cap-shaped Vendrascospira frykmani displays a polygonal pattern with low, raised, rounded centres and depressed surrounding margins on the internal mould (Peel 2023; Fig. 6), which superficially resembles the apical muscle scar attachment area of laterally compressed Eotebenna.
Vendrasco et al. (2010) described a pair of small, elongate muscle scars located on the supra-apical (dorsal) surface immediately adjacent to the apex in an internal mould of the tall, slender helcionelloid Yochelcionella snorkorum Vendrasco, Porter, Kouchinsky, Li, and Fernandez, 2010, from the Gowers Formation (Miaolingian, middle Cambrian) in Australia. The scars occur on the early growth stage (protoconch) prior to the development of the prominent comarginal rugae (Fig. 8E). While their location close to the apex is reminiscent of the muscle attachment scars in Eotebenna danica (Fig. 8F), only two large scars are present (length 100 µm) in Yochelcionella snorkorum compared to the approximately 25 smaller scars (up to 20 µm) in Eotebenna danica. Additionally, the muscle field in Eotebenna danica lies across the median dorsal plane of symmetry on the supra-apical surface, which is unique among known helcionelloid muscle scars. However, a prominent muscle scar on the supra-apical surface (dorsum), astride the median plane of symmetry, is characteristic of rostroconch univalve molluscs such as the late Cambrian–Silurian Ribeiria Sharpe, 1853, (Fig. 9), as illustrated by Pojeta and Runnegar (1976) and Polechova (2015). The placement of an additional sub-apical scar in Riberia recalls the muscle scar in Anhuiconus and Securiconus, but these helcionelloids lack the prominent transverse pegma that defines rostrococonchs. Ribeiria has a slender elongate shell that is similar in proportions to Eotebenna viviannae but its inferred orientation in life is sub-horizontal, paralleling that of most bivalves and in strong contrast to the nearly vertical orientation of Eotebenna (Peel 1991a, b). As noted by Peel (2006), and subsequently confirmed by anatomical study of present day scaphopods (Sigwart et al. 2016), Eotebenna and Yochelcionella essentially grow upwards from the plane of the aperture following the same adaptive trend subsequently realized in scaphopods. This is reflected in the location of their muscle scars at the apex of the narrow shells (Fig. 8E, F), in a similar position to the attachment scars in scaphopods (Simone 2009).
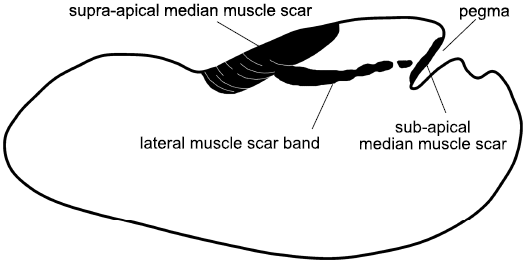
Fig. 9. Muscle scars on internal mould of the rostroconch Ribeiria Sharpe, 1853, in lateral view. Both the anterior and posterior median muscle scars lie across the median dorsal plane of symmetry, the former attached to the transverse pegma preserved as a deep cleft on the internal mould. The posterior scar is a uniform attachment area, often ornamented with transverse growth lines, unlike the multiple small scars of Eotebenna (based on Pojeta and Runnegar 1976; Polechova 2015).
Peel (2023) commented that the described variation in the patterns of helcionelloid muscle scars suggested that the existence of several distinct evolutionary lineages likely is obscured by the morphologically rather featureless, cap-shaped, limpet-like shells of most taxa. It is also relevant that several groups sharing similar shell form to the helcionelloids have been removed from the paraphyletic class, notably some groups of probable aculiferans (Vendrasco et al. 2009; Peel 2020a–c, 2021a). Recognition of the strongly coiled Protowenella Runnegar and Jell, 1976, as a hyolith and not a helcionelloid by Peel (2021c) was a particularly strong demonstration of the difficulty of assigning morphologically simple shells. It is important to recall that the pattern of muscle scars in univalve molluscs is dependent on their mechanical function and the morphology of the shell, as well as their systematic position (Bandel 1982; Harper and Rollins 1982, 2000; Peel 1991a, b; 2020a, b, 2023; Peel and Horný 1999).
Two groups of helcionelloids based on muscle scar patterns were recognized by Peel (2023), although muscle scars are not yet known in most helcionelloid taxa. In the Vendrascospira–Hensoniconus group, two pairs of large scars occur on the lateral areas (Fig. 8A, C). In the Bemella communis–Figurina figurina group, the dominant muscle scars are narrow elongate bands along the supra-apical surface (Fig. 8D). Securiconus sp. and Anhuiconus microtuberus (Fig. 8B) define a third group in which a single muscle scar crosses the median dorsal plane of symmetry on the sub-apical surface, below the apex; the presence of other muscle scars in these genera has not been established.
The location of muscle scars described here in Eotebenna danica and Eotebenna viviannae permits the recognition of a fourth group, together with Yochelcionella snorkorum, based on the grouping of the scars on the supra-apical surface at the apex (Fig. 8E, F). The similarity in position is not surprising since both genera are placed within the Family Yochelcionellidae but the detailed morphology of the known scar patterns in the two genera is different. The laterally compressed shell of Eotebenna danica shows similarities with species of Mellopegma Runnegar and Jell, 1976, some species of stenothecid helcionelloids, species of the tuarangiid Pseudomyona Runnegar, 1983, and some rostroconch species, suggesting a similar mode of life (Pojeta and Runnegar 1976; Vendrasco et al. 2011a; Peel 2021a).
The quandary facing malacologists is how these muscle patterns might be manifested from the ancestral helcionelloids in the pathways of early evolution of mollusc crown groups, if at all. Current information is insufficient to provide resolution to questions such as the suggestion by Waller (1998) that helcionelloids should be divided into an exogastric line leading to bivalves and the endogastric line proposed by Peel (1991a, b). While muscle scars are well known in the earliest bivalves Fordilla and Pojetaia (Pojeta 1975, 2000; Runnegar and Bentley 1983), they are not yet recognized in their supposed ancestral helcionelloids Watsonella Grabau, 1900, and Anabarella Vostokova, 1962. The upright shells of the Eotebenna–Yochelcionella lineage provide a parallel adaptation to the development of the scaphopods. The latter were likely derived from conocardiid rostroconchs (Engeser and Riedel 1996; Peel 2006), but these in turn are descendants of endogastric helcionelloids (Peel 2004, 2006). It is open to speculation as to what extent muscle emplacement on the supra-apical surface of helcionelloids might foreshadow the serialized dorsal muscle patterns in the exogastric tryblidiid and hypseloconid tergomyans since agreement is lacking even concerning the basic relative orientation of the shell in the two groups, with helcionelloids variously regarded as exogastric or endogastric, or a mixture of both (Runnegar and Pojeta 1974; Runnegar and Jell 1976; Peel 1991a, b; Geyer 1994; Waller 1998; Vendrasco 2012; Parkhaev 2017).
Conclusions
Muscle attachment scars are described on internal moulds of Eotebenna viviannae Peel 1991a and Eotebenna danica sp. nov. from the middle Cambrian (Guzhangian, Miaolingian) of Bornholm, Denmark. The cluster of small scars at the apex of the laterally compressed univalve shells is unique within helcionelloid molluscs. Comparison with described muscle scar patterns in other Cambrian helcionelloids suggests the existence of at least four lineages within the paraphyletic Class Helcionelloida, but recognition of their scope and relation to mollusc crown groups is obscured by the morphological simplicity of helcionelloid shells and the current paucity of information concerning musculature in the group as a whole.
Acknowledgements
Jerzy Dzik (Institute of Paleobiology PAS, Warszawa, Poland), Michael J. Vendrasco (Pasadena City College, USA) and the editors are thanked for constructive reviews of the manuscript.
References
Axheimer, N., Eriksson, M.E., Ahlberg, P., and Bengtsson, A. 2006. The middle Cambrian cosmopolitan key species Lejopyge laevigata and its biozone: new data from Sweden. Geological Magazine 143: 447–455. Crossref
Babin, C. 1977. Étude comparée des genres Babinka Barrande et Coxiconchia Babin (Mollusques Bivalves de l’Ordovicien) intérêt phylogénétique. Géobios 10: 51–79. Crossref
Babin, C. and Farjat, A.D. 1994. Unusual accessory muscle scar patterns in two species of Palaeoneilo, a paleotaxodont bivalve from the Devonian of Bolivia. Journal of Paleontology 68: 1041–1047. Crossref
Bandel, K. 1982. Morphologie und Bildung der frühontogenetischen Gehäuse bei conchiferan Mollusken. Facies 7: 1–198. Crossref
Barrande, J. 1881. Systême Silurien du centre de la Bohême, Vol. 6, Classe des Mollusques. Ordre des Acéphalés. 342 pp. Charles Bellmann, Paris.
Bengtson, S., Conway Morris, S., Cooper, B.J., Jell, P.A., and Runnegar, B.N. 1990. Early Cambrian fossils from South Australia. Memoirs of the Association of Australasian Palaeontologists 9: 1–364.
Berg-Madsen, V. 1985. Middle Cambrian biostratigraphy, fauna and facies in southern Baltoscandia. Acta Universitatis Upsaliensis, Abstracts of Uppsala Dissertations from the Faculty of Science 781: 1–37.
Cuvier, G. 1797. Tableau élementaire de l’historie naturelle des animaux. 710 pp. Baudouin, Paris. Crossref
Dong, W., Huang, J., Liu, C., Wang, H., Zhang, G., Xie, L., and Zhang, R. 2022. Characterization of the myostracum layers in molluscs reveals a conservative shell structure. Frontiers in Marine Science 9: 862929. Crossref
Driscoll, E.G. 1964. Accessory muscle scars, an aid to protobranch orientation. Journal of Paleontology 38: 61–66.
Dzik, J. and Mazurek, D. 2013. Affinities of the alleged earliest Cambrian gastropod Aldanella. Canadian Journal of Zoology 91: 914–923. Crossref
Elicki, O. and Gürsu, S. 2009. First record of Pojetaia runnegari Jell, 1980 and Fordilla Barrande, 1881 from the Middle East (Taurus Mountains, Turkey) and critical review of Cambrian bivalves. Paläontologische Zeitschrift 83: 267–291. Crossref
Engeser, T. and Riedel, F. 1996. The evolution of the Scaphopoda and its implications for the systematics of the Rostroconchia (Mollusca). Mitteilungen Geologische-Paläontologische Institut der Universität Hamburg 79: 117–138.
Etheridge, R. 1878. Notes on a few Silurian fossils from Ayrshire. Proceedings of the Royal Physical Society of Edinburgh 4: 167–176.
Geyer, G. 1994. Middle Cambrian molluscs from Idaho and early conchiferan evolution. New York State Museum Bulletin 481: 69–86.
Geyer, G. 2019. A comprehensive Cambrian correlation chart. Episodes 42 (4): 1–12. Crossref
Grabau, A.W. 1900. Palaeontology of the Cambrian terranes of the Boston basin. Occasional Papers of the Boston Society of Natural History 4: 601–694.
Gubanov, A.P. and Peel, J.S. 2000. Cambrian monoplacophoran molluscs (Class Helcionelloida). American Malacological Bulletin 15: 139–145.
Gubanov, A.P., Kouchinsky, A., Peel, J.S., and Bengtson, S. 2004. Middle Cambrian molluscs of “Australian” aspect from northern Siberia. Alcheringa 28: 1–20. Crossref
Hall, J. 1847. Palaeontology of New York, Vol. 1, Containing Descriptions of the Organic Remains of the Lower Division of the New York System (Equivalent to the Lower Silurian Rocks of Europe). 338 pp. Van Benthuysen, Albany.
Harper, J.A. and Rollins, H.B. 1982. Recognition of Gastropoda and Monoplacophora in the fossil record. Proceedings of the Third North American Paleontological Convention 1: 227–232.
Harper, J.A. and Rollins, H.B. 2000. The bellerophont controversy revisited. American Malacological Union 15: 147–156.
Hinz-Schallreuter, I. 1997. Leben im Kambrium – die Welt der Mikrofossilien. In: M. Zwanzig and H. Löser (eds.), Berliner Beiträge zur Geschiebeforschung, 5–23. Cpress Verlag, Dresden.
Horný, R.J. 1963. Lower Palaeozoic Monoplacophora and patellid Gastropoda (Mollusca) of Bohemia. Sborník Ústředniho Ústavu Geologickeho 28: 7–83.
Isakar, M. and Peel, J.S. 2007. Lower Cambrian helcionelloid molluscs from Estonia. GFF 129: 255–262. Crossref
Jell, P.A. 1980. Earliest known pelecypod on Earth—a new Early Cambrian genus from South Australia. Alcheringa 4: 233–239. Crossref
Knod, R. 1908. Devonische Faunen Boliviens. Neues Jahrbuch für Mineralogie, Geologie und Paläontologie, Beilage Band 25: 493–600.
Koken, E. and Perner, J. 1925. Die Gastropoden des baltischen Untersilurs. Mémoires de l’Académie des Sciences de Russie, Série. 8, Classe Physico-mathématique 37: 1–326.
Kouchinsky, A. 1999. Shell microstructures of the Early Cambrian Anabarella and Watsonella as new evidence on the origin of the Rostroconchia. Lethaia 32: 173–180. Crossref
Kouchinsky, A.V. 2000a. Shell microstructures in Early Cambrian molluscs. Acta Palaeontologica Polonica 45: 119–150.
Kouchinsky, A.V. 2000b. Skeletal microstructures of hyoliths from the Early Cambrian of Siberia. Alcheringa 24: 65–81. Crossref
Krasilova, I.N. 1977. Fordillids (Bivalvia) from the early Palaeozoic of the Siberian Platform [in Russian]. Paleontologičeskij žurnal 1987 (4): 24–30.
Kristof, A., de Oliveira, A.L., Kolbin, K.G., and Wanninger, A. 2015. Neuromuscular development in Patellogastropoda (Mollusca: Gastropoda) and its importance for reconstructing ancestral gastropod bodyplan features. Journal of Zoological Systematics and Evolutionary Research 54: 22–39. Crossref
Kříž, J. 1995. Coxiconchia Babin, 1966 from the Llanvirn of the Prague Basin (Bivalvia, Ordovician, Bohemia) and the function of some “accessoric” muscles in recent and fossil Bivalvia. Věstník Českého geologického ústavu 70: 45–50.
Landing, E., Geyer, G., Jirkov, I.A., and Schiaparelli, S. 2021. Lophotrochozoa in the Cambrian evolutionary radiation and the Pelagiella problem. Papers in Palaeontology 7: 2227–2244. Crossref
Lemche, H. 1957. A new living deep-sea mollusk of the Cambro-Devonian class Monoplacophora. Nature 179: 413–416. Crossref
Li, L., Betts, M.J., Yun, H., Pan, B., Topper, T.P., Li, G., Zhang, X., and Skovsted, C.B. 2023a. Fibrous or prismatic? A comparison of the lamello-fibrillar nacre in early Cambrian and modern lophotrochozoans. Biology 12 (113): 1–15. Crossref
Li, L., Topper, T.P., Betts, M.J., Dorjnamjaa, D., Altanshagai, G., Enkhbaatar, B., Li, G., and Skovsted, C.B. 2023b. Calcitic shells in the aragonite sea of the earliest Cambrian. Geology 51: 8–12. Crossref
Li, L., Zhang, X., Skovsted, C.B., Yun, H., Li, G., and Pan, B. 2019. Shell microstructures of the helcionelloid mollusc Anabarella australis from the lower Cambrian (Series 2) Xinji Formation of North China. Journal of Systematic Paleontology 17: 1699–1709. Crossref
Li, L., Zhang, X., Skovsted, C.B., Yun, H., Pan, B., and Li, G. 2021. Revisiting the molluscan fauna from the Cambrian (Series 2, stages 3–4) Xinji Formation of North China. Papers in Palaeontology 7: 521–564. Crossref
Lindberg, D.R. 2009. Monoplacophorans and the origin and relationships of mollusks. Evolution: Education and Outreach 2: 191–203. Crossref
Lindstrom, G. 1880. Fragmenta Silurica e dono Caroli Henrici Wegelin. 60 pp. Samson and Wallin, Stockholm.
Lindström, G. 1884. On the Silurian Gastropoda and Pteropoda of Gotland. Kongliga svenska Vetenskaps-Akademiens Handlingar 19 (6): 1–250. Crossref
McAlester, A.L. 1965. Systematics, affinities and life habits of Babinka, a transitional Ordovician lucinoid bivalve. Palaeontology 8: 231–246.
Missarzhevsky, V.V. [Missarževskij, V.V.] 1989. Oldest skeletal fossils and stratigraphy of Precambrian and Cambrian boundary beds [in Russian]. Trudy Geologičeskogo Instituta Akademii Nauk SSSR 443: 1–237.
Montfort, P.D. de 1808. Conchyliologie systématique, et classification méthodique des coquille; offrant leurs figures, leur arrangement générique, leurs descriptions caractéristiques, leurs noms; ainsi que leur synonymie en plusieurs langues. 2, Coquilles univalves, non cloisonnées. 409 pp. F. Schoell, Paris.
Nielsen, A.T. and Schovsbo, N.H. 2007. Cambrian to basal Ordovician lithostratigraphy in southern Scandinavia. Bulletin of the Geological Society of Denmark 53: 47–92. Crossref
Page, L.R. 1997. Larval shell muscles in the abalone Haliotis kamtschatkana. Biology Bulletin 193: 30–46. Crossref
Parkhaev, P.Y. 2001. The functional morphology of the Cambrian univalved mollusks—Helcionellids. Paleontological Journal 35: 470–475.
Parkhaev, P.Y. 2002. Phylogenesis and the system of the Cambrian univalved mollusks. Paleontological Journal 36: 25–36.
Parkhaev, P.Y. 2006. New data on the morphology of ancient gastropods of the genus Aldanella Vostokova, 1962 (Archaeobranchia, Pelagielliformes). Paleontological Journal 40: 244–252. Crossref
Parkhaev, P.Y. 2014a. Protoconch morphology and peculiarities of the early ontogeny of the Cambrian helcionelloid mollusks. Paleontological Journal 48: 369–379. Crossref
Parkhaev, P.Y. 2014b. Structure of shell muscles in the Cambrian gastropod genus Bemella (Gastropoda: Archaeobranchia: Helcionellidae). Paleontological Journal 48: 17–25. Crossref
Parkhaev, P.Y. 2017. On the position of Cambrian Archaeobranchians in the system of the Class Gastropoda. Paleontological Journal 51: 453–463. Crossref
Parkhaev, P.Y. and Karlova, G.A. 2011. Taxonomic revision and evolution of Cambrian mollusks of the genus Aldanella Vostokova, 1962 (Gastropoda: Archaeobranchia). Paleontological Journal 45: 1145–1205. Crossref
Peel, J.S. 1989. A Lower Cambrian Eotebenna (Mollusca) from Arctic North America. Canadian Journal of Earth Science 26: 1501–1503. Crossref
Peel, J.S. 1991a. Functional morphology of the Class Helcionelloida nov., and the early evolution of the Mollusca. In: A.M. Simonetta and S. Conway Morris (eds.), The Early Evolution of Metazoa and The Significance of Problematic Taxa, 157–177. Cambridge University Press, Cambridge.
Peel, J.S. 1991b. The Classes Tergomya and Helcionelloida, and early molluscan evolution. Bulletin Grønlands Geologiske Undersøgelse 161: 11–65. Crossref
Peel, J.S. 1993. Muscle scars and mode of life of Carinaropsis (Bellerophontoidea, Gastropoda) from the Ordovician of Tennessee. Journal of Paleontology 67: 528–534. Crossref
Peel, J.S. 2004. Pinnocaris and the origin of scaphopods. Acta Palaeontologica Polonica 49: 543–550.
Peel, J.S. 2006. Scaphopodization in Palaeozoic molluscs. Palaeontology 49: 1357–1364. Crossref
Peel, J.S. 2019. Muscle scars in euomphaline gastropods from the Ordovician of Baltica. Estonian Journal of Earth Sciences 68: 88–100. Crossref
Peel, J.S. 2020a. Muscle scars, mode of life and systematics of Pollicina (Mollusca) from the Ordovician of Baltica. Estonian Journal of Earth Sciences 69: 20–36. Crossref
Peel, J.S. 2020b. The Dala thumb: shell morphology and failed predation in Pollicina cyathina (Gastropoda) from the Ordovician of Dalarna, Sweden. GFF 142: 139–146. Crossref
Peel, J.S. 2020c. The oldest palaeoloricate mollusc (Cambrian Series 2, Stage 4; North Greenland) and its bearing on aculiferan evolution. Bulletin of Geosciences 95: 127–144. Crossref
Peel, J.S. 2021a. An outer shelf shelly fauna from Cambrian Series 2 (Stage 4) of North Greenland (Laurentia). Journal of Paleontology 95: 1–41. Crossref
Peel, J.S. 2021b. In-place operculum demonstrates that the middle Cambrian Protowenella is a hyolith and not a mollusc. Alcheringa 45: 385–394. Crossref
Peel, J.S. 2021c. Pseudomyona from the Cambrian of North Greenland (Laurentia) and the early evolution of bivalved molluscs. Bulletin of Geosciences 96: 195–215. Crossref
Peel, J.S. 2023. Muscle scars in Miaolingian helcionelloids from Laurentia and the diversity of muscle scar patterns in Cambrian univalve molluscs. Alcheringa [published online, https://doi.org/10.1080/03115518. 2023.2243501] Crossref
Peel, J.S. and Horný, R.J. 1999. Muscle scars and systematic position of the Ordovician limpets Archinacella and Barrandicella gen. nov. (Mollusca). Journal of the Czech Geological Society 44: 97–115.
Peel, J.S. and Kouchinsky, A. 2022. Middle Cambrian (Miaolingian Series, Wuliuan Stage) molluscs and mollusc-like microfossils from North Greenland (Laurentia). Bulletin of the Geological Society of Denmark 70: 69–104. Crossref
Pojeta J.P., Jr. 1975. Fordilla troyensis Barrande and early bivalve phylogeny. Bulletins of American Paleontology 63: 363–384.
Pojeta J.P., Jr. 2000. Cambrian Pelecypoda (Mollusca). American Malacological Bulletin 15: 157–166.
Pojeta J.P., Jr. and Runnegar, B. 1976. The paleontology of rostroconch mollusks and the early history of the phylum Mollusca. Professional Papers, US Geological Survey 968: 1–88. Crossref
Polechová, M. 2013. Bivalves from the Middle Ordovician Šárka Formation (Prague Basin, Czech Republic). Bulletin of Geosciences 88: 427–461. Crossref
Polechová, M. 2015. The youngest representatives of the genus Ribeiria Sharpe, 1853 from the late Katian of the Prague Basin (Bohemia). Estonian Journal of Earth Sciences 64: 84–90. Crossref
Ponder, W.F., Lindberg, D.R., and Ponder, J.M. 2020. Biology and evolution of the Mollusca, Volume 2. 870 pp. CRC Press, Boca Raton. Crossref
Rouault, R. 1851. Mémoires sur le terrain paléozoïque des environs de Rennes. Bulletin de la Société géologique de France 8: 358–399.
Runnegar, B. 1981. Muscle scars, shell form and torsion in Cambrian and Ordovician univalved molluscs. Lethaia 14: 311–322. Crossref
Runnegar, B. 1983. Molluscan phylogeny revisited. Memoir of the Association of Australasian Palaeontologists 1: 121–144.
Runnegar, B. 1985. Shell microstructures of Cambrian molluscs replicated by phosphate. Alcheringa 9: 245–257. Crossref
Runnegar, B. and Bentley, C. 1983. Anatomy, ecology and affinities of the Australian Early Cambrian bivalve Pojetaia runnegari Jell. Journal of Paleontology 57: 73–92.
Runnegar, B. and Jell, P.A. 1976. Australian Middle Cambrian molluscs and their bearing on early molluscan evolution. Alcheringa 1: 109–138. Crossref
Runnegar, B. and Pojeta, J., Jr. 1974. Molluscan phylogeny: the paleontological viewpoint. Science 186: 311–317. Crossref
Sharpe, D. 1853. Appendix B: Description of the new species of Zoophyta and Mollusca. In: C. Ribeiro (ed.), Carboniferous and Silurian Formations of the Neighborhood of Bussaco in Portugal. Quarterly Journal of the Geological Society of London 9: 146–158.
Sigwart, J.D., Sumner-Rooney, L.H., Dickey, J., and Carey, N. 2016. The scaphopod foot is ventral: more evidence from the anatomy of Rhabdus rectius (Carpenter, 1864) (Dentaliida: Rhabdidae). Molluscan Research 37: 79–87. Crossref
Simone, L.R.L. 2009. Comparative morphology among representatives of main taxa of Scaphopoda and basal protobranch Bivalvia (Mollusca). Papéis Avulsos de Zoologia (São Paulo) 49: 405–457. Crossref
Skawina, A. and Dzik, J, 2011. Umbonal musculature and relationships of the Late Triassic filibranch unionid bivalves. Zoological Journal of the Linnean Society 163: 863–883. Crossref
Skovsted, C.B. 2004. Mollusc fauna of the Early Cambrian Bastion Formation of North-East Greenland. Bulletin of the Geological Society of Denmark 51: 11–37. Crossref
Soot-Ryen, H. 1969. A new species of Babinka (Bivalvia) from the lower Ordovician of Öland, Sweden. Palaeontology 12: 173–177.
Thomas, R.D.K., Runnegar, B., and Matt, K. 2020. Pelagiella exigua, an early Cambrian stem gastropod with chaetae: lophotrochozoan heritage and conchiferan novelty. Palaeontology 63: 601–627. Crossref
Ushatinskaya, G.T. and Parkhaev, P.Y. 2005. Preservation of imprints and casts of cells of the outer mantle epithelium in the shells of Cambrian brachiopods, mollusks, and problematics. Palaeontological Journal 39: 251–263.
Vendrasco, M. 2012. Early evolution of molluscs. In: A. Fyodorov and H. Yakovlev (eds.), Mollusks: Morphology, Behaviour, and Ecology, 1–43. Nova Science, New York.
Vendrasco, M.J. and Checa, A.G. 2015 Shell microstructure and its inheritance in the calcitic helcionellid Mackinnonia. Estonian Journal of Earth Sciences 64: 99–104. Crossref
Vendrasco, M.J., Checa, A.G., and Heimbrock, W.P. 2019. Remarkable preservation of shell microstructures from the Late Ordovician of the Cincinnati Arch region, USA, and the success of nacre among Ordovician mollusks. Journal of Paleontology 93: 658–672. Crossref
Vendrasco, M.J., Checa, A.G., and Kouchinsky, A.V., 2011a. Shell microstructure of the early bivalve Pojetaia and the independent origin of nacre within the Mollusca. Palaeontology 54: 825–850. Crossref
Vendrasco, M.J., Kouchinsky, A.V., Porter, S.M., and Fernandez, C.Z. 2011b. Phylogeny and escalation in Mellopegma and other Cambrian molluscs: Palaeontologia Electronica 14: 1–44.
Vendrasco, M.J., Li, G., Porter, S.M., and Fernandez, C.Z. 2009. New data on the enigmatic Ocruranus–Eohalobia group of Early Cambrian small skeletal fossils. Palaeontology 52: 1373–1396. Crossref
Vendrasco, M.J., Porter, S.M., Kouchinsky, A., Li, G., and Fernandez, C.Z. 2010. New data on molluscs and their shell microstructures from the Middle Cambrian Gowers Formation, Australia. Palaeontology 53: 97–135. Crossref
Vendrasco, M.J., Rodríguez-Navarro, A.B., Checa, A.G., Devaere, L., and Porter, S.M., 2015. To infer the early evolution of mollusc shell microstructures. Key Engineering Materials 672: 113–133. Crossref
Vostokova, V.A. 1962. Cambrian gastropods from Siberia and Taymyr [in Russian]. Trudy Naučno-Issledovatel’skogo Instituta Geologii Arktiki 28: 51–74.
Waller, T.R. 1998. Origin of the molluscan class Bivalvia and a phylogeny of major groups. In: P.A. Johnston and J.W. Haggart (eds.), Bivalves—An Eon of Evolution, 1–45. University of Calgary Press, Calgary.
Weidner, T. and Nielsen, A.T. 2009. The Middle Cambrian Paradoxides paradoxissimus Superzone on Öland, Sweden. GFF 131: 253–268. Crossref
Weidner, T., Rushton, A.W.A., and Ebbestad, J.O.R. 2014. A paradoxidid–agnostoid fauna from the mid-Cambrian (Stage 5) of the Caledonian Lower Allochthon on Tåsjöberget, Ångermanland, Sweden. GFF 136: 513–530. Crossref
Westergård, A.H. 1946. Agnostidea of the middle Cambrian of Sweden. Sveriges Geologiska Undersökning Series C 477: 1–160.
Williams, A. and Wright, A.D. 1970. Shell structure of the Craniacea and other calcareous inarticulate brachiopods. Special Papers in Palaeontology 7: 15–51.
Wingstrand, K. 1985. On the anatomy and relationships of Recent Monoplacophora. Galathea Report 16: 7–94.
Winrow, P. and Sutton, M.D. 2012. Epithelial cell moulds in acrotretoid brachiopods. Historical Biology 24: 557–565. Crossref
Yochelson, E.L. 1988. A new genus of Patellacea (Gastropoda) from the Middle Ordovician of Utah: the oldest known example of the superfamily. New Mexico Bureau of Mines and Mineral Resources Memoir 44: 195–200.
Zhou, B. and Xiao, L. 1984. Early Cambrian monoplacophorans and gastropods from Huainan and Huoqiu counties, Anhui Province [in Chinese]. Professional Papers of Stratigraphy and Palaeontology, Chinese Academy of Geological Sciences 13: 125–140.
Acta Palaeontol. Pol. 68 (4): 625–638, 2023
https://doi.org/10.4202/app.01101.2023