


Early Tremadocian cephalopods from Santa Rosita Forma- tion in NW Argentina: the oldest record for South America
MARCELA CICHOWOLSKI, N. EMILIO VACCARI, ALEXANDER POHLE, DANIEL A. MORÓN ALFONSO, ROMAIN VAUCHER, and BEATRIZ G. WAISFELD
Cichowolski, M., Vaccari, N.E., Pohle, A., Morón Alfonso, D.A., Vaucher, R., and Waisfeld, B.G. 2023. Early Tremadocian cephalopods from Santa Rosita Formation in NW Argentina: the oldest record for South America. Acta Palaeontologica Polonica 68 (4): 583–601.
We describe early Tremadocian (Kainella meridionalis Biozone) cephalopods from the Cordillera Oriental, Jujuy, NW Argentina. They consist of numerous small specimens collected at the Quebrada de Arenal, Trancas section, near the town of Tilcara, in the Alfarcito Member of the Santa Rosita Formation. All but three specimens were assigned to a new species of Ellesmeroceras (Family Ellesmeroceratidae), E. humahuacaensis sp. nov., based on its slightly endogastric curvature, the characteristics of the siphuncle and chambers dimensions. Micro CT scanning of one specimen aided in the description of the apex and facilitated the construction of a 3D model of the species. A single, similar specimen was assigned to Ellesmeroceras sp. pending the availability of additional material. Two specimens differ from the rest, being exogastric with a lower angle of expansion. They are tentatively assigned to Bassleroceras sp. This material indicates that Cambrian and early Tremadocian cephalopods are not as different as previously thought. “Diversification” and “extinction” events during the late Cambrian may be attributed to taxonomic “over-splitting” and taphonomic and/or sampling biases, respectively. These specimens are currently the oldest recorded in the Central Andean Basin and of West Gondwana, and probably represent the first migration of cephalopods into the region, when the water column was still poorly colonized. During the middle Tremadocian, subsequent immigrations and originations of several cephalopod orders accounted for a rise in diversity and expansion into new niches during this interval. Some of these taxa persisted into the middle Floian, at which time, a second increase in diversity is recorded. Ellesmeroceras humahuacaensis sp. nov. is interpreted as a sub-vertical nektobenthic organism.
Key words: Cephalopoda, Ellesmeroceratidae, Ellesmeroceras, stem cephalopods, Tremadocian, Ordovician, Santa Rosita Formation, Cordillera Oriental.
Marcela Cichowolski [mcicho@gl.fcen.uba.ar; ORCID: https://orcid.org/0000-0002-9079-6350 ] and Daniel A. Morón Alfonso [paleokarzis@gmail.com; ORCID: https://orcid.org/0000-0002-8865-8370 ], Instituto de Estudios Andinos “Don Pablo Groeber” (IDEAN), Universidad de Buenos Aires-CONICET, Intendente Güiraldes 2160, Ciudad Universitaria-Pabellón II C1428EGA, CABA, Argentina.
N. Emilio Vaccari [evaccari@unc.edu.ar; ORCID: https://orcid.org/0000-0002-2965-1566 ] and Beatriz G. Waisfeld [bwaisfeld@unc.edu.ar; ORCID: https://orcid.org/0000-0001-9474-6855 ], Centro de Investigaciones de Ciencias de la Tierra (CICTERRA), Universidad Nacional de Córdoba-CONICET, Av. Vélez Sarsfield 1699, X5016GCB, Córdoba, Argentina.
Alexander Pohle [alexander.pohle@rub.de; ORCID: https://orcid.org/0000-0001-6979-1048 ], Institute of Geology, Mineralogy, and Geophysics, Ruhr University Bochum, Universitätsstrasse 150, 44801 Bochum, Germany.
Romain Vaucher [romain.vaucher88@gmail.com; ORCID: https://orcid.org/0000-0003-3051-4128 ], Department of Earth Sciences, University of Geneva, Rue des Maraîchers 13, 1205 Geneva, Switzerland; Institute of Earth Sciences (ISTE), University of Lausanne, Geopolis, CH-1015 Lausanne, Switzerland.
Received 24 August 2023, accepted 4 November 2023, available online 11 December 2023.
Copyright © 2023 M. Cichowolski et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Cephalopoda first appeared in the fossil record during the Cambrian. Plectronoceras cambria, from the late Cambrian of China, is considered the first unambiguous cephalopod (Kröger et al. 2011; Pohle et al. 2022; Landing et al. 2023). Following their appearance, late Cambrian and earliest Ordovician cephalopod occurrences were restricted to the palaeotropical realm (Kröger 2013; Fang et al. 2019). Although Furongian cephalopod faunas are particularly well-known in North and South China (Chen et al. 1979a, b; Chen and Qi 1982; Chen and Teichert 1983a, b; Li 1984), their taxonomy is in need of revision as they are likely to be “over-split” in species in the original publications (Dzik 1984; Holland 1987; Hewitt 1989; King and Evans 2019; Pohle and Jell 2019). Chinese Cambrian cephalopods are very abundant and diverse, particularly when compared to those from Laurentian regions (Flower 1954, 1964; Chen and Teichert 1983a; Landing and Kröger 2009). The assignment of Cambrian ages to cephalopods records described from Siberia and Kazakhstan by Korde (1949) and Malinovskaya (1964) are considered dubious (Landing and Kröger 2009; Fang et al. 2019). However, Dzik (2020) reported several specimens from Siberia that he considered conspecific with Korde’s (1949) material and assigned them to the latest Furongian. Additionally, Pohle and Jell (2019) reported the existence of a rich and numerous latest Cambrian–earliest Ordovician cephalopod fauna from Australia, collected but not described by Mary Wade during the 1980s (mentioned in Wade 1988; Wade and Stait 1998). This collection fills the gap that previously existed in the fossil record of cephalopods, as since they are almost completely absent from uppermost Cambrian rocks worldwide (“the Trempealeauan eclipse” of Chen and Teichert 1983a). Australian cephalopods are from that age, extending into the earliest Ordovician (Pohle and Jell 2019). See a schematic illustration in Fig. 1.

Fig. 1. Cambrian and early Tremadocian cephalopod records from different localities within the paleotropical belt (compiled from literature). Abbreviations: Fm., formation; Mb., Member.
The orders Plectronocerida, Protactinocerida, and Yanhecerida are known exclusively from the Furongian (late Cambrian). Protactinocerida is likely synonymous with Plectronocerida, and perceived differences between them are mainly an artefact of the oblique sections used in the study of this material (Wade 1988; Wade and Stait 1998; Pohle and Jell 2019; Mutvei 2020; Pohle et al. 2022). The order Ellesmerocerida first appears in the upper Cambrian (basal part of Stage 10) and is the only group to cross the Cambrian/Ordovician boundary represented by the genera Ectenolites and Clarkoceras (Chen and Teichert 1983a; Kröger 2013; Fang et al. 2019).
Earliest Ordovician ellesmeroceratids have been reported mainly from Laurentia (Ulrich et al. 1944; Unklesbay 1954; Unklesbay and Young 1956; Flower 1964; Kröger and Landing 2007) but also China (Chen and Teichert 1983a), Tarim (Troedsson 1937), possibly from Kazakhstan (Chen and Teichert 1983b), and Australia (Wade and Stait 1998; Pohle and Jell 2019). In every case, the earliest Tremadocian cephalopods are found in carbonatic, often thrombolitic facies (Kröger et al. 2009). Hence, the cephalopod record from the Cordillera Oriental is the first from early Tremadocian siliciclastic facies from west Gondwana.
While lower Tremadocian strata in this basin (Kainella meridionalis Trilobite Biozone, Tr1; Fig. 2) are widespread and have been intensely studied for their palaeontological content (e.g., Harrington and Leanza 1957; Meroi Arcerito et al. 2015; Benedetto and Carrasco 2002; Vaccari and Waisfeld 2010; Balseiro et al. 2011; Mángano et al. 2013; Tortello et al. 2013, Zeballo et al. 2013; Benedetto and Muñoz 2015; Serra et al. 2021), the presence of cephalopods was, hitherto, unknown. Cecioni (1965) reported some species that would have been collected from the early Tremadocian “Chañarcito Limestones” of Harrington and Leanza (1957), on the right margin of the Purmamarca River, 4 km upstream of Purmamarca town. However, a lack of coincidence exists between the geographical location and the stratigraphical age and formation to which Cecioni’s (1965) material has been assigned. The Chañarcito Formation crops out near Purmamarca railway station (Harrington and Leanza 1957), and far from the Purmamarca River locality. Furthermore, no cephalopods have been found in these horizons, which are not limestone but essentially black shale beds (Harrington and Leanza 1957). Based both on the lithology and the geographical location, it is most probable that Cecioni’s (1965) species come from the Coquena Formation, and are associated both with the Asaphellus nazarenensis and Notopeltis ortomethopa trilobite biozones, indicative of middle Tremadocian age (e.g., Tortello et al. 2016; Meroi Arcerito et al. 2018). No earliest Tremadocian cephalopods have been previously reported from South America.
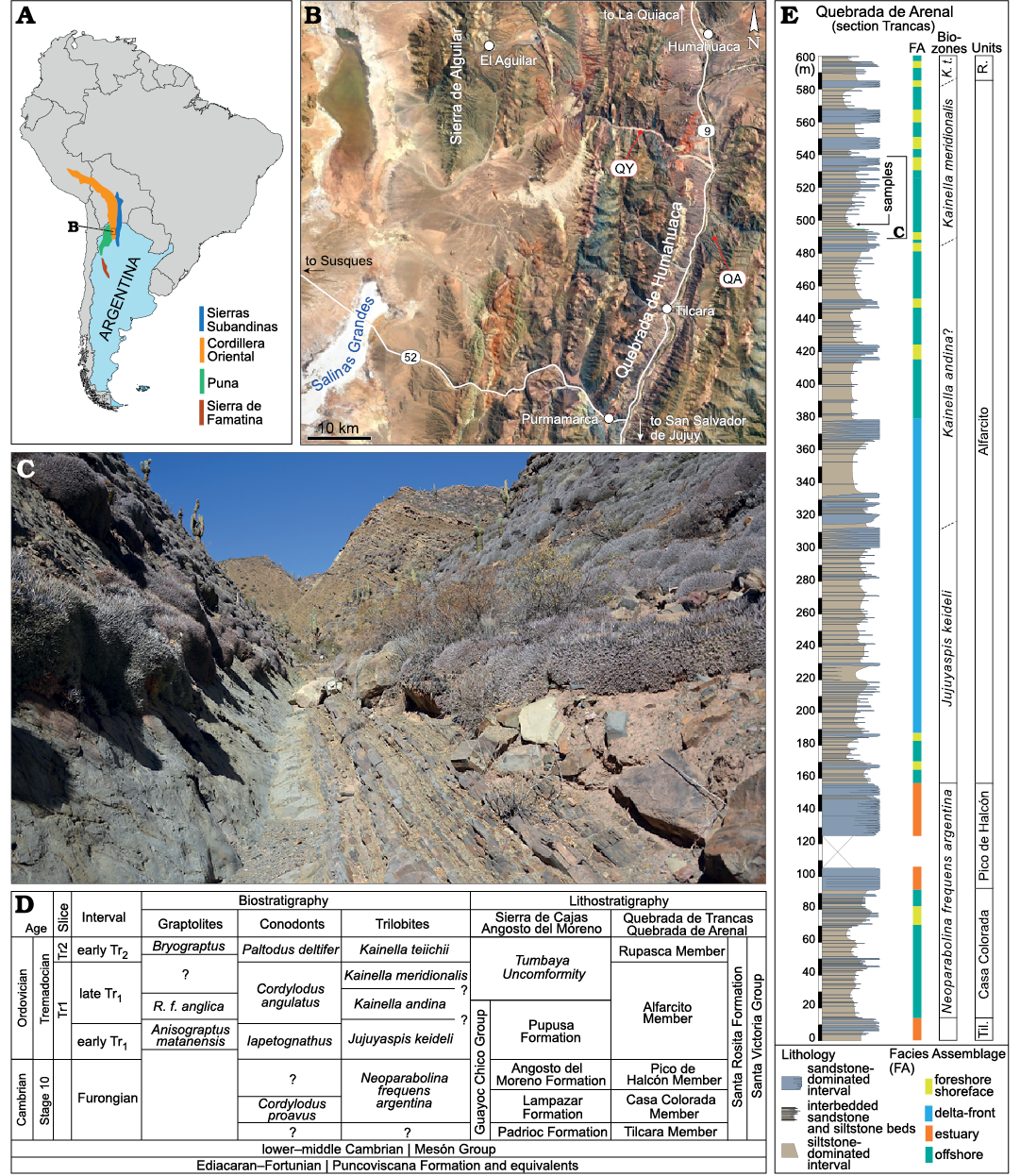
Fig. 2. A. Map of South America showing the main Paleozoic geological provinces of NW Argentina. B. Map of the study area (Jujuy Province, Argentina). The satellite image from Google Earth. QA, Quebrada de Arenal (-23.474677, -65.337646); QY, Quebrada de Yacoraite (-23.3321972, -65.457527). C. General view of the outcrop where the specimens were collected along the Quebrada de Arenal. This interval is characterized by sandstone interbedded with a general interval of siltstone. D. Regional biostratigraphy and lithostratigraphy correlated with the map intervals used. Modified and updated from Balseiro and Waisfeld (2013) and Vaucher et al. (2020). R. f. anglica, Rhabdinopora flabelliformis anglica. E. Stratigraphic section of the Quebrada de Arenal with the main facies assemblages (FA) that defined the section. Modified from Vaucher et al. (2020). The position where the samples were collected is indicated and belongs to Kainella merdionalis Trilobite Biozon. Til., Tilcara Member; R., Ruspaca Member; K. t., Kainella teiichii.
Cephalopod occurrences of this age are rare globally and usually consist exclusively of ellesmeroceratids (Kröger and Zhang 2009). Here, we report the presence of more than 400 specimens of cephalopods mostly preserved in a single lenticular shell bed (gutter cast) at Quebrada de Arenal, Trancas section (Alfarcito Member, Santa Rosita Formation, Cordillera Oriental; Fig. 2). Most of the specimens are assigned to the family Ellesmeroceratidae Kobayashi, 1934.
Institutional abbreviations.—CEGH-UNC, Cátedra de Estratigrafía y Geología Histórica, Universidad Nacional de Córdoba, Córdoba, Argentina; CPBA, Colección de Paleontología de la Facultad de Ciencias Exactas y Naturales de la Universidad de Buenos Aires, Argentina.
Other abbreviations.—RCL, relative cameral length; SCI, septal concavity index.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: lsid:zoobank.org:pub:DAE0282E-6CB3-49F2-BBFE- B5421EF76B2F.
Geological setting
The Santa Rosita Formation is superbly and extensively exposed in the Cordillera Oriental, northwest Argentina (provinces of Salta and Jujuy; Fig. 2). It was deposited during the Furongian (Age 10) and the early Early Ordovician (Tremadocian; Tr1–Tr2) (e.g., Astini 2003; Buatois et al. 2006; Vaucher et al. 2020). This formation is subdivided into six members, from base to top: Tilcara Member (Furongian), Casa Colorada Member (Furongian), Pico de Halcón Member (Furongian), Alfarcito Member (Tr1), Rupasca Member (Tr2), and Humacha Member (Tr2) (Fig. 2). These members record a complex depositional history, including fluvial, tide-dominated estuarine, river- and wave-dominated shallow-marine environments (Astini 2003; Buatois and Mángano 2003; Buatois et al. 2006; Vaucher et al. 2020). More specifically, the deposition of the Aflarcito Member was initiated in a river-dominated shallow-marine environment (Jujuyaspis keideli Biozone) and gradually evolved (during the Kainella andina Biozone) into a wave-dominated shallow-marine environment (Vaucher et al. 2020). The cephalopod remains were found in the upper part of the Alfarcito Member (Kainella meridionalis Biozone). This interval consists of siltstone interbedded with hummocky to micro hummocky cross-stratified, very fine- to fine-grained sandstone. The upper part of the Alfarcito Member was deposited below the fair-weather wave base in open marine upper offshore environments, reflecting low-energy suspension fall-out conditions punctuated by storm events (Vaucher et al. 2020). The shell bed bearing the cephalopods occurs as the infill of an erosive structure (gutter cast) within a fine-grained siltstone package. The assemblage present in the infill exhibits moderate diversity and is dominated by cephalopods associated with trilobite remains, as well as subordinate brachiopods, large gastropods, and bivalves. The shell concentration is matrix-supported with only occasional contacts among shells. The matrix, consisting of siltstone to very fine-grained sandstone bears fragmented bioclastic debris. The internal structure is largely simple, with the bioclasts arranged roughly parallel to the base of the shell bed, with no preferred orientation of the clasts.
The co-occurrence of bioclasts of different fossil groups with such different morphologies suggests low shape selection. Size selection is also low, with sizes ranging from less than one mm to ca. 30 mm. Fragmentation is low, in the case of the cephalopods, mainly affecting the apical and adoral regions. The fragmented bioclasts associated with well-preserved remains of different shapes and preservation potentials (e.g., trilobites, cephalopods) suggest a mixture of allochthonous and para-autochthonous material that was accumulated and buried by a storm event. Despite previous interpretations of intermediate palaeolatitudes for the NW Argentina Basin during the early Palaeozoic (e.g., Astini 2003), recently, its position during the Early Ordovician was estimated near 24 ± 10°S (Spagnuolo et al. 2012), and near 30±4°S (Torsvik et al. 2012; Cocks and Torsvik 2021). This paleolatitude corresponds to the edge of the palaeotropical belt and records the oldest cephalopod fauna outward of the coetaneous carbonate settings.
Material and methods
The studied specimens come from the Early Ordovician Alfarcito Member of the Santa Rosita Formation (Fig. 2). Apart from one specimen, they were collected mainly at the Quebrada de Arenal locality, Trancas section (Fig. 2), near the village of Tilcara. One specimen, collected previously, came from the Quebrada de Yacoraite (Fig. 2), also in the Department of Tilcara, Jujuy. Except for the specimen from the Quebrada de Yacoraite, the material collected and described herein is housed at the Centro de Investigaciones en Ciencias de la Tierra (CICTERRA) with the prefix CEGH-UNC. The remaining specimen is deposited in the Colección de Paleontología de la Facultad de Ciencias Exactas y Naturales de la Universidad de Buenos Aires, with the prefix CPBA. There are ca. 460 specimens of cephalopods distributed along 22 fragments of rock of different sizes from the same gutter cast, within the Kainella meridionalis Biozone (Vaucher et al. 2020; Fig. 2E). One fragment was heated to make it more susceptible to fracturing and then it was broken up to reveal more specimens. Some of the resulting fragments correspond to each other as a result of this process. Specimens were counted from each rock fragment using a binocular microscope and the relative location of each individual was recorded. Multiple counting of specimens is unavoidable as many of the fragments contain part and counterpart of the same specimen. The final number is thus an approximation. For each fragment of rock, those specimens that could be measured and/or illustrated were assigned a letter in order to differentiate between them. Those specimens that could not be measured or were not illustrated take the number of the sample in which they are embedded. In the case of the measurements of the siphuncle diameter (Table 1), where the section measured corresponds to a specimen that is not in the table of measurements (Table 2) or the plates and has no assigned letter, we append “-” or “--” to indicate that this is the case.
Table 1. Ratio between siphuncle diameter and conch diameter versus conch diameter, taken of the available cross sections in different specimens. In bold indicated identical measures in different individuals, where the section measured correspondes to a specimen that is not in Table 2 or the plates and therefore it has no corresponded letter, we append “-” or “--” after specimen number to differentiate between measures of the same sample rock, avoiding that the specimens from the same sample are taken as the same ones.
|
Specimen number |
CD |
DS/CD |
|
27496a |
1.2 |
0.25 |
|
27479- |
1.5 |
0.4 |
|
27490- -- |
1.5 |
0.46 |
|
27484b |
1.9 |
0.31 |
|
27494b |
2 |
0.35 |
|
27495b |
2 |
0.25 |
|
27479b |
2.2 |
0.22 |
|
27494- |
2.2 |
0.45 |
|
27479-- |
2.3 |
0.3 |
|
27485-- |
2.4 |
0.25 |
|
27486- |
2.4 |
0.25 |
|
27479c |
2.5 |
0.32 |
|
27484- |
2.5 |
0.32 |
|
27484-- |
2.5 |
0.32 |
|
27490-- |
2.5 |
0.32 |
|
27495a |
2.5 |
0.32 |
|
27482- |
2.6 |
0.27 |
|
27485- |
2.8 |
0.25 |
|
27490- |
2.8 |
0.25 |
|
27489b |
2.8 |
0.28 |
|
27489- |
2.8 |
0.35 |
|
27491a |
2.8 |
0.35 |
|
27481b |
3 |
0.2 |
|
27480-- |
3 |
0.23 |
|
27497- |
3 |
0.26 |
|
27483- |
3 |
0.33 |
|
27484a |
3 |
0.33 |
|
27499- |
3 |
0.33 |
|
27482-- |
3.2 |
0.22 |
|
27485a |
3.2 |
0.25 |
|
27483a |
3.4 |
0.29 |
|
27491d |
3.4 |
0.29 |
|
27489-- |
3.5 |
0.23 |
|
27491- |
4 |
0.35 |
|
27496b |
4 |
0.35 |
|
Average |
2.64 |
0.29 |
|
Mean |
2.67 |
0.3 |
|
Standard deviation |
0.64 |
0.06 |
|
Median |
2.8 |
0.3 |
The conchs are preserved in random orientations, with variously oriented sections, i.e., transverse, oblique or longitudinal (Fig. 3). Some of the conchs were prepared using pneumatic air scribes and needles under a binocular microscope until a significant part of the shell was visible. The samples were photographed both dry and underwater to increase contrast. They were photographed using a Canon Power Shot S50 digital camera mounted on a Leica MZ75 binocular microscope. The most complete specimens were coated with ammonium chloride to enhance contrast. We measured as many specimens as possible using a digital caliper with a resolution of 0.1 mm (Table 2). Most character definitions follow Pohle et al. (2022: see supplementary information).
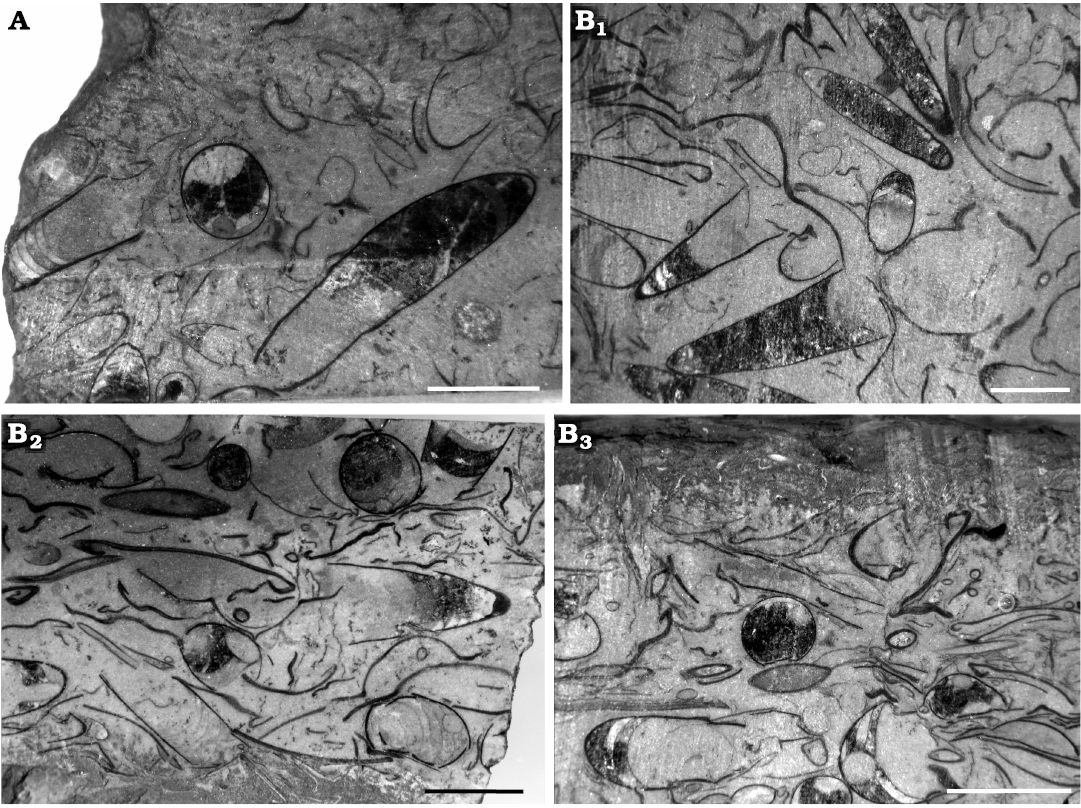
Fig. 3. Polished sections of samples from the lower Tremadocian (Ordovician) of Quebrada de Arenal, Trancas section, Cordillera Oriental, Jujuy, Argentina, showing the random orientation of the conchs of Ellesmeroceras humahuacaensis sp. nov. A. CEGH-UNC 27489, showing diagonal and transverse views of specimens. B. CEGH-UNC 27491. B1, several cephalopods tangentially cut, and fragments of shell material. B2, some almost transverse and oblique sections of cephalopods and a variety of undetermined shell material. B3, transverse and sagittal cuts of cephalopods and a variety of randomly oriented shell material. Scale bars 5 mm.
Micro CT data were collected from CEGH-UNC 27495 using a SkyScan 1272 scanner at the Facultad de Odontología, Universidad de Buenos Aires (Argentina), with scanning parameters: 90 kV voltage, 111 µA current, and 2268 ms exposure time. The tomographic data set (i.e., volume) obtained has the following dimensions (X = 1333, Y = 896, Z = 1344 slides), with a resolution of 19.81 µm isotropic voxel size, and it is housed at the Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires (Buenos Aires, Argentina). Examination of this volume showed that the sample contains abundant fragmentary shell material and at least three specimens preserved as 3D structures. For a more detailed evaluation, the latter specimens were isolated into three separate sub-volumes. These volumes were transformed and reoriented using 3D grids to obtain optimal planes for their measurement. For this process, we used 3D Slicer (Fedorov et al. 2012). Originally, the micro-CT scanning was performed to examine the nature of the connecting rings of the specimen CEGH-UNC 27495b, since in external view the siphuncle showed a cyrtocerinid aspect. However, the connecting rings were shown to be thin and did not expand inward as initially thought. Therefore, we focused on CEGH-UNC 27495a which is rather well preserved, and although it lacks the living chamber, exposes what seems to be at least part of the embryonic shell. Further virtual models were generated using Blender 2.81 (Blender Online Community, 2022) based on the visualization of the specimens from the original tomographic dataset (Fig. 4). Direct reconstruction of a specific specimen was unviable due to poor contrast and lack of continuity between the structures (i.e., variable diagenetic alteration within the specimens). The virtual model was oriented vertically based on the results of Peterman et al. (2019).
Table 2. Measurements of Ellesmeroceras humahuacaensis sp. nov. LF, length of fragment; Dv Diaor, dorsoventral diameter at adoralmost end; Lat. Diaor, lateral diameter at adoralmost end; Dv Diaap, dorsoventral diameter at adapicalmost end; Lat. Diaap, lateral diameter at adapicalmost end; Si. Dia., siphuncle diameter (ratio of the siphuncle diameter to the conch diameter, expressed as a percentage); L. Cham., length of the chambers, measured as the distance between two adjacent suture lines. Usually, a range of measures is considered (a maximum and a minimum value); Cham./Conch. Dia, number of camerae within a distance equivalent to the maximum preserved conch diameter; L. Li. Cham., length of the preserved portion of the living chamber; W Li. Cham b: width of the living chamber at its base; L. Phrag., length of the preserved portion of phragmocone; Dv Exp., dorsoventral expansion rate [rate of expansion of the conch in the dorsoventral plane, calculated as (Dv Dia. 1 - Dv Dia. 2)/distance between 1 and 2]; AA dv, apical angle based on dorsoventral expansion rate (arctangents of the expansion rate); Lat. Exp., lateral expansion rate, calculated in the same form as for the Dv Exp. but using Lat. Dia.); AA lat., Apical angle based on lateral expansion rate; LM, distance over which apical angle is measured (i.e. distance between diameter 1 and 2); SCI, septal concavity index (ratio of the distance between the plane of the suture and the culmination of the septum to the diameter of the phragmocone); RCL., relative cameral length (ratio of the distance between adjacent sutures and the conch diameter). * approximate measures due to bad preservation or breakage; ** measured in the adapical end of the straight part; *** measure taken considering only the straight part of the conch.
|
Specimen number |
LF (mm) |
Dv Diaor (mm) |
Lat. Diaor (mm) |
Dv Diaap (mm) |
Lat. Diaap (mm) |
Si. Dia. (%) |
L. Cham. (mm) |
Cham./Conch Dia |
L. Li. Cham. (mm) |
W Li. Cham b |
L. Phrag. (mm) |
Dv Exp. |
AA dv (°) |
Lat. Exp. |
AA lat. (°) |
LM (mm) |
SCI |
RCL |
|
CEGH-UNC 27479 a |
9.4 |
|
3.4* |
|
2.4* |
|
0.5 |
6.8 |
5 |
2.8 |
4.4 |
|
|
0.1 |
5.7 |
9 |
|
0.15 |
|
CEGH-UNC 27479 b |
4.6 |
|
|
2.2 |
2.4 |
22 |
0.5 |
|
0 |
|
4.6 |
|
|
|
|
|
|
|
|
CEGH-UNC 27479 c |
10 |
|
|
2.5 |
|
32 |
0.5 |
|
4 |
|
6 |
|
|
|
|
|
|
|
|
CEGH-UNC 27480 b |
10 |
|
3.3 |
|
2 |
|
0.4 |
|
4 |
|
6 |
|
|
0.13 |
|
|
|
|
|
CEGH-UNC 27481 b |
3.7 |
|
|
3 |
|
20 |
0.4 |
8.25 |
0 |
|
3.7 |
|
|
|
|
|
0.26 |
|
|
CEGH-UNC 27483 a |
5 |
3.4 |
|
|
|
29 |
0.6 |
|
0 |
|
5 |
|
|
0.1 |
5.6 |
|
0.28 |
0.17 |
|
CEGH-UNC 27483 b |
6.5 |
|
|
|
|
|
0.4 |
|
2.3 |
2.2 |
4.2 |
|
|
|
|
|
0.27 |
0.2 |
|
CEGH-UNC 27484 a |
9 |
|
|
3 |
|
33 |
0.4 |
|
4 |
2.7 |
5 |
|
|
|
|
|
|
0.13 |
|
CEGH-UNC 27485 a |
7.4 |
3.6 |
|
2.8 |
|
25 |
0.5 |
7.2 |
4.2 |
3.2 |
3.2 |
0.1 |
5.7 |
|
|
|
|
0.14 |
|
CEGH-UNC 27485 b |
7.4 |
|
2.6 |
|
2 |
|
0.4* |
|
|
|
|
|
|
0.15 |
8.5 |
4 |
|
0.15 |
|
CEGH-UNC 27487 a |
13.5 |
|
3.5 |
|
2.2 |
|
0.5 |
7 |
6 |
3.2 |
7.5 |
|
|
0.14 |
8 |
9 |
|
0.16 |
|
CEGH-UNC 27488 a |
7.5 |
|
2.5 |
|
2.1 |
|
|
|
7.5 |
|
0 |
|
|
0.05 |
2.9 |
|
0.33 |
0.14 |
|
CEGH-UNC 27488 b |
11.5 |
|
3.3* |
|
1.5 |
|
0.5 |
|
4 |
2.8 |
7.5 |
|
|
0.18 |
10 |
7 |
|
0.18 |
|
CEGH-UNC 27490 a |
17* |
|
3 |
|
2 |
|
0.5 |
6 |
5* |
2.9 |
12* |
|
|
0.16 |
9 |
13.2 |
0.3 |
0.16 |
|
CEGH-UNC 27491 a |
9 |
3.2 |
|
2.7 |
|
35 |
0.5 |
6.4 |
|
|
|
0.09 |
5.7 |
|
|
4.5 |
|
0.15 |
|
CEGH-UNC 27491 b |
13 |
|
3 |
|
1.8 |
|
0.5 |
6 |
5 |
2.8 |
8 |
|
|
0.08 |
5 |
|
|
0.15 |
|
CEGH-UNC 27491 c |
7.5 |
3 |
|
2 |
|
|
|
|
|
|
|
0.13 |
7.4 |
|
|
7.5 |
|
|
|
CEGH-UNC 27491 d |
10.5 |
3.4 |
|
2.2 |
|
29 |
0.5 |
6.8 |
4.6 |
3 |
5.9 |
0.11 |
6.3 |
|
|
|
|
0.14 |
|
CEGH-UNC 27494 a |
13 |
3 |
|
1.8** |
|
|
0.4 |
7.5 |
3.5 |
3 |
9.5 |
0.12 |
6.8*** |
|
|
10 |
|
0.13 |
|
CEGH-UNC 27494 b |
9.5 |
3.5* |
|
2 |
|
35 |
0.4 |
8.7 |
5.5 |
2.8 |
4 |
0.15* |
8.5 |
|
|
9.5 |
|
0.11 |
|
CEGH-UNC 27494 c |
10 |
|
2.5 |
|
0.5 |
|
0.5 |
5 |
3 |
2.4 |
7 |
|
|
0.2 |
11 |
10 |
0.22 |
0.2 |
|
CEGH-UNC 27494 d |
14 |
|
3.2 |
|
0.7 |
|
0.6 |
5.3 |
5.5 |
2.8 |
8.5 |
|
|
0.18 |
10 |
14 |
|
0.18 |
|
CEGH-UNC 27495 b |
7 |
|
2.7 |
|
2 |
25 |
0.4 |
6.7 |
0 |
|
7 |
|
|
0.1 |
5.7 |
7 |
|
0.15 |
|
CEGH-UNC 27496 a |
8.6 |
|
2.4 |
|
1 |
|
0.4–0.5 |
5.3 |
2.7 |
2.8 |
5.9 |
|
|
0.16 |
9 |
8.6 |
|
0.18 |
|
CEGH-UNC 27500 a |
8 |
|
3 |
|
2 |
|
0.4 |
7.5 |
2.7 |
|
5.3 |
|
|
0.12 |
7 |
8 |
|
0.13 |
|
CEGH-UNC 27501 a |
14 |
4.8 |
|
1.6/2.5** |
|
|
0.4–0.6 |
9.6 |
3.3 |
4.3 |
10.7 |
0.22/0.23*** |
12/13*** |
|
|
14/10*** |
|
0.1 |
|
Average |
9.18 |
3.4 |
2.8 |
2.48 |
1.7 |
28.5 |
0.47 |
6.87 |
3.49 |
2.9 |
5.85 |
0.11 |
6.7 |
0.13 |
7.49 |
8.6 |
0.27 |
0.15 |
Systematic palaeontology
The systematic and phylogenetic relationships within early cephalopods have been actively discussed in recent years and continue to be so. There is an ongoing project regarding the revised version of the Treatise on Invertebrate Paleontology Part K, Nautiloidea (King and Evans 2019), and several proposals on fossil cephalopod systematics have been recently published (e.g., King and Evans 2019; Pohle et al. 2022; Hoffmann et al. 2022). Pohle et al. (2022) proposed a Bayesian methods-based phylogeny that will be useful as the base of the new classification scheme. Here we follow the scheme proposed by Pohle et al. (2022: table 2), but see also discussions in Mutvei (2015) and King and Evans (2019).
Class Cephalopoda Cuvier, 1797
Order Ellesmerocerida Flower in Flower and Kummel, 1950
Family Ellesmeroceratidae Kobayashi, 1934
Remarks.—In terms of the number of described genera, the Ellesmeroceratidae is likely the top family among early Palaeozoic cephalopods; probably because of its status as a wastebasket taxon, to which many early cephalopods were assigned (Pohle et al. 2022). As already indicated and discussed in previous studies (Flower 1964; Kröger and Landing 2007; Evans 2011), it is an arduous task to differentiate among the genera of the family, especially those that are “simpler”, or less “specialized” (sensu Evans 2011), due to the gradational boundaries between them in terms of rate of expansion and degree of curvature (see Flower 1964: fig. 8; Evans 2011: text-fig. 4). Following the analysis by Pohle et al. (2022: fig. 2), the family is most probably para or polyphyletic, although generally, ellesmeroceratid phylogenetic relationships are volatile and contain large uncertainties (Pohle et al. 2022).
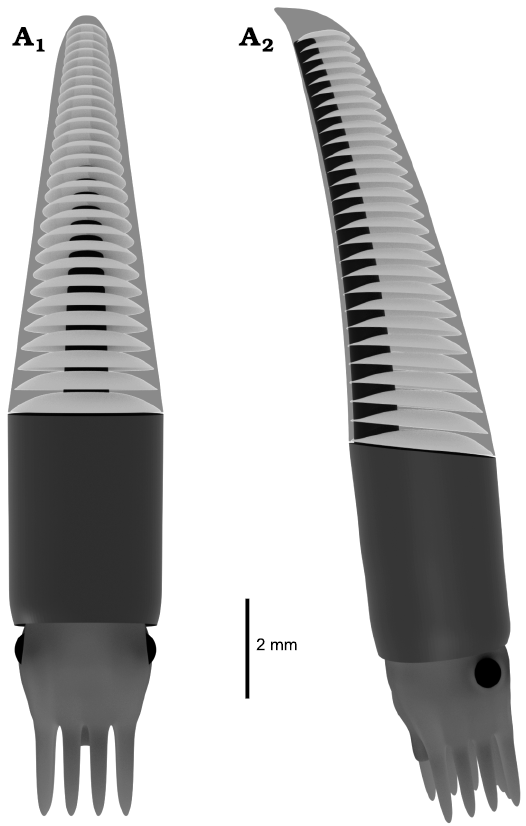
Fig. 4. Virtual reconstruction of the ellesmeroceratid cephalopod Ellesmeroceras humahuacaensis sp. nov. from the lower Tremadocian, Lower Ordovician of Quebrada de Arenal, Trancas section, Cordillera Oriental, Jujuy, Argentina based on composite data of the CT scan analysis of sample CEGH-UNC 27495 (three specimens), in dorsal (A1) and lateral (A2) views.
Despite the previous statements, on the basis of their general morphology and siphuncle characteristics, all the specimens (except two) from the Alfarcito Member of the Santa Rosita Formation are here assigned to the Family Ellesmeroceratidae. The slightly endogastrically curved apical conch, gently undulating suture, marginal, relatively large siphuncle with loxochoanitic septal necks and thick, straight connecting rings, combined with the very short phragmocone chambers support this assignment (Chen and Teichert 1983b; Kröger and Landing 2007). However, due to the considerations above, the generic assignment is more elusive.
Genus Ellesmeroceras Foerste, 1921
Type species: Ellesmeroceras scheii Foerste, 1921, from the Lower Ordovician at Victoria Head, Bache Peninsula, Arctic Canada.
Diagnosis.—Nearly orthoconic, smooth Ellesmeroceratidae with subcircular to compressed cross-section and lateral sutural lobes. Growth axis in early growth stages endogastrically curved, straight in later growth stages. Siphuncle marginal, at the concave side of the shell curvature with a diameter approximately ranging from one-fifth to one-third of the conch cross-section. Siphuncular segments concave or straight, consist of thick connecting rings. Septal necks in some forms short and orthochoanitic, in others hemichoanitic to slightly loxochoanitic. Endosiphuncular diaphragms known. (After Kröger and Landing 2007).
Remarks.—The assignment to this genus was principally based on the morphology of the conchs that are endogastrically curved apically, becoming straight later in ontogeny The apical angle, which is intermediate, is higher than in Ectenolites but lower than in Eremoceras. Our 3D specimens look very similar to the sketches of Ellesmeroceras of Flower (1964: figs. 7, 11 and 12). Since the erection of the genus, many species of Ellesmeroceras have been described. There has also been a flux of species reassigned to other genera or from other genera (e.g., Flower 1964). This reflects the general difficulties in assessing the Ellesmeroceratidae as a whole (see discussion above), and composition of the genus is likely to remain in flux until a comprehensive revision is carried out.
Ellesmeroceras humahuacaensis sp. nov.
Figs. 5–7.
Zoobank LSID: urn:lsid:zoobank.org:act:3AE0518B-5574-4C39-A113- 220AE67E718DEtymology: In reference to the outcrops along the Quebrada de Humahuaca, Jujuy where species was found.
Type material: Holotype: CEGH-UNC 27494a, almost complete three dimensionally preserved specimen. Paratypes: CEGH-UNC 27484a (longitudinal polished section of a fragment including part of the living chamber and part of the phragmocone), CEGH-UNC 27489b (longitudinal polished section of part of the phragmocone), and CEGH-UNC 24795a (adorally incomplete phragmocone of a three dimensionally preserved specimen). All from the type locality and horizon.
Type locality: Quebrada de Arenal, Trancas section, 4 km SSE from the locality of Huacalera, 13 km NNE from Tilcara town. Quebrada de Humahuaca, Cordillera Oriental, Jujuy, Argentina.
Type horizon: Horizon Are Verde+3m, Alfarcito Member, 495 m from the base of the Santa Rosita Formation, Trancas section, lower Tremadocian, Ordovician.
Material.—CEGH-UNC 27479a–c and ca. 36 more specimens numbered under 27479, but without specific letters as they were not measured or illustrated; CEGH-UNC 27480 (ca. seven specimens including b but excepting a); CEGH-UNC 27481a, b, and approximately seven more specimens; CEGH-UNC 27482 (ca. 11 specimens); CEGH-UNC 27483a and ca. 34 other specimens; CEGH-UNC 27484a, b, and approximately eight more specimens; CEGH-UNC 27485a, b, and ca. 21 other specimens, CEGH-UNC 27486 (ca. 12 specimens); CEGH-UNC 27487a and ca. seven other specimens; CEGH-UNC 27488a, b and ca. 14 other specimens; CEGH-UNC 27489a–e and ca. 26 more specimens; CEGH-UNC 27490 a and ca. 37 more specimens; CEGH-UNC 27491a–d and ca. 73 more specimens; CEGH-UNC 27492 (ca. eight specimens); CEGH-UNC 27493 (ca. four specimens); CEGH-UNC 27494 a–d and ca. 55 more specimens; CEGH-UNC 27495a, b and two more specimens; CEGH-UNC 27496a, c, and 27 more specimens; CEGH-UNC 27498 (ca. ten specimens); CEGH-UNC 27499 (ca. four specimens); CEGH-UNC 27500a and 21 more specimens; CEGH-UNC 27501a and 14 other specimens; CPBA 23610. All from the type locality and horizon except CPBA 23610 from the Quebrada de Yacoraite.
Diagnosis.—Very small Ellesmeroceras with circular conch cross-section. Siphuncle marginal on the concave side, with a width of approximately one-quarter to one-third of the conch diameter. Septal necks loxochoanitic and probably mesochoanitic, with thick and straight connecting rings. Around a quarter of the total length of the conch is curved endogastrically at the apical end. The expansion rate varies throughout ontogeny, being usually higher in the initial stages than in later ones. Shell with transverse growth lines. Suture line transverse with lateral lobes.
Description.—Very small conchs, the maximum length of a preserved fragment of phragmocone is ca. 14 mm, including part of the living chamber, whose basal diameter is 2.8 mm, and almost complete adapically, with 0.8 mm in dorsoventral diameter (Fig. 5A). In some specimens, the living chamber seems to be slightly constricted (Fig. 5D). The relative body chamber length is medium, ca. 2.5 mm. The apical part (approximately a quarter of the total length) is gently endogastrically curved, becoming straighter adorally (Figs. 5A, 6). The siphuncle is marginal and its relative diameter ranges between 20% and 46% of the conch diameter (average 29%, mean 30%, standard deviation 0.06, median 30%; Tables 1 and 2). It has constant loxochoanitic and probably mesochoanitic or hemichoanitic septal necks, and straight and thick connecting rings. The septal neck transition (sensu Pohle et al. 2022) is gradual (Fig. 7B, F, G). The conch cross-section is circular to very slightly compressed (circular sensu Pohle et al. 2022; Fig. 5D), and the sutures are almost straight, generally with a wide lateral lobe (Fig. 5B, D). The chambers are short (Figs. 5E, 6), ranging from 0.4 to 0.6 mm in length, average value 0.47 mm, and constant throughout ontogeny (relative cameral depth short, Table 2). The expansion rate is rather variable during ontogeny but is usually low to moderate (see Table 2). At least some of the variation arises from the variable lengths measured along the conch in each specimen sampled (Table 2: LM). The shell surface is generally smooth, with faint transverse growth lines (Fig. 5F, G). The embryonic conch (visible in the reconstruction through the micro-CT scan images) appears to be conical and slightly endogastric (Fig. 5). However, it is probably incomplete. The apicalmost chamber preserved is small and short, with a height of ca. 0.2 mm and a length of ca. 0.15 mm. The diameter is ca. 0.6 mm (Fig. 6A3).
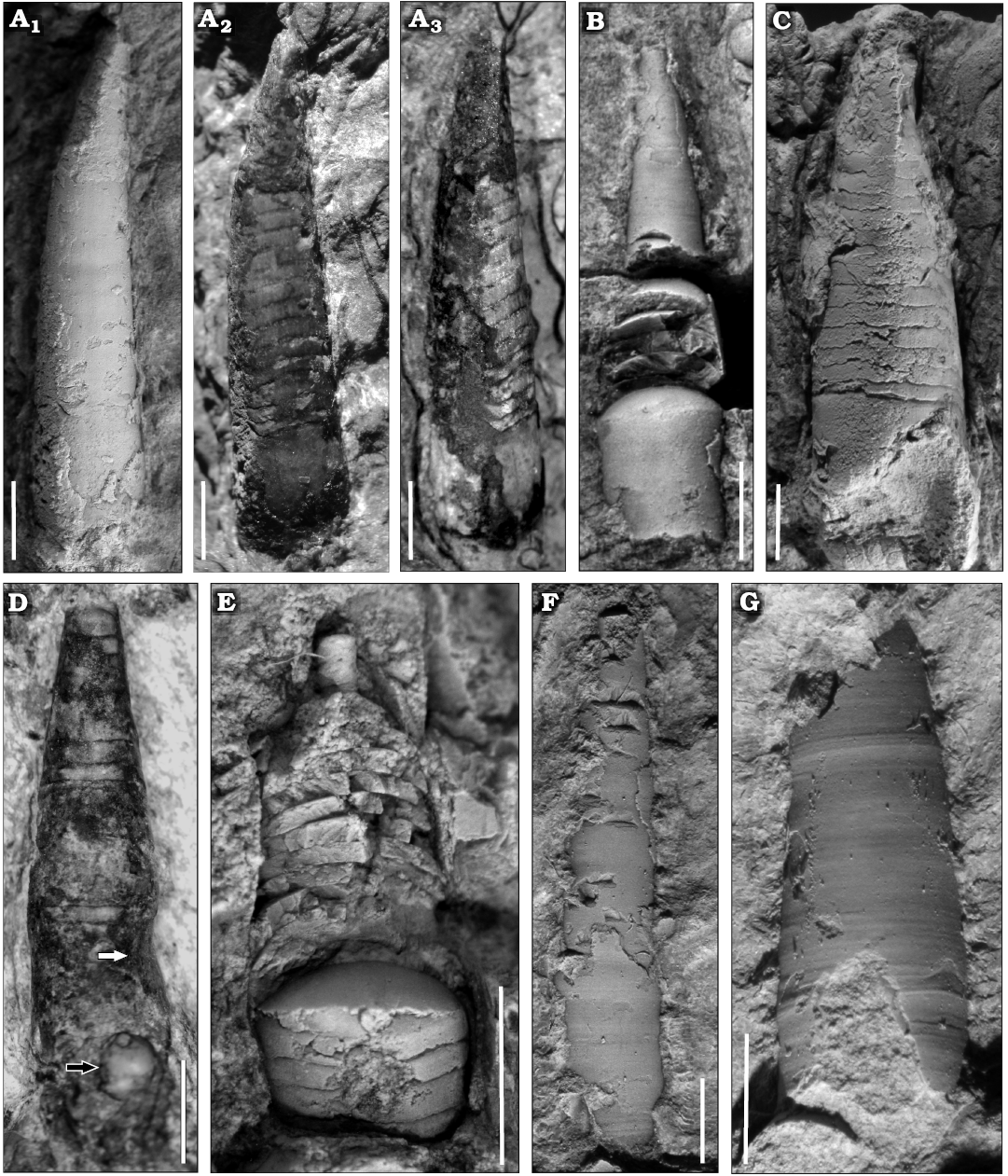
Fig. 5. Ellesmeroceratid cephalopod Ellesmeroceras humahuacaensis sp. nov. from the lower Tremadocian (Ordovician) of Quebrada de Arenal, Trancas section, Cordillera Oriental, Jujuy, Argentina. A. CEGH-UNC 27494a in lateral view (coated with ammonium chloride A1, without coating A2), intermediate view between lateral and dorsal (A3). Note the sutural lateral lobes and the bent apical part of the conch. B. CEGH-UNC 27494c in dorsal view. Note the specimen is crossed by a fracture in the rock that was fixed. C. CEGH-UNC 27501 in dorsal view with the apical part rather deformed. D. CEGH-UNC 27496a in dorsal view. E. CEGH-UNC 27496c in dorsal view, partially broken externally. F. CEGH-UNC 27491b in dorsal view, with shell wall. G. CEGH-UNC 27491c in external view, showing the growth lines. Scale bars 2 mm.
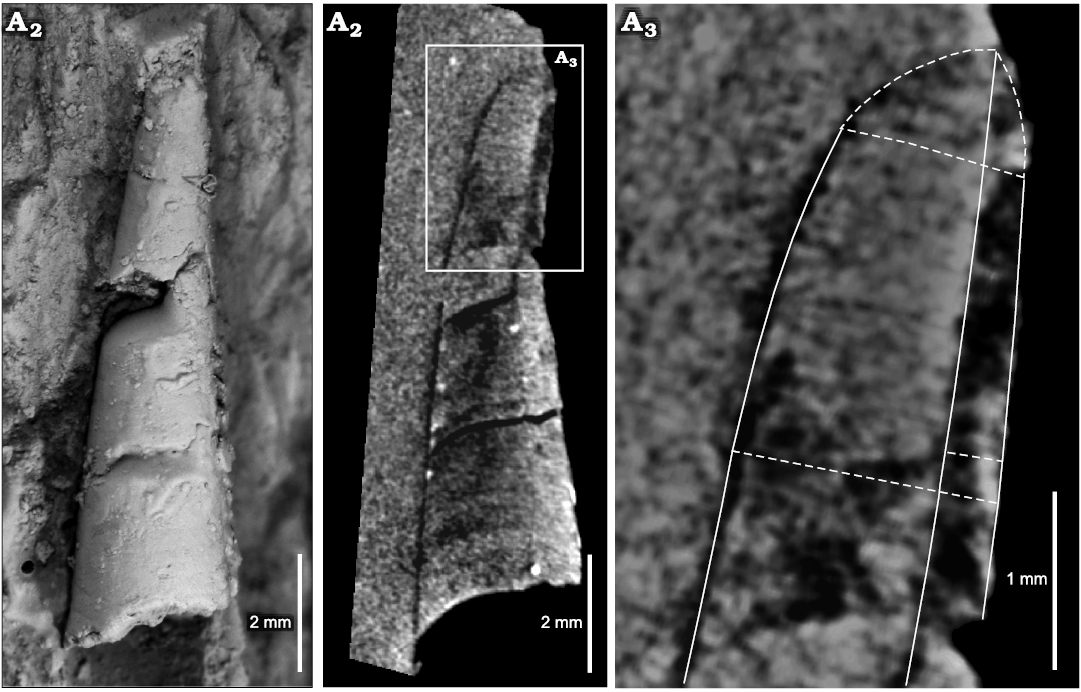
Fig. 6. Ellesmeroceratid cephalopod Ellesmeroceras humahuacaensis sp. nov. (paratype CEGH-UNC 27495a) from the lower Tremadocian (Ordovician) of Quebrada de Arenal, Trancas section, Cordillera Oriental, Jujuy, Argentina. A1, external lateral (slightly oblique) view of the coated specimen; A2, longitudinal micro-CT section; A3, detail of the apical part and adjacent chambers viewed in the micro-CT scan.
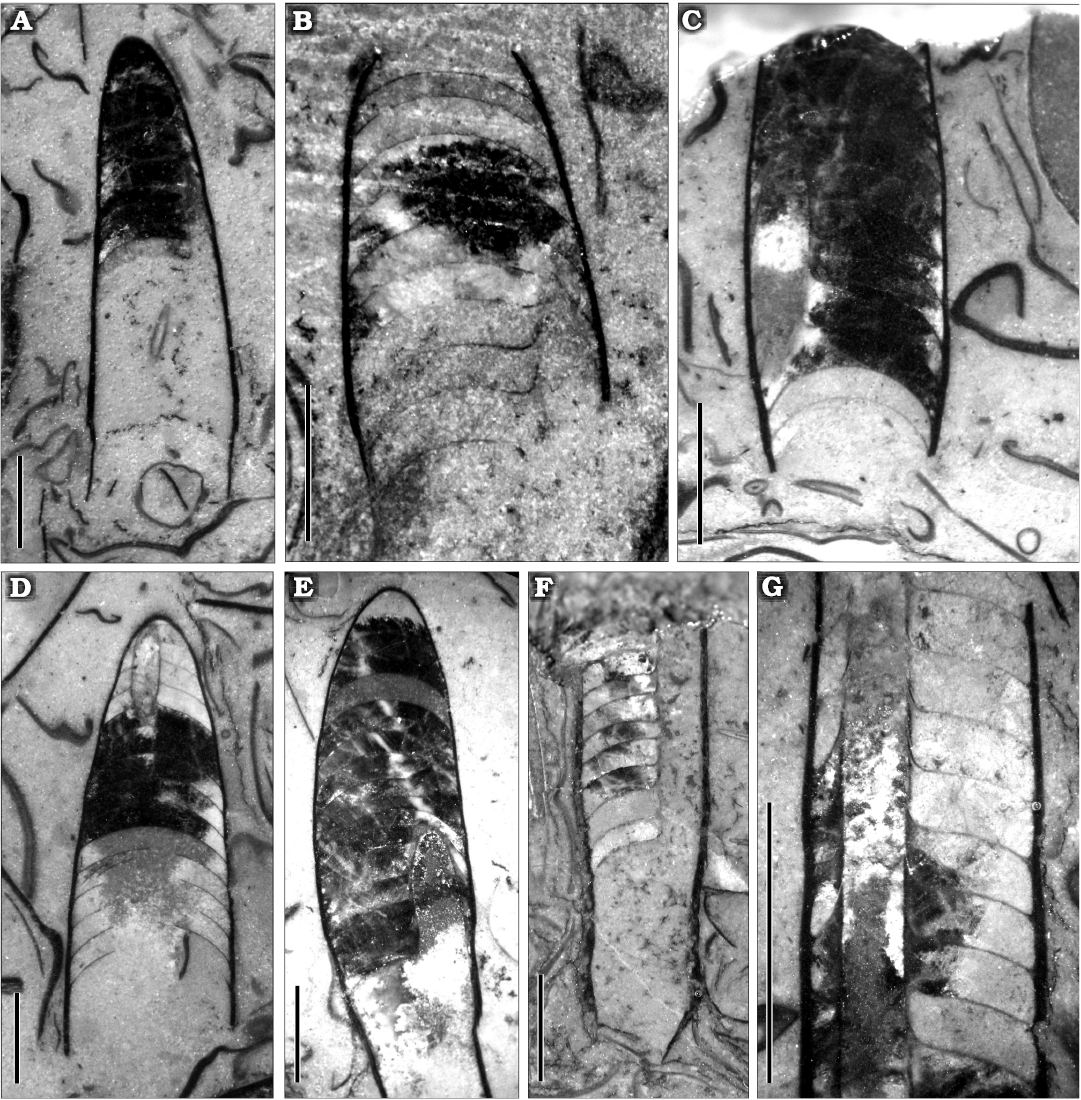
Fig. 7. Polished sections with different orientations of the ellesmeroceratid cephalopod Ellesmeroceras humahuacaensis sp. nov. from the lower Tremadocian (Ordovician) of Quebrada de Arenal, Trancas section, Cordillera Oriental, Jujuy, Argentina. A. CEGH-UNC 27489a, sagittal and somewhat oblique cut showing part of the siphuncle, the living chamber and the camerae. B. CEGH-UNC 27489b, sagittal and oblique cut of part of phragmocone showing septal necks. C. CEGH-UNC 27489c, sagittal and oblique cut of part of phragmocone broken apically showing cameral depth. D. CEGH-UNC 27489d, oblique cut of a fragment of conch with some broken septa and part of the siphuncle visible apically. E. CEGH-UNC 27489e, oblique cut of part of a conch in which the siphuncle is partially visible. F. CEGH-UNC 24784a, nearly longitudinal cut showing the siphuncle with septal necks and connecting rings. G. CEGH-UNC 24784b, detail of siphuncle in an oblique section. Scale bars 2 mm.
Remarks.—The enormous quantity of specimens found in the small fragments of rocks shows substantial variability in preservation and completeness. At first sight, all look very similar although different parts are visible in each one. There are just three specimens that are different and will be described below. The remaining specimens are provisionally included in the new species, E. humahuacaensis, based on a parsimonious view, and pending the availability of better-preserved material. Therefore, the new species is represented by nearly 460 conchs and conch fragments from the Alfarcito Member of the Santa Rosita Formation, NW Argentina. We consider that most of our specimens are adults or subadults because the presence of a high number of septa in the phragmocones, and the length of the straight part of the conch following the curved apical part.
As with our material, the frequently incomplete preservation of the conchs of coetaneous species often impedes comparison between different taxa.
Our species is not assigned to Ectenolites because this genus has a more slender conch, with a smaller proportion of the apical portion curved; nor to Eremoceras, it is cyrtoconic at all growth stages and possesses a larger apical angle (Kröger and Landing 2007). Although Evans (2011) recognised that the presence and morphology of the initial part of the phragmocone can be essential to the distinction between different ellesmeroceratid genera, he suggested, based on plots of several characters measured in Ellesmeroceras, Ectenolites, and Eremoceras, that without information regarding the curvature, they may not be distinguishable.
Ellesmeroceras humahuacaensis sp. nov. is unique in its tiny size when compared to other species of the genus. Ellesmeroceras scheii, from the Lower Ordovician of Ellesmere Island (Canada), the description of which was based on a single specimen, is larger (estimated length of complete specimen of ca. 30 mm), has a ventral saddle, and seems to have shorter septal necks (Flower 1964: pl. 25: 8). However, the holotype is poorly preserved, and the nature of the apical portion is not known (Ulrich et al. 1944).
The siphuncle becomes slightly removed from the conch wall in Ellesmeroceras bridgei during its ontogeny. In addition, the species possesses a conspicuous high and narrow dorsal saddle, while the shells are larger than those of E. humahuacaensis (Flower 1941).
Ruthenoceras elongatum from the Cambrian/Ordovician boundary of Siberia (Korde 1949; Dzik 2020) is rather larger (reaching a length of ca. 90 mm in more complete specimens) and is highly variable in several traits, including the inclination of septa and curvature of the conch. Our material shows some similarities to specimens illustrated by Dzik (2020: fig. 5d, e), which he considered to be juveniles. Those are curved at all growth stages and are more strongly curved, while also possessing a greater rate of conch expansion.
Stratigraphic and geographic range.—Santa Rosita Formation, Alfarcito Member, lower Tremadocian, Lower Ordovician. Quebrada de Humahuaca, Jujuy, Argentina.
Ellesmeroceras sp.
Fig. 8A.
Material.—CEGH-UNC 27496b from the Alfarcito Member, Santa Rosita Formation, early Tremadocian, Lower Ordovician, Quebrada de Arenal, Trancas section, Jujuy, Argentina.
Description.—The specimen consists of a small, slightly cyrtoconic, endogastric fragment of phragmocone of ca. 20 mm long and lacking the apical portion. It is 4 mm in diameter at the adoral end and 2.5 mm in diameter adapically (Fig. 8A). It is estimated that the complete conch could easily reach 30 mm in length. The chambers are short, ranging from 0.4 to 0.6 mm, with an RCL of 0.12 or eight chambers within a distance equivalent to the maximum diameter. The sutures are sinuous (Fig. 8A1, A2). The siphuncle has a diameter 35% that of the conch diameter at the same point of measurement, is marginal and ventral. The expansion rate is low, with an apical angle of 5.7°. The shell wall is not preserved. In the adoral part of the phragmocone, seems to be an inflexion followed by a slight increase in the expansion rate (see arrow in Fig. 8A1). This could be a pathological trait or a diagnostic character, but as we only have the single specimen, it is impossible to evaluate its significance.
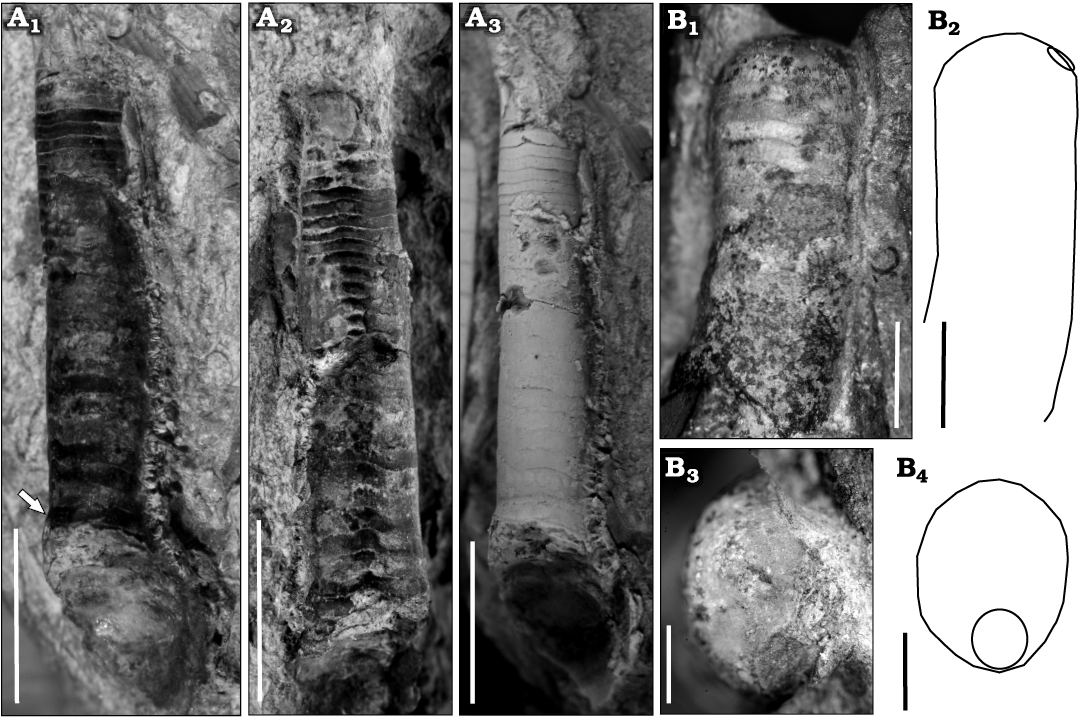
Fig. 8. Early Tremadocian (Ordovician) cephalopods from Quebrada de Arenal, Trancas section, Cordillera Oriental, Jujuy, Argentina. A. Ellesmeroceratid Ellesmeroceras sp. (CEGH-UNC 27496b) in oblique-lateral (A1) and oblique-ventral (A2) view of the uncoated specimen, and oblique-lateral view of the coated specimen (A3). Arrow points to the siphuncle. Note the sinuosity of the suture lines. B. Bassleroceratid Bassleroceras? sp. (CEGH-UNC 27497) in lateral view (B1, B2) showing exogastric curvature, low expansion rate, and external siphuncle, in apical view of the apicalmost septum preserved (B3, B4), showing the slightly compressed shape and the siphuncle position. Photographs (B1, B3) and schematic drawings (B2, B4) Scale bars: A, 5 mm, B1, B2, 2 mm; B3, B4, 1 mm.
Remarks.—Despite the similarities to E. humahuacaensis, the notably larger size of the conch and the sinuosity of the suture line lead us to regard this specimen as a separate taxon. Until other material exhibiting additional characters is available, we prefer to leave this specimen in open nomenclature. Nevertheless, the slightly cyrtoconic, endogastric conch and the very short chambers suggest that it may be assigned to Ellesmeroceras.
Order undetermined
Family Bassleroceratidae Ulrich, Foerste, Miller, and Furnish, 1944
Remarks.—The family Bassleroceratidae has been assigned both to the Ellesmerocerida, the Tarphycerida, and the later discarded order Bassleroceratida (Evans 2011: 22, and discussion and references therein). We followed here the proposal by Pohle et al. (2022), in which the family is located within the subclass but not included in any particular order, although Kröger and Pohle (2021) included the family within Ellesmerocerida. See Evans (2011) for further discussion about the family Bassleroceratidae.
Genus Bassleroceras Ulrich and Foerste, 1935
Type species: Orthoceras perseus Billings, 1865, from the St. Armand Limestone, near Phillipsburg, Missisquoi County, Quebec, Canada. Tremadocian, Lower Ordovician.
Bassleroceras? sp.
Fig. 8B.
Material.—CEGH-UNC 27497 and 27480a, from the Alfarcito Member, Santa Rosita Formation, lower Tremadocian, Lower Ordovician, Quebrada de Arenal, Trancas section, Jujuy, Argentina.
Description.—CEGH-UNC 27497 is a 7.3 mm long fragment of a slightly exogastrically curved conch, of which 3.2 mm correspond to part of the living chamber and 4.1 mm to part of the phragmocone. It has an oral dorsoventral diameter of ca. 3 mm, an apical dorsoventral diameter of c 2.6 mm, and an apical lateral diameter of ca. 2.1 mm. The cross-section is slightly compressed (0.8), and the siphuncle is rather large, ventral and marginal (Fig. 8B), with a diameter 34 % of that the conch diameter. The chambers are very short, ca. 0.3 mm long (Fig. 8B1). There are 10 chambers in a length equivalent to the conch diameter, i.e., an RCL of 0.1. At 2.3°, the expansion rate is very low. CEGH-UNC 27480a is a 7.4 mm long fragment of a slightly exogastric conch, of which 4 mm corresponds to part of the living chamber and 3.4 mm to the phragmocone. Adapically, the dorsoventral diameter is ca. 3 mm, and the siphuncle is 1 mm wide, or 33% of that the conch diameter. The chambers are 0.3 mm long, indicating an RCL of 0.08. The SCI is 0.16.
Remarks.—The slightly exogastric curvature of these specimens, along with the very low expansion rate of the conch, suggests that they can be assigned to the Basslerocertidae. We would need more material in order to investigate internal structures and determine more precisely the taxonomic assignment of these specimens. Compare with this material, Lawrenceoceras Ulrich, Foerste, Miller, and Furnish, 1944, has a smaller siphuncle relative to the conch diameter, and it is not completely marginal (Kröger and Pohle 2021). In Anguloceras the septa slope steeply apicad from the venter to the dorsum ( Unklesbay and Young 1956). In Avaoceras the siphuncle is submarginal ( Ulrich et al. 1944). Hitherto, the oldest reported bassleroceratids were late Tremadocian in age (Evans 2011). The questionable Bassleroceras from the Balnakeil Formation (Evans 2011) and the two species of Bassleroceras from the Rochdale Formation (Kröger and Landing 2008) are both slightly younger than the Argentine specimens described herein. The formers are from the Paltodus deltifer–Macerodus dianae Conodont Zone (which is lower Tr2). If the presence of the group in the Alfarcito Member of the Cordillera Oriental, Jujuy, is confirmed, it would be one of the oldest records. It is interesting to note that similar forms, but yet undescribed, appear to occur in the earliest Tremadocian of Australia (AP unpublished data).
The age of the specimens further highlights the problematic state of ellesmerocerid taxonomy, as it is also difficult to differentiate between bassleroceratids and certain late Cambrian ellesmerocerids such as the Huaiheceratidae and the Xiaoshanoceratidae, which apparently also had an exogastric conch. The Xiaoshanoceratidae supposedly have a depressed cross-section, while the only character distinguishing the Huaiheceratidae from the Bassleroceratidae is the thinner connecting ring of the former. In our opinion, these characteristics are questionable, because the xiaoshanoceratids are only known from randomly oriented cross-sections (Chen and Teichert 1983a) and a depressed cross-section may also be produced by an oblique cut, meanwhile the connecting rings are frequently missed and/or altered during diagenesis.
Discussion
Palaeogeographic distribution of Cambrian and earliest Ordovician cephalopods.—After the first appearance of cephalopods in North China, in the Yenchou Member of the Fengshan Formation (upper Jiangshanian, Furongian), the Wanwankou Member hosted what Teichert (1988: 24) called “the Wanwankou Explosion’’, as numerous new families, genera and species appeared, both in North and South China. In contrast, the uppermost Chinese Cambrian (upper part of Stage 10) is nearly devoid of cephalopods (Chen and Teichert 1983a). From a current perspective, it appears likely that the late Cambrian diversity peak for cephalopods is a product of extensive “over-splitting” of taxa (Holland 1987; Hewitt 1989; King and Evans 2019; Pohle et al. 2022).
Landing and Kröger (2009) referred to the occurrence of a plectronocerid from the upper Cambrian Minaret Formation of Antarctica, apparently reported by Webers et al. (1992). However, in that work, there is no mention of a plectronocerid or other cephalopods, although some monoplacophorans, ancestral to cephalopods, were described. Late Cambrian cephalopods were also reported from Siberia (Korde 1949; Dzik 2020) and Kazakhstan (Malinovskaya 1964), although their precise ages were uncertain. After revision (Flower 1954; Dzik 2020) these Siberian cephalopods were assigned to a single species of ellesmeroceratid, Ruthenoceras elongatum Korde, 1949. Dzik (2020) described R. elongatum on the basis of nearly 150 specimens. We consider that the variability is high enough to suspect the presence of more than one taxon. The age of the fauna corresponds approximately to the Cambrian/Ordovician boundary (Dzik 2020). The age of the cephalopods from Kazakhstan described by Malinovskaya (1964) are dubious because the trilobites of the associated fauna were never illustrated and could be from the earliest Ordovician (Landing and Kröger 2009).
The late Cambrian cephalopods of Laurentia have lower diversities than those from China. They have been reported from Texas, Nevada, and eastern New York. The Texan specimens include very small plectronoceratids and slender conchs of ellesmeroceratids (no more than a few centimetres long) (Flower 1954, 1964). Fossils from Nevada are too poorly preserved for generic identification, and the same applies to those from New York State, but are described as rather small cyrtoconic conches (Landing and Kröger 2009). All these records are from the latest Cambrian.
An abundant association of late Cambrian cephalopods from Queensland, Australia, was reported by Pohle and Jell (2019). They come from the interval in which, in China, cephalopods are scarce. The youngest specimens are from the early Tremadocian. The geographic and stratigraphic distribution of these early cephalopods are shown in Fig. 1.
Besides Australia and probable records from Kazakhstan, early Tremadocian (tripartite division) cephalopods are found in North and South China (Chen et al. 1979a, b; Teichert 1988; Kröger 2013), Tarim (Troedsson 1937), Sino-Korean Platform (Kröger 2013), and especially in Laurentia (Ulrich et al. 1943, 1944; Unklesbay 1954; Unklesbay and Young 1956; Flower 1964; Kröger and Landing 2007). Faunas from different basins in Laurentia, but especially those from the Tribes Hills and Tanyard Formations, are remarkable because of the diversity of the Ellesmeroceratidae, ranging from orthoconic longicones to cyrtocones and brevicones forms. This contrasts strongly with our assemblage from NW Argentina, with its very low diversity, and the vast majority of specimens assigned to Ellesmeroceras humahuacaensis sp. nov. In this context, our fauna is probably more similar to those from Kazakhstan, Tarim and Australia, also dominated by simple ellesmeroceratids, and although these assemblages are slightly more diverse, they are still rather homogeneous.
Teichert (1988: 24) stated ten main characteristics of Cambrian cephalopods that he considered set them apart from later cephalopods, although he admitted that the earliest Ordovician faunas are very much like their Cambrian predecessors except for a generally larger average size. Our specimens, however, are very small; equivalent in size to the earliest plectronocerids from China. In fact, the general conch size distribution within Cambrian and lower Tremadocian strata presents a complex pattern which is difficult to analyse because the uncertain ages of some records. A further problem in distinguishing between Cambrian and early Tremadocian cephalopods is that the taxonomy of the Cambrian forms has largely relied on internal characters (many species are exclusively known from thin sections; see, e.g., Chen et al. 1979a, b; Chen and Teichert 1983a; Li 1984), while the external morphologies of these taxa remain poorly known, whereas earliest Ordovician forms (particularly from Laurentia) are known from both their external form, but also, to a great extent, internally (e.g., Ulrich et al. 1943, 1944; Flower 1964; Kröger and Landing 2007). Considering all those records from the upper Cambrian and lowest Ordovician, the proposed eclipse of cephalopods in the latest Cambrian and the Ordovician revival reported by Teichert (1988) can perhaps be attributed to sampling and/or taphonomic biases rather than a real extinction event. This would also compare to patterns seen in other organisms, as it becomes increasingly clear that the “Furongian biodiversity gap” arises from inadequate sampling and environmental fluctuations (e.g., Harper et al. 2019; Deng et al. 2023; Du et al. 2023).
For the late Cambrian as well as for the earliest Tremadocian, all known cephalopod records come from the paleotropical belt, between 0° and 30° south latitude (Cocks and Torsvik 2021; Fig. 9). If we consider the surface circulation pattern based on simulations by Pohl et al. (2016), localities with early cephalopods are well interconnected via ocean currents. Based on this pattern, the presence of late Cambrian cephalopods in Antarctica would be expected. Through Antarctica, platform areas of South America and Australia were connected (Cocks and Torsvik 2021), which could have acted as migratory routes for cephalopods from the Equator to the south tropic of 30° south latitude.
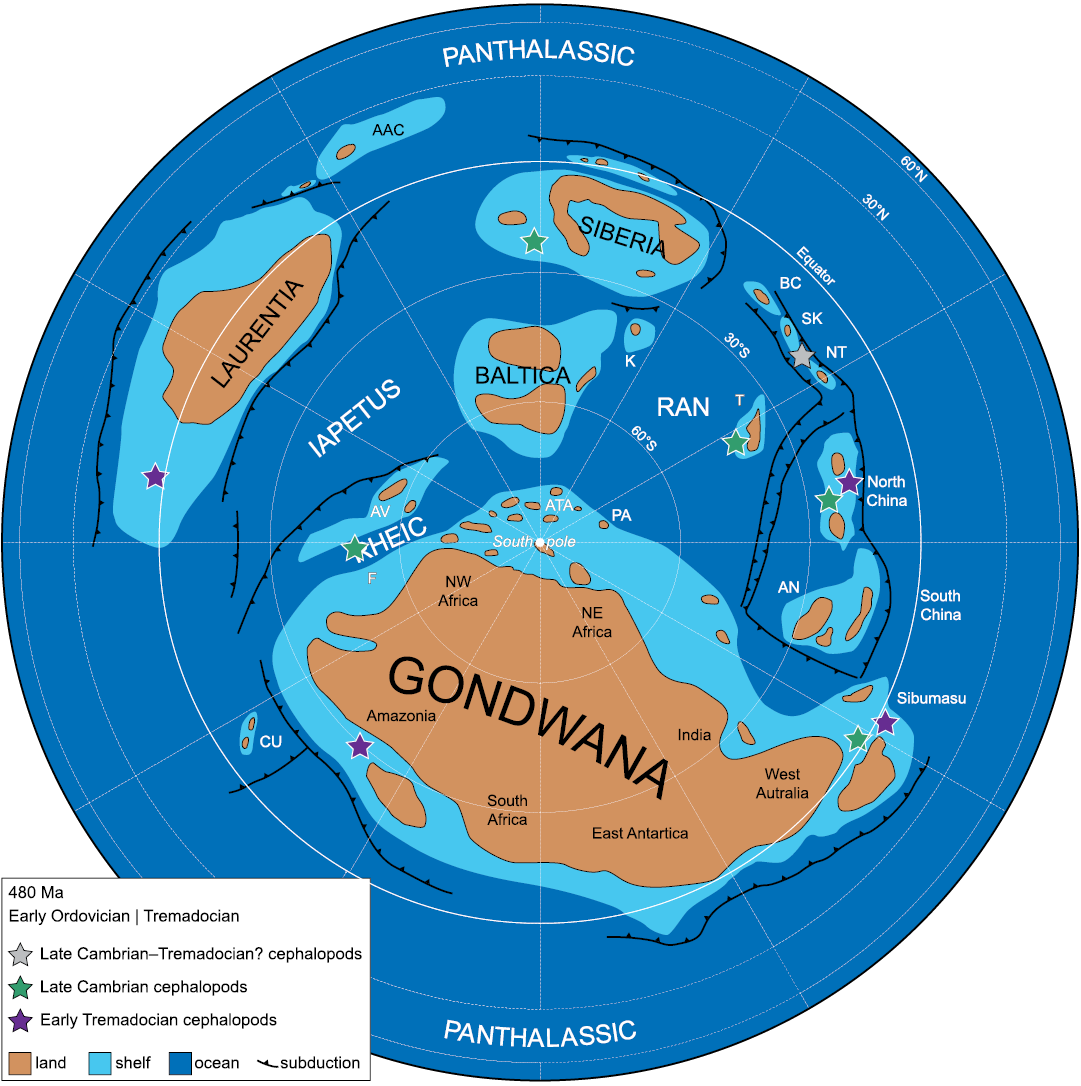
Fig. 9. Palaeogeographic map for the Tremadocian, with the location of latest Cambrian and earliest Tremadocian cephalopods (simplified from Cocks and Torsvik 2021). Abbreviations: AAC, Arctic-Alaska Chukotka; AN, Annamia; ATA, Armorican Terrane Assemblage; AV, Avalonia; BC, Boshchekul- Chingiz; CU, Cuyania; F, Florida; K, Kara; K-O, Kolyma-Omolon; NT, North Tien Shan (including Ch-Ili); PA, Palaeo-Adria; SK, Stepnyak, Selety, and Kokchetav; T, Tarim.
Early Ordovician cephalopods evolution in the NW Argentina Basin.—The cephalopods described herein are from the upper part of stage slice Tr1 (of Bergström et al. 2009) of the Tremadocian (Fig. 2). They represent the first immigration of the group into the Central Andean Basin, probably coming from the East, through the platform areas of Australia and Antarctica (see Pohl et al. 2016 and Cocks and Torsvik 2021). The high dominance of ellesmeroceratids is typical of that age. The exogastric, bassleroceratid-like forms, are a minority and, according to our observations regarding field trips and available collections, they wouldn’t have prospered in the basin. During the middle Tremadocian, the diversity of the cephalopods in NW Argentina increased, reflecting the immigration of taxa and local originations. These include the endemic ellesmeroceratid Purmamarcoceras kobayashii, a rather small (ca. 45 mm long incomplete), endogastric form with a marginal siphuncle and depressed conch section (Cecioni 1965). Immigrants are represented by several groups. One conspicuous element is the cyrtocerinid Saloceras cf. sericeum (Salter in Ramsay, 1866), whose affinities are peri-Gondwanic, especially with Avalonia (Evans 2005; Cichowolski and Vaccari 2011). Endoceratoids also arrived, represented in part by isolated and large undetermined siphuncles (MC unpublished data), and mainly by the proterocameroceratid Protocyptendoceras fuenzalidae that persists into the Floian (Cichowolski 2009). Proterocameroceratids, along with cytocerinids, belong to the Peri-Gondwana Realm of Kröger (2013), i.e., Saloceras Realm of Kröger and Evans (2011), with a long-range, spanning since the middle Tremadocian to the middle Floian (Fl2) (Cichowolski et al. 2014). Also from the middle Tremadocian, MC unpublished material indicates the arrival of some orthoceratoids, such as rioceratids and protocycloceratids. Finally, Cecioni (1965) reported from the same beds as Protocyptendoceras and Purmaracoceras, the presence of Clarkoceras and Robsonoceras. Unfortunately, this material cannot be located. The presence in our material of unstudied rioceratids supports Cecioni’s (1965) recording of Robsonoceras in this interval. More material is needed to develop a more complete scenario of middle Tremadocian cephalopod groups from the southern Central Andean Basin.
During the Floian, the cyrtocerinids increase in diversity. Local originations include the record of Margaritoceras, endemic to the Central Andean Basin (common in Bolivia as well, Cecioni and Flower 1985), and other endemic species of Saloceras (Cichowolski et al. 2014). Although the taxonomy of the Floian material is still under study, the diversity of endoceratoids and protocycloceratids also seems to rise. The diversity patterns of Early Ordovician cephalopods of the basin will be better understood as further collection yields more complete material, permitting the study of internal characters. Most specimens from the Acoite Formation (Floian) are internal moulds. Interestingly, the family Ellesmeroceratidae has not so far been recorded from Floian strata.
Palaeoecology of E. humahuacaensis.—Based on the thin, densely spaced and weakly concave septa, Westermann (1998) considered the early ellesmeroceratids restricted to shallow waters. In addition, as these conchs had no effective counterweights for neutral equilibrium, their poise would have been sub-vertical, capable of vertical migrations and “hopping” bottom feeding. In a recent investigation of the hydrostatics of Palaeozoic ectocochleate cephalopods, Peterman et al. (2019) included Plectronoceras. Their results indicate that the conch was stable with the aperture facing downwards, and the endogastric phragmocone curving upwards, corroborating Westermann’s (1998) conclusions. These, and similar forms would have been slightly negatively buoyant (Westermann 1998; Peterman et al. 2019) and would have needed to permanently swim to stay buoyant, due to the small cameral volume capacity relative to the body. Consequently, the animal would have been mainly neritic and demersal, as sustainable active locomotion would be too energetic-demanding to remain in the water columns (Peterman et al. 2019). Considering our material of E. humahuacaensis, with its lower apical angle relative to the species of Plectronoceras used by Peterman et al. (2019), and its higher cameral depth (SCI), it seems possible that this species had greater buoyancy and, then, a higher potential to disperse, especially in juveniles as the deeper camerae appear to be more apicad in position. The result of Peterman et al. (2019) aligns with the data on the paleoenvironmental distribution of this morphotype (Fig. 4), which is usually restricted to neritic settings (Kröger et al. 2009). Ellesmeroceras humahuacaensis sp. nov. comes from strata deposited in shallow marine settings (upper offshore, see Geological setting), thus agreeing with previous interpretations on the mode of life. Servais et al. (2015), however, stated that early cephalopods would have been planktonic drifters due to early wide distribution and shell characters, but did not explain which characters were responsible for the planktonic habit.
It is interesting to note that benthic communities thriving in the late Cambrian–early Tremadocian seas of Andean Gondwana are by far dominated by trilobites. Subordinate groups include sessile filter feeders such as rhynchonelliformean and linguliformean brachiopods (Benedetto 2003), isolated echinoderms (eocrinoids and stylophorans, Nohejlova et al. 2018) and sparse gastropods. These communities were generally of low diversity and exhibited a very simple ecological structure, with intergrading assemblages, dominated by widespread generalist trilobites (Waisfeld and Balseiro 2016; Serra et al. 2019). Selected literature records for this interval indicated the incipient colonization of the pelagic realm by phytoplankton (e.g., acritarchs, Rubinstein et al. 2003), zooplankton (e.g., graptolites and phyllocarids, Zeballo et al. 2005; Manca 1991), and nektobenthos (e.g., conodonts, Zeballo and Albanesi 2013). By contrast, no previous records of organisms living low in the water column, above the sediment/water interface were so far reported. In this context, the record of ellesmeroceratids of largely demersal life habit, inhabiting the water column near the sea floor, can be viewed as the initial partitioning of this ecospace (near bottom habitats) in the offshore environment. This scenario evolved during the middle Tremadocian (e.g., Kröger et al. 2009). The appearance of endoceratoids, cyrtocerinids and orthoceratoids (Cecioni 1965; Cichowolski et al. 2022) indicates an increasingly complex marine ecosystem. This new fauna would have been able to colonize the water column, both as vertical migrants, as well as horizontal swimmers (Westermann 1998).
Acknowledgements
David Evans (Natural England, Bridgwater, UK) and Björn Kröger (Finnish Museum of Natural History, Helsinki, Finland) are sincerely thanked for their constructive reviews that greatly improved an early version of this manuscript. In particular, we thank DE for his help with the English language. He is also thanked for his help with the interpretation of the siphuncle in some photos and for his permanent support. We are in debt with Augusto Rapalini (Instituto de Geología de Buenos Aires, Argentina) for the palaeomagnetic and palaeolatitude information of the studied strata. Martina Aubrechtová and Vojtĕk Turek (Czech Academy of Sciences, Prague, Czechia) are thanked for their help with the embryonic conch interpretation. The authors thank Agustina Toscano (Universidad de Buenos Aires, Argentina) who helped with the photos of the exogastric specimen. Founding for this project was provided by PICT 2016 0558 (to BGW), and PUE: 22920160100051. This is the contribution R-472 to the Instituto de Estudios Andinos “Don Pablo Groeber”.
References
Astini, R.A. 2003. The Ordovician Proto-Andean basins. In: J.L. Benedetto (ed.), Ordovician Fossils of Argentine, 1–74, Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba, Córdoba.
Balseiro, D. and Waisfeld, B.G. 2013. Evenness and diversity in Upper Cambrian–Lower Ordovician trilobite communities from the Central Andean Basin (Cordillera Oriental, Argentina). Palaeontology 57: 531–546. Crossref
Balseiro, D., Waisfeld, B.G., and Buatois, L.A. 2011. Unusual trilobite biofacies from the Lower Ordovician of the Argentine Cordillera Oriental: new insights into olenid palaeoecology. Lethaia 44: 58–75. Crossref
Benedetto, J.L. 2003. Brachiopods. In: J.L. Benedetto (ed.), Ordovician Fossils of Argentina, 187–272. Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba, Córdoba.
Benedetto, J.L. and Carrasco, P. 2002. Tremadoc (earliest Ordovician) brachiopods from the Purmamarca region and the Sierra de Mojotoro, Cordillera Oriental of northwestern Argentina. Geobios 35: 647–661. Crossref
Benedetto, J.L. and Muñoz, D.F. 2015. Linguloidean brachiopods from the Lower Ordovician (Tremadocian) of northwestern Argentina. Bulletin of Geosciences 90: 417–430. Crossref
Bergström, S.M., Chen, X., Gutiérrez-Marco, J.C., and Dronov, A. 2009. The new chronostratigraphic classification of the Ordovician System and its relations to major regional series and stages and to δ31C chemostratigraphy. Lethaia 42: 97–107. Crossref
Billings, E. 1865. Palaeozoic fossils. Containing descriptions and figures of new or little known species of organic remains from the Silurian rocks. Geological Survey of Canada, Montreal 1: 169–394. Crossref
Blender Online Community 2022. Blender, a 3D Modelling and Rendering Package [available online, https://www.blender.org/download/releases/2-81/]
Buatois, L.A. and Mángano, M.G. 2003. Sedimentary facies, depositional evolution of the Upper Cambrian–Lower Ordovician Santa Rosita formation in northwest Argentina. Journal of South American Earth Sciences 16: 343–363. Crossref
Buatois, L.A., Zeballo, F.J., Albanesi, G.L., Ortega, G., Vaccari, N.E., and Mángano, M.G. 2006. Depositional environments and stratigraphy of the Upper Cambrian–Lower Ordovician Santa Rosita Formation at the Alfarcito Area, Cordillera Oriental, Argentina: Integration of biostratigraphic data within a sequence stratigraphic framework. Latin American Journal of Sedimentology and Basin Analysis 13: 1–29.
Cecioni, G. 1965. Contribución al conocimiento de los nautiloideos Eo-Paleozoicos argentinos. Parte II: Robsonoceratidae, Ellesmeroceratidae, Proterocameroceratidae, Baltoceratidae. Boletín del Museo de Historia Natural, Santiago de Chile 29: 1–24. Crossref
Cecioni, G. and Flower, R.H. 1985. Bathmoceratidae (Nautiloideos, Ordovícico) de Sud América. Neues Jahrbuch für Geologie und Palaöntologie Abhandlungen 170: 343–357. Crossref
Cichowolski, M. 2009. A review of the endocerid cephalopod Protocyptendoceras from the Floian (Lower Ordovician) of the Eastern Cordillera, Argentina. Acta Palaeontologica Polonica 54: 99–109. Crossref
Cichowolski, M. and Vaccari, N.E. 2011. The oldest record of Eothinoceratidae (Ellesmerocerida, Nautiloidea): Middle Tremadocian of the Cordillera Oriental, NW Argentina. Geological Journal 46: 42–51. Crossref
Cichowolski, M., Vaccari, N.E., and Waisfeld, B.G. 2022. Cefalópodos tremadocianos del Noroeste Argentino. Resúmenes de la Reunión Anual de Comunicaciones de la Asociación Paleontológica Argentina, 103. Asociación Paleontológica Argentina, Salta.
Cichowolski, M., Waisfeld, B.G., Vaccari, N.E., and Marengo, L. 2014. A review of the nautiloid Family Eothinoceratidae from the Floian of the Central Andean Basin of NW Argentina and Southern Bolivia. Geological Journal 50: 764–782. Crossref
Chen, J.Y. and Qi, D.L. 1982. Upper Cambrian cephalopods from western Zhejiang. Geological Society of America Special Papers 187: 137–142. Crossref
Chen, J.Y. and Teichert, C. 1983a. Cambrian cephalopods from China. Palaeontographica A 181: 1–102.
Chen, J.Y. and Teichert, C. 1983b. Cambrian cephalopods. Geology 11: 647–650. Crossref
Chen, J.Y., Zou, X.P., Chen, T.E., and Qi, D.L. 1979a. Late Cambrian Ellesmerocerida (Cephalopoda) of North China [in Chinese, with English abstract]. Acta Palaeontologica Sinica 18: 103–122.
Chen, J.Y., Zou, X.P., Chen, T.E., and Qi, D.L. 1979b. Late Cambrian cephalopods of North China—Plectronocerida, Protactinocerida (ord. nov.) and Yanhecerida (ord. nov) [in Chinese, with English abstract]. Acta Palaeontologica Sinica 18: 1–24.
Cocks, L.R.M. and Torsvik, T.H. 2021. Ordovician palaeogeography and climate change. Gondwana Research 100: 53–72. Crossref
Cuvier, G 1797 Tableau élémentaire de l’histoire naturelle des animaux. 593 pp. Deterville, Paris. Crossref
Deng, Y., Fan, J., Yang, S., Shi, Y., Lu, Z., Xu, H., Sun, Z., Zhao, F. and Hou, Z. 2023. No Furongian Biodiversity Gap: Evidence from South China. Palaeogeography, Palaeoclimatology, Palaeoecology 618: 111492. Crossref
Du, M., Li, H., Tan, J., Wang, Z., and Wang, W. 2023. The bias types and drivers of the Furongian Biodiversity Gap. Palaeogeography, Palaeoclimatology, Palaeoecology 612: 111394. Crossref
Dzik, J. 1984. Phylogeny of the Nautiloidea. Palaeontologia Polonica 45: 1–219.
Dzik, J. 2020. Variability of conch morphology in a cephalopod species from the Cambrian to Ordovician transition strata of Siberia. Acta Palaeontologica Polonica 65: 149–165. Crossref
Evans, D.H. 2005. The Lower and Middle Ordovician Cephalopod Faunas of England and Wales. Monograph of the Palaeontographical Society, London 158: 1–81. Crossref
Evans, D.H. 2011. The Lower Ordovician cephalopod faunas of the Durness Group, North-West Scotland Monograph of the Palaeontographical Society, London 165: 1–131. Crossref
Fang, X., Kröger, B., Zhang, Y.D., Zhang, Y.B. and Chen, T.E. 2019. Palaeogeographic distribution and diversity of cephalopods during the Cambrian–Ordovician transition. Palaeoworld 28: 51–57. Crossref
Fedorov, A., Beichel, R., Kalpathy-Cramer, J., Finet, J., Fillion-Robin, J.C., Pujol, S., Bauer, C., Jennings, D., Fennessy, F., Sonka, M., Buatti, J., Aylward, S., Miller, J.V., Pieper, S., and Kikinis, R. 2012. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magnetic Resonance Imaging 30: 1323–1341. Crossref
Flower, R.H. 1941. Cephalopods from the Seward Peninsula of Alaska. Bulletin of American Paleontology 27 (102): 1–22.
Flower, R.H. 1954. Cambrian Cephalopods. New Mexico Institute of Mining and Technology, State Bureau of Mines and Mineral Resources, Bulletin 40: 1–51.
Flower, R.H. 1964.The nautiloid order Ellesmeroceratida (Cephalopoda). New Mexico Institute of Mining and Technology, State Bureau of Mines and Mineral Resources, Memoire 12: 1–164. Crossref
Flower, R.H. and Kummel, B. 1950. Classification of the Nautiloidea Journal of Paleontology 24: 604–615.
Foerste, A.F. 1921. Notes on Arctic Ordovician and Silurian cephalopods. Bulletin of the Scientific Laboratories of Denison University 19: 247–306.
Harper, D.A.T., Topper, T.P., Cascales-Miñana, B., Servais, T., Zhang, Y.D., and Ahlberg, P. 2019. The Furongian (late Cambrian) Biodiversity Gap: Real or apparent? Palaeoworld 28: 4–12. Crossref
Harrington, H.J. and Leanza, A.F. 1957. Ordovician trilobites of Argentina. Department of Geology, University of Kansas Special Publication 1: 1–276.
Hewitt, R.A. 1989. Recent growth of nautiloid and ammonite taxonomy. Paläontologische Zeitschrift 63: 281–296. Crossref
Hoffmann, R., Howarth, M.K., Fuchs, D., Klug, C., and Korn, D. 2022. The higher taxonomic nomenclature of Devonian to Cretaceous ammonoids and Jurassic to Cretaceous ammonites including their authorship and publication. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 305: 187–197. Crossref
Holland, C.H. 1987. The nautiloid cephalopods: a strange success: President’s anniversary address 1986. Journal of the Geological Society 144: 1–15. Crossref
King, A.H. and Evans, D.H. 2019. High-level classification of the nautiloid cephalopods: a proposal for the revision of the Treatise Part K. Swiss Journal of Palaeontology 138: 65–85. Crossref
Kobayashi, T. 1934. The Cambro-Ordovician formations and faunas of South Chosen. Part II. Lower Ordovician faunas. Journal of the Faculty of Science, Tokyo University, Geology, Mineralogy 43: 521–585.
Korde, K.B. 1949. Nautiloidea of the Upper Cambrian of the Angara [in Russian]. Doklady Akademia Nauk SSSR 49: 671–673.
Kröger, B. 2013. Chapter 27: Cambrian–Ordovician cephalopod palaeogeography and diversity. In: D.A.T. Harper and T. Servais (eds.), Early Palaeozoic Biogeography and Palaeogeography. Geological Society, London, Memoirs 38: 429–448. Crossref
Kröger, B. and Evans, D.H. 2011. Review and palaeoecological analysis of the late Tremadocian–early Floian (Early Ordovician) cephalopod fauna of the Montagne Noire, France. Fossil Record 14: 5–34. Crossref
Kröger, B. and Landing, E. 2007. The earliest Ordovician cephalopods of eastern Laurentia—ellesmerocerids of the Tribes Hill Formation, eastern New York. Journal of Paleontology 81: 841–857. Crossref
Kröger, B. and Landing, E. 2008. Onset of the Ordovician cephalopod radiation—evidence from the Rochdale Formation (middle Early Ordovician, Stairsian) in eastern New York. Geological Magazine 145: 490–520. Crossref
Kröger, B. and Pohle, A. 2021. Early–Middle Ordovician cephalopods from Ny Friesland, Spitsbergen—a pelagic fauna with Laurentian affinities. European Journal of Taxonomy 783: 1–102. Crossref
Kröger, B. and Zhang, Y.B. 2009. Pulsed cephalopod diversification during the Ordovician. Palaeogeography, Palaeoclimatology, Palaeoecology 273: 174–183. Crossref
Kröger, B., Servais, T., and Zhang, Y. 2009. The origin and initial rise of pelagic cephalopods in the Ordovician. PLoS ONE 4(9): e7262. Crossref
Kröger, B., Vinther, J., and Fuchs, D., 2011. Cephalopod origins and evolution: a congruent picture emerging from fossils, development and molecules. Bioessays 33: 602–613. Crossref
Landing, E. and Kröger, B. 2009. The oldest cephalopods from east Laurentia. Journal of Paleontology 83: 123–127. Crossref
Landing, E., Kröger, B., Westrop, S.R., and Geyer, G. 2023. Proposed Early Cambrian cephalopods are chimaeras, the oldest known cephalopods are 30 m.y. younger. Communications Biology 6: 32. Crossref
Li, L.Z. 1984. Cephalopods from the upper Cambrian Siyangshan Formation of western Zhejiang. In: Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (ed.), Stratigraphy and Palaeontology of Systemic Boundaries in China Cambrian–Ordovician Boundary 1, 187–265. Anhui Science and Technology Publishing House, Hefei.
Malinovskaya, V.D. 1964. Upper Cambrian nautiloids from the Maly Karatau Ridge [In Russian]. Paleontologičeskij Žurnal 1964: 56–62.
Manca, N. 1991. Organismos planctónicos en el Tremadociano inferior de los alrededores de la Quebrada de Humahuaca. Provincia de Jujuy, Argentina. Revista del Instituto de Geología y Minería 8: 141–150.
Mángano, M.G., Buatois, L.A., and Muñiz Guinea, F. 2013. Ichnology of the Alfarcito Member (Santa Rosita Formation) of northwestern Argentina: animal-substrate interactions in a lower Paleozoic wave-dominated shallow sea. Ameghiniana 42: 641–668
Meroi Arcerito, F.R., Waisfeld, B.G., and Balseiro, D. 2015. Diversification of Asaphellus Callaway, 1877 (Asaphidae: Trilobita) during the Tremadocian in South West Gondwana (Cordillera Oriental, Argentina). Geodiversitas 37: 131–150. Crossref
Meroi Arcerito, F.R., Waisfeld, B.G., Vaccari, N.E, and Muñoz, D.F., 2018. High-resolution trilobite biostratigraphy for the Early late Tremadocian (Tr2) interval (Early Ordovician) Santa Rosita formation, Argentine Cordillera Oriental. Ameghiniana 55: 531–553. Crossref
Mutvei, H. 2015. Characterization of two new superorders Nautilosiphonata and Calciosiphonata and a new order Cyrtocerinida of the subclass Nautiloidea; siphuncular structure in the Ordovician nautiloid Bathmoceras (Cephalopoda). GFF 137: 164–174. Crossref
Mutvei, H. 2020. Restudy of some plectronocerid nautiloids (Cephalopoda) from the late Cambrian of China; discussion on nautiloid evolution and origin of the siphuncle. GFF 142: 115–124. Crossref
Nohejlova, M., Nardin, E., Lefebvre, B., Vaucher, R., Vaccari, N.E., Muñoz, D.F. and Waisfeld, B.G. 2018. A new Echinodermata fauna from the early Tremadocian (Lower Ordovician) of Jujuy Province, Argentina. In: 16th International Echinoderm Conference, Nagoya, Japan, 98. Nagoya University, Nagoya.
Peterman, D.J., Barton, C., and Yacobucci, M. 2019. The hydrostatics of Paleozoic ectocochleate cephalopods (Nautiloidea and Endoceratoidea) with implications for modes of life and early colonization of the pelagic zone. Palaeontologia Electronica: art. 22.2.24. Crossref
Pohl, A., Nardin, E., Vandenbroucke, T.R.A., and Donnadieu, Y. 2016. High dependence of Ordovician ocean surface circulation on atmospheric CO2 levels. Palaeogeography, Palaeoclimatology, Palaeoecology 458: 39–51. Crossref
Pohle, A. and Jell, P.A. 2019. Filling the gap: a forgotten fauna of Cambrian nautiloid cephalopods from Australia and its implications. In: Abstracts of the 17th Swiss Geoscience Meeting, 138. Fribourg University, Fribourg.
Pohle, A. Kröger, B., Warnock, R.C.M., King, A.H., Evans, D.H., Aubrechtová, M., Cichowolski, M., Fang, X., and Christian Klug. 2022. Early cephalopod evolution clarified through Bayesian phylogenetic inference. BMC Biology 20: 88. Crossref
Ramsay, A.C. 1866. The Geology of North Wales, with an appendix on the fossils by J.W. Salter. Memoir of the Geological Survey of Great Britain 3: 1–381.
Rubinstein, C.V., Mángano, M.G., and Buatois, L.A. 2003. Late Cambrian acritarchs from the Santa Rosita Formation: Implications for the Cambrian–Ordovician boundary in the Eastern Cordillera, northwest Argentina. Revista Brasileira de Paleontología 6: 43–48.
Serra, F., Balseiro, D. and Waisfeld, B.G. 2019. Diversity patterns in upper Cambrian to Lower Ordovician trilobite communities of north-western Argentina. Palaeontology 62: 677–695. Crossref
Serra, F., Balseiro, D., Vaucher, R., and Waisfeld, B.G. 2021. Structure of trilobite communities along a delta-marine gradient (Lower Ordovician; Northwestern Argentina). Palaios 36: 39–52. Crossref
Servais, T., Perrier, V., Danelian, T., Klug, C., Martin, R., Munnecke, A., Nowak, H., Nützel, A., Vandenbroucke, T.R.A., Williams, M., and Rasmussen, C.M.Ø. 2015. The onset of the ‘Ordovician Plankton Revolution’ in the late Cambrian. Palaeogeography, Palaeoclimatology, Palaeoecology 458: 12–28. Crossref
Spagnuolo, C.M., Rapalini, A.E., and Astini, R.A. 2012. Assembly of Pampia to the SW Gondwana margin: A case of strike-slip docking? Gondwana Research 21: 406–421. Crossref
Teichert, C. 1988. Main features of cephalopod evolution. In: M.R. Clarke and E.R. Trueman (eds.), The Mollusca, 11–79. Academic Press, San Diego. Crossref
Torsvik, T.H., Van der Voo, R., Preeden, U., Mac Niocaill, C., Steinberger, B., Doubrovine, P.V., van Hinsbergen, D.J.J., Domeier, M., Gaina, C., Tohver, E., Meert, J.G., McCausland, P.J.A., and Cocks, L.R.M. 2012. Phanerozoic polar wander, palaeogeography and dynamics. Earth Science Reviews 114: 325–368. Crossref
Tortello, M.F., Zeballo, F.J., and Esteban, S.B. 2013. Trilobites Tremadocianos en facies de lutitas oscuras del Miembro Alfarcito (Formación Santa Rosita), Quebrada de Moya, Jujuy, Argentina. Ameghiniana 50: 137–152. Crossref
Tortello, M.F., Benítez, M.H., and Esteban, S.B. 2016. Trilobites and sedimentary facies of the upper Coquena Formation (late Tremadocian; Notopeltis orthometopa Zone), Cordillera Oriental, northwestern Argentina. Association of Australasian Palaeontologists; Australasian Palaeontological Memoirs 49: 1–19.
Troedsson, G.T. 1937. On the Cambro-Ordovician faunas of western Quruq tagh, eastern T’ien-shan. Palaeontologia Sinica, New Series B2: 1–74.
Ulrich, E.O. and Foerste, A.F. 1935. New genera of Ozarkian and Canadian cephalopods. Denison University Journal of Scientific Laboratories, Bulletin 30: 259–290. Crossref
Ulrich, E.O., Foerste, A.F., and Miller, A.K. 1943. Ozarkian and Canadian cephalopods. Part II: Brevicones. Geological Society of America Special Papers 59: 1–157. Crossref
Ulrich E.O., Foerste A.F., Miller A.K., and Unklesbay A.G. 1944. Ozarkian and Canadian cephalopods. Part III: Longicones and Summary. Geological Society of America Special Papers 58: 1–226.
Unklesbay, A.G. 1954. Nautiloids from the Tanyard Formation of central Texas. Journal of Paleontology 28: 637–656.
Unklesbay, A.G. and Young, R.S. 1956. Early Ordovician nautiloids from Virginia. Journal of Paleontology 30: 481–491.
Vaccari, N.E. and Waisfeld, B.G. 2010. Kainella Walcott, 1925 (Trilobita, Ordovícico Temprano) en el noroeste de Argentina y sur de Bolivia. Paleontología sistemática. Ameghiniana 47: 273–292. Crossref
Vaucher, R, Vaccari, N.E., Balseiro, D., Muñoz, D.F., Dillinger, A., Waisfeld, B.G., and Buatois, L.A. 2020. Tectonic controls on late Cambrian–Early Ordovician deposition in Cordillera Oriental (Northwest Argentina). International Journal of Earth Sciences 109: 1897–1920. Crossref
Wade, M. 1988. Nautiloids and their descendants: cephalopod classification in 1986. New Mexico Bureau of Mining and Mineral Resources, Memoir 44: 15–25.
Wade, M. and Stait, B. 1998. Subclass Nautiloidea. Introduction and fossil record. In: P.L. Beesley, G.J.B. Ross, and A. Wells (eds.), Mollusca: The Southern Synthesis, Part A, 485–493. Australian Biological Resources Study, Canberra.
Waisfeld, B.G. and Balseiro, D. 2016: Decoupling of local and regional dominance in trilobite assemblages from northwestern Argentina: new insights into Cambro-Ordovician ecological changes. Lethaia 49: 379–392. Crossref
Webers, G.F., Pojeta, J., and Yochelson, E.L. 1992. Cambrian mollusks from the Minaret Formation. In: G.F. Webers, C. Craddock, and J.F. Splettoesser (eds.), Geology and Paleontology of the Ellsworth Mountains, West Antarctica. Geological Society of America Memoir 170: 181–294. Crossref
Westermann, G.E.G. 1998. Life habits of nautiloids. In: E. Savazzi (ed.), Functional Morphology of the Invertebrate Skeleton, 263–298. John Wiley & Sons, Chichester.
Zeballo, F.J. and Albanesi, G.L. 2013. New conodont species and biostratigraphy of the Santa Rosita Formation (late Furongian–Tremadocian) in the Tilcara Range, Cordillera Oriental of Jujuy, Argentina. Geological Journal 48: 170–193. Crossref
Zeballo, F.J., Albanesi, G.L., and Ortega, G. 2005. Conodontes y graptolitos de las formaciones Alfarcito y Rupasca (Tremadociano) en el área de Alfarcito, Tilcara, Cordillera Oriental de Jujuy, Argentina. Parte1: Bioestratigrafía. Ameghiniana 42: 39–46.
Acta Palaeontol. Pol. 68 (4): 583–601, 2023
https://doi.org/10.4202/app.01103.2023