


A new thalassematid echiuran worm from the Middle Ordovician Castle Bank Biota of Wales, UK
JOSEPH P. BOTTING and LUCY A. MUIR
Botting, P.J. and Muir, L.A. 2023. A new thalassematid echiuran worm from the Middle Ordovician Castle Bank Biota of Wales, UK. Acta Palaeontologica Polonica 68 (4): 571–581.
Echiurans (spoonworms) are a very distinctive group of polychaete annelids that had long been considered to constitute a separate phylum. Their fossil record is extremely limited, although trace fossils that have been suggested to be attributable to them date back as far as the Cambrian Period. The oldest body fossils are from the Carboniferous Mazon Creek Biota, and preserve only limited morphological detail. New material from the Middle Ordovician (Darrivilian, Didymograptus murchisoni Biozone) Castle Bank Biota of Wales shows fine detail of the morphology of a new taxon, Llwygarua suzannae gen. et sp. nov., including several details that indicate an assignment to the derived family Thalassematidae, allied to the speciose genus Ochetostoma. These details include proboscis morphology, anterior setae, and muscle organisation within the trunk. An additional specimen is described in open nomenclature, as it may be either a distinct species, or a juvenile of Llwygarua suzannae gen. et sp. nov. with a relatively elongated proboscis. These worms demonstrate a very early and previously unrecognised diversification of the echiuran grown group, further supporting an early diversification of Annelida as a whole.
Key words: Annelida, Echiura, Thalassematidae, Darriwilian, Middle Ordovician, United Kingdom.
Joseph P. Botting [acutipuerilis@yahoo.co.uk; ORCID 0000-0003-0388-8677 ], Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, 39 East Beijing Road, Nanjing 210008, China; Department of Natural Sciences, Amgueddfa Cymru-National Museum Wales, Cathays Park, Cardiff CF10 3NP, UK.
Lucy A. Muir [lucy@asoldasthehills.org; ORCID 0000-0001-6324-2259 ], Department of Natural Sciences, Amgueddfa Cymru-National Museum Wales, Cathays Park, Cardiff CF10 3NP, UK.
Received 20 September 2023, accepted 13 November 2023, available online 29 December 2023.
Copyright © 2023 J.P. Botting and L.A. Muir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Echiurans were long considered to be a distinct phylum, but are now resolved as a morphologically divergent group within the polychaete annelids, with their sister group being the Capitellidae (Parry et al. 2014, 2016; Goto et al. 2020). Their fossil record is limited and ambiguous, with the oldest reasonably convincing example being Coprinoscolex ellogimus from the Pennsylvanian (Carboniferous) Mazon Creek Biota (Jones and Thompson 1977). Trace fossils assigned to echiurans include some from the Neogene (Izumi and Yoshizawa 2016), and possibly also more generally distributed ichnofossils such as Zoophycos (Kotake 1992), Treptichnus-like burrows containing particular pellet morphologies (Orłowski and Żylińska 1996) and certain star-shaped trace fossils (Ohta 1984). Such trace fossils may suggest a long record for echiurans, potentially extending back to the Cambrian, but are invariably inconclusive, and other organisms may have generated similar traces; detailed body fossils are therefore essential for tracing echiuran evolution.
As echiurans are entirely soft-bodied, exceptional preservation is required to generate recognisable fossils. Even in the Chengjiang Biota, which preserves many of the extant “worm” phyla (Ma et al. 2010), no echiurans have been recognised. Their cuticles are relatively thick, suggesting that they should have been fossilized in some Burgess Shale-type faunas if they were present at the time, but so far none have been recognized. Extant echiurans include some unusual and potentially distinctive morphological features, however, particularly in relation to the form of the relatively soft proboscis and muscle bands, and the presence of a pair of anterior setae (Stephen and Edmonds 1972). Some of the potentially more preservable features such as the anal hooks that are seen in many taxa are likely derived features that are not present in Coprinoscolex ellogimus, are not universally present in modern taxa, and represent adaptations to burrowing (Orłowski and Żylińska 1996).
This paper describes a new echiuran genus and species based on body fossils with details of the distinctive proboscis, anterior setae and musculature from the Darriwilian (Middle Ordovician) Castle Bank Burgess Shale-type fauna of Wales, UK. One additional echiuran specimen is described in open nomenclature.
Institutional abbreviation.—NMW, Amgueddfa Cymru National Museum Wales, Cardiff, UK.
Nomenclatoral acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: urn:lsid:zoobank.org:pub:98D701B1-231A-409F-8CD2-ABBBA3264C45.
Geological setting
The specimens described here are from the Darriwilian, Middle Ordovician (Didymograptus murchisoni Biozone) Castle Bank Biota of central Wales (Fig. 1). Previous papers have described sponges (Botting 2021; Botting and Ma 2022; Botting et al. 2023a) and opabiniid-like arthropods (Pates et al. 2022), with an introduction to the assemblage and sedimentology provided by Botting et al. (2023b). The Castle Bank location lies within the Gilwern Volcanic Formation of the Builth-Llandrindod Inlier (Schofield et al. 2004). This area records the entire history of a volcanic island sequence (Botting and Muir 2008) through approximately ten million years, with richly fossiliferous horizons at all levels. Castle Bank falls into the upper part of the Gilwern Volcanic Formation, a short distance stratigraphically below the Cwm Amliw Tuff Formation, and the biostratigraphic age is constrained by abundant graptolites (Botting et al. 2023b). Details of the Castle Bank site are not being published to protect the fauna from uncontrolled collecting, but exact locality information is deposited with the specimens in Amgueddfa Cymru National Museum Wales and is thus available to researchers.
The Castle Bank deposits represent the waning phase of volcanism, with eruptions still continuing in the south of the inlier at this time (Schofield et al. 2004). Interpretation of the palaeoenvironment indicates a water depth around storm wave base, probably between 50 and 100 m below sea level, with burial under low-oxygen conditions (Botting et al. 2023b). The sediment consists of a sequence of finely laminated siltstone and mudstone beds, micaceous siltstone, and volcanic ash layers, with the background fossil assemblage composed dominantly of planktonic graptolites and small phosphatic brachiopod valves.
Fossils with exceptional preservation, showing carbon-film preservation of even labile tissues such as internal organs, are primarily limited to an interval 20 cm thick, known as horizon A3–A4 (Botting et al. 2023b). This interval is composed dominantly of thin (up to 4 cm thick) event beds composed of transported micaceous silt, and contains locally abundant remains of entirely soft-bodied organisms, including the material described here.
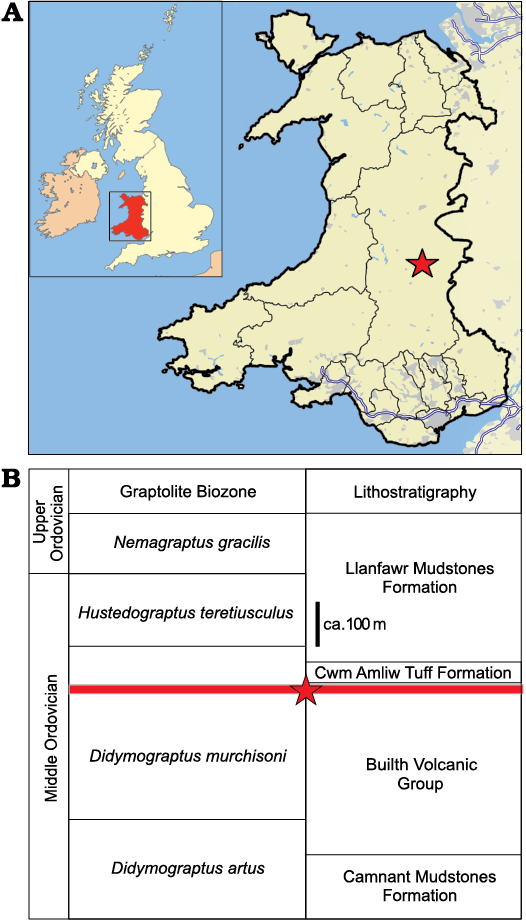
Fig. 1. A. Location of Castle Bank within Wales (highlighted within UK, inset). B. Stratigraphic position within the sequence of the Builth-Llandrindod Inlier. Asterisk indicates Castle Bank locality.
Material and methods
Imaging of the specimens was carried out with a GXCAM HICHROME AF MET 2 megapixel digital camera (GX Microscopes, Wickhambrook, Suffolk, UK) attached to a Leica S8 APO stereomicroscope (Leica, Wetzlar, Germany), illuminated primarily with a Leica LED 3000 RL cross-polarising ring light. Specimens were photographed dry, without water or alcohol; this is because the relatively coarse-grained and unmetamorphosed siltstone is prone to cracking when wetted and dried, and it was not safe immerse it. Images were processed (brightness and contrast) with Open Office, and stacking of images was performed with Helicon Focus 8. All studied fossil material is housed in Amgueddfa Cymru National Museum Wales, Cardiff (UK).
Systematic palaeontology
Phylum Annelida Lamarck, 1802
Class Polychaeta Grube, 1850
Subclass Echiura Sedgwick, 1898
Suborder Echiurida Sedgwick, 1898
Family Thalassematidae Forbes and Goodsir, 1841
Genus Llwygarua nov.
ZooBank LSID: urn:lsid:zoobank.org:act:7D02B25D-F3F1-476E-8196- 387F4BAFF9EE
Etymology: From Welsh Llwy garu, a love spoon: a traditional carved wooden spoon, given as a gift of romantic intent, in Wales; in reference to echiurans (spoon worms). Pronounced “H-loui-garry-a”. Gender feminine.
Type species: Llwygarua suzannae sp. nov., see below. By monotypy.
Diagnosis.—As for the type species.
Remarks.—Recognition of the new genus as an echiuran is based on the presence of several key features, including the presence and form of the proboscis, diagnostic muscle-band arrangement, anterior setal pair and the presence of a differentiated caudal region. The combination of characters that have been preserved is fully diagnostic of the Echiurida; in particular, no other worms show the same bilobed proboscis morphology or pair of anterior setae, and the musculature is also highly distinctive.
No described fossil echiurans show even an approximate similarity to the new genus. The closest similarity is to the extant thalassematids such as Ochetostoma Rüppell and Leuckart, 1828 (e.g., Fig. 2A), but assignment of an Ordovician fossil to a living genus would be highly contentious, and the current morphological classification of genera within the family appears unreliable (Goto et al. 2020). Some of the key characters that are applied in living taxa (e.g., the number and arrangement of nephridia) cannot be assessed, so such an assignment would be based on incomplete characters. There are also some possibly unique characters in the fossil specimens described here, such as the reticulate structure of the proboscis, that support separation. Overall, diagnosis of the new species and genus is based on the limited characters that are preserved, and these cannot be used to separate it easily from extant thalassematids; although a distinct generic assignment is preferred, it remains possible that future discoveries and advances in classification of modern taxa will support a reassignment of Llwygarua gen. nov. to an extant genus.
Stratigraphic and geographic range.—Darriwilian, Middle Ordovician (Didymograptus murchisoni Biozone) of Castle Bank, near Llandrindod, Wales, UK.
Llwygarua suzannae sp. nov.
Figs. 2B, 3, 4.
ZooBank LSID: urn:lsid:zoobank.org:act:ADCFAAFC-D007-496D-AC8A-3809F1CA6F7E
Etymology: After Suzanne Douel, a retired biology teacher who has supported research on the Castle Bank fauna since its discovery.
Type material: Holotype, NMW.2021.3G.99, complete specimen in lateral view. Paratype, NMW.2021.3G.100 , complete specimen with folded proboscis from the type locality and horizon.
Type locality: Castle Bank, near Llandrindod, Wales, UK.
Type horizon: Didymograptus murchisoni Biozone, Darriwilian, Middle Ordovician.
Material: Type material and probably also NMW.2021. 3G.101, complete specimen in dorsal view, with fine details lacking, from the type locality and horizon.
Diagnosis.—Trunk widest at anterior, tapering to short, bluntly pointed caudal tip, which curves slightly ventrally; no obvious anal hooks; proboscis approximately half length of trunk and nearly as broad, bilobed with slightly irregular margins and with short anterior projections either side of gutter along the midline; texture of proboscis weakly reticulate. Pair of stout, straight anterior setae. Longitudinal trunk muscles form approximately 8–12 broad bands; oblique transverse musculature finer but also fasciculated.
Description.—Worm with trapezoidal trunk and broad, bilobed proboscis. Holotype (Fig. 2B) preserved in oblique lateral view, and paratype (Fig. 3) mostly lateral with proboscis folded over and with lobes flattened out to either side. The holotype is 7 mm long and 1.5 mm wide, and the paratype is 5.5 mm long and 1.2 mm wide. The additional specimen is also 5.5 mm long and 1.2 mm wide. The significant information is derived from the holotype and paratype. The specimen NMW.2021.3G.101 (Fig. 4) shows the same proportions and body shape (including narrowed caudal region), but is complicated by wrinkling and lacks the fine detail to confirm the assignment.
Trunk broadest close to anterior end, tapering gradually towards posterior, then more rapidly in the posteriormost third (change in angle of taper much stronger on ventral side than dorsal). Posterior termination bluntly pointed, bending ventrally (Fig. 2B1). In the paratype, there are several irregular projections (Fig. 3A4) that may result from decay of this region, and possibly reflect some internal structures of the anal region, but are too indistinct to be clearly interpreted.
Surface of trunk is wrinkled, presumably due to compaction of the cuticle, which shows no distinct texture and was apparently smooth. Anterior ventral setal pair visible in holotype (one distinct, the other probably present but partly overlapping compressed margin; Fig. 2B4). Setae straight, at least 0.3 mm long (base uncertain), and relatively robust with blunt end.
Multiple dark longitudinal bands are visible in the trunk of the paratype (Fig. 3A1, A2), and less clearly in the holotype (Fig. 2B1); these are interpreted as longitudinal subdermal muscle bands. The number of these cannot be counted accurately due to the superposition of the two sides of the body wall, but the estimated number is 8–12. Finer oblique to transverse lines are superimposed on the primary muscle bands in the paratype, forming narrow bundles (probably of at least 2–3 fibres) at around 70° to the longitudinal structures (Fig. 3A2). Oblique structures only locally visible, but are aligned with steps in trunk outline (Fig. 3A1) and therefore appear to have exerted some control of deformation during burial; holotype shows similarly-angled fabric.
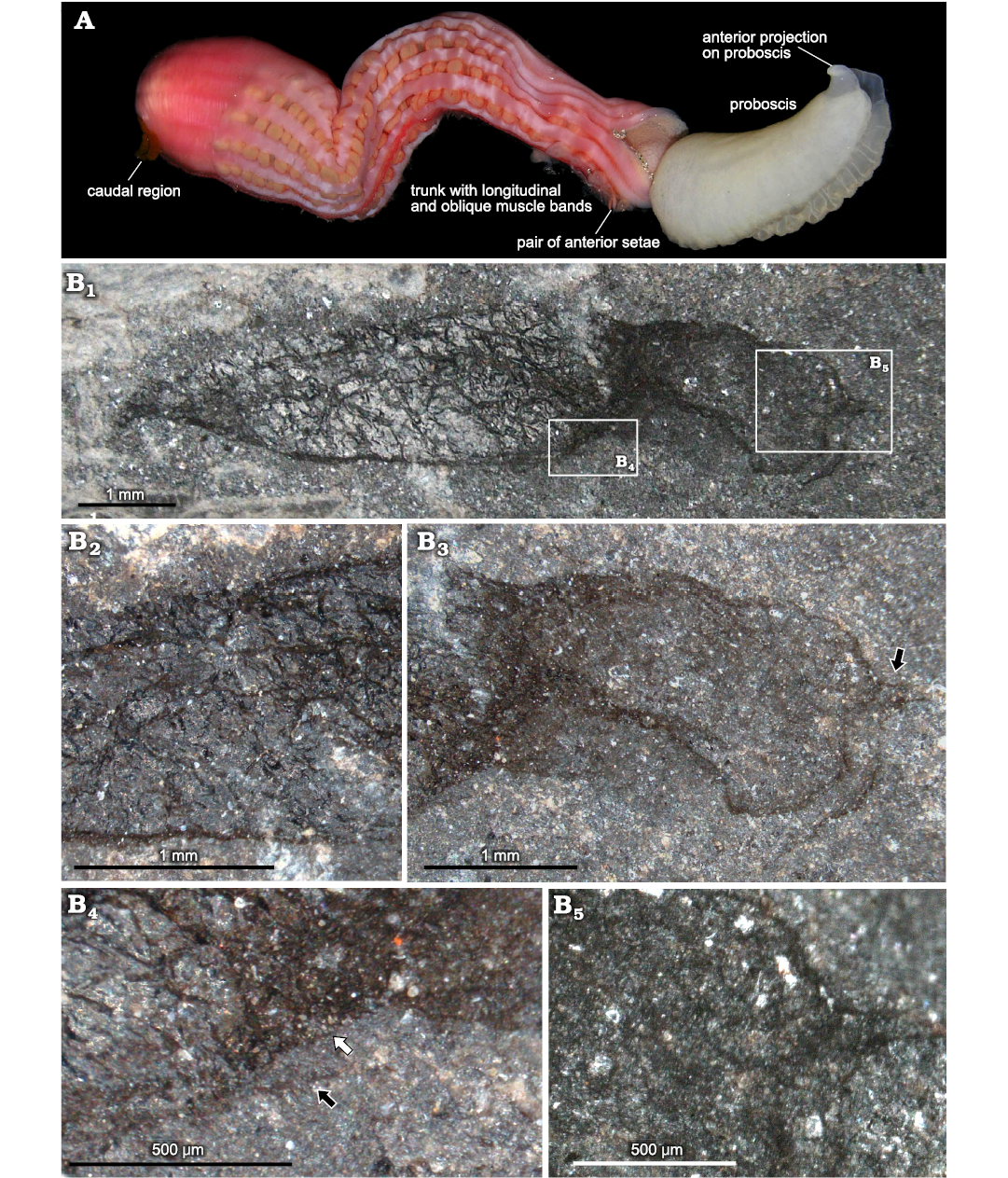
Fig. 2. Recent thalassematid echiuran (A) and a fossil equivalent (B). A. ?Ochetostoma sp., highlighting significant features for comparison with the fossils (photograph courtesy of Arthur Anker), typical length 10–20 cm. B. Holotype of Llwygarua suzannae gen. et sp. nov., from the Darriwilian (Middle Ordovician) of Castle Bank, Wales, UK, NMW.2021.3G.99 showing overall specimen in slightly oblique lateral view (B1); enlargement with anterior ventral part of trunk (B4), showing one anterior seta (white arrow) and probably second seta (black arrow); detail of proboscis in cross-polarised light (B5), showing reticulate structure and anterior projections adjacent to midline; central region of trunk with distinctive wrinkling of cuticle in response to compression (B2), and proboscis in plane-polarised light (B3), with anterior projections arrowed.

Fig. 3. Thalassematid echiuran Llwygarua suzannae gen. et sp. nov., from the Darriwilian (Middle Ordovician) of Castle Bank, Wales, UK, paratype NMW.2021.3G.100. A1, overall view; A2, close up showing detail of central region of trunk, with muscle arrangement of longitudinal and oblique muscle bundles (arrowed); A3, proboscis with anterior projections arrowed, A5, close up showing reticulate structure; A4, caudal region with irregular structures possibly resulting from damage.
Proboscis (Figs. 2B3, B5, 3A3) with midline separating two lobes, approximately half as long as trunk, and around 80% as wide (depending on angle of flattening). Margin slightly undulating and finely crenulated (Fig. 3A5), but on larger scale effectively straight until broadly rounded apex (Fig. 2B3). Based on angles of flattening, the two lobes curl inwards ventrally. Internal darker strands mark the midline between lobes (Figs. 3A3, 4A2), around the expected location of the gutter leading to the mouth. Either side of the midline, short (0.1–0.2 mm) sharply rounded projections extend from the apex (Figs. 2B3, B5, 3A3); these projections are also weakly preserved in the additional specimen (Fig. 4A3). The lobes of the proboscis show a distinct polygonal reticulation of darker lines (Figs. 2B5, 3A5), similar in preservation to that of nervous tissue in arthropods (Botting et al. 2023b).

Fig. 4. Thalassematid echiuran Llwygarua suzannae gen. et sp. nov., from the Darriwilian (Middle Ordovician) of Castle Bank, Wales, UK, NMW.2021.3G.101. A1, overall view of worm in probably dorsal view; A2, proboscis and midline structures; A3, detail of apex of proboscis, the anterior projections faintly visible (arrowed); A4, counterpart of A1; A5, close up of A4 showing caudal region.
Remarks.—The new species is reconstructed in Fig. 5. There are no comparable fossil echiurans with which this species could be confused. Coprinoscolex ellogimus Jones and Thompson, 1977, from the Carboniferous Mazon Creek Biota shows little fine detail but is different in shape (tapered at both ends), with a much smaller, fan-like proboscis and no anterior setae (Jones and Thompson 1977). Those authors used the lack of setae to argue that the species should be assigned to the extant echiuran family Bonellidae, but this is not strong evidence. The specimens typically also contain Tomaculum-like, cylindrical pellets, for which there is no evidence in the Castle Bank Biota. Such pellets could be produced by various worms, including annelids and priapulids (Hu et al. 2021), and the assignment of Coprinoscolex ellogimus to the echiurans was based primarily on the proboscis, body form and convoluted gut. Whilst this is reasonably compelling, there is little similarity of Coprinoscolex ellogimus to Llwygarua suzannae gen. et sp. nov.
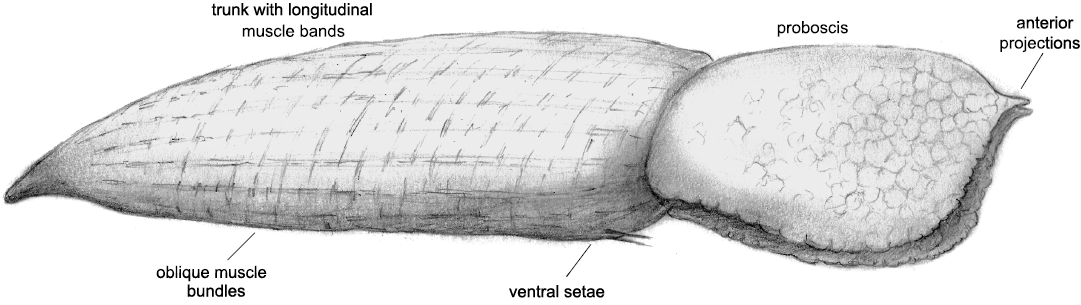
Fig. 5. Reconstruction of Llwygarua suzannae gen. et sp. nov.; observed size range: 5.5 to 7 mm long.
The most challenging feature to interpret in the new material is the reticulation on the proboscis, which has no obvious counterpart in living echiurans. However, the structures and sculpture of modern echiuran probosces are diverse, including a variety of ridged forms (Maiorova and Adrianov 2018), and it is possible that this feature represents either a surface texture, or an internal organisation of, e.g., nerves or blood vessels. No such features are known in the very limited material of other echiuran fossils, however, and such anatomical details as could explain the structures are not preserved in Coprinoscolex ellogimus; at this stage the true interpretation of the reticulation is unknown.
Stratigraphic and geographic range.—Type horizon and locality only.
Echiura gen. et sp. indet.
Fig. 6.
Material.—NMW.2021.3G.102, complete specimen in lateral view, from level A3–A4 at the type locality and horizon for Llwygarua suzannae gen. et sp. nov.
Description.—A single specimen preserved in lateral view. Length 2.1 mm, of which 1.3 mm is the trunk, and 0.8 mm the more flexible, wrinkled proboscis (which is otherwise preserved with similar appearance to the trunk). Maximum dorsoventral width 0.3 mm, constant in anterior half then gradually tapering to sharply rounded tip. Caudal apex showing three or four possible blunt projections, up to 0.08 mm long, directly obliquely backwards on the ventral surface (Fig. 6A3, A4).
Proboscis maximum width almost as wide as trunk, sinuous, narrowing immediately above junction with trunk, but then expanding slightly to 0.3 mm, before narrowing to apex that is truncate and 0.2 mm wide (Fig. 6A1). Proboscis marked by irregular reticulation (slightly elongated longitudinally) of polygonal cells up to 0.07 mm long (Fig. 6A2). Margins of proboscis with fine serration or tuberculation. Apical margin apparently slightly frayed, with diverging strands from reticulation. No setae or muscles preserved.
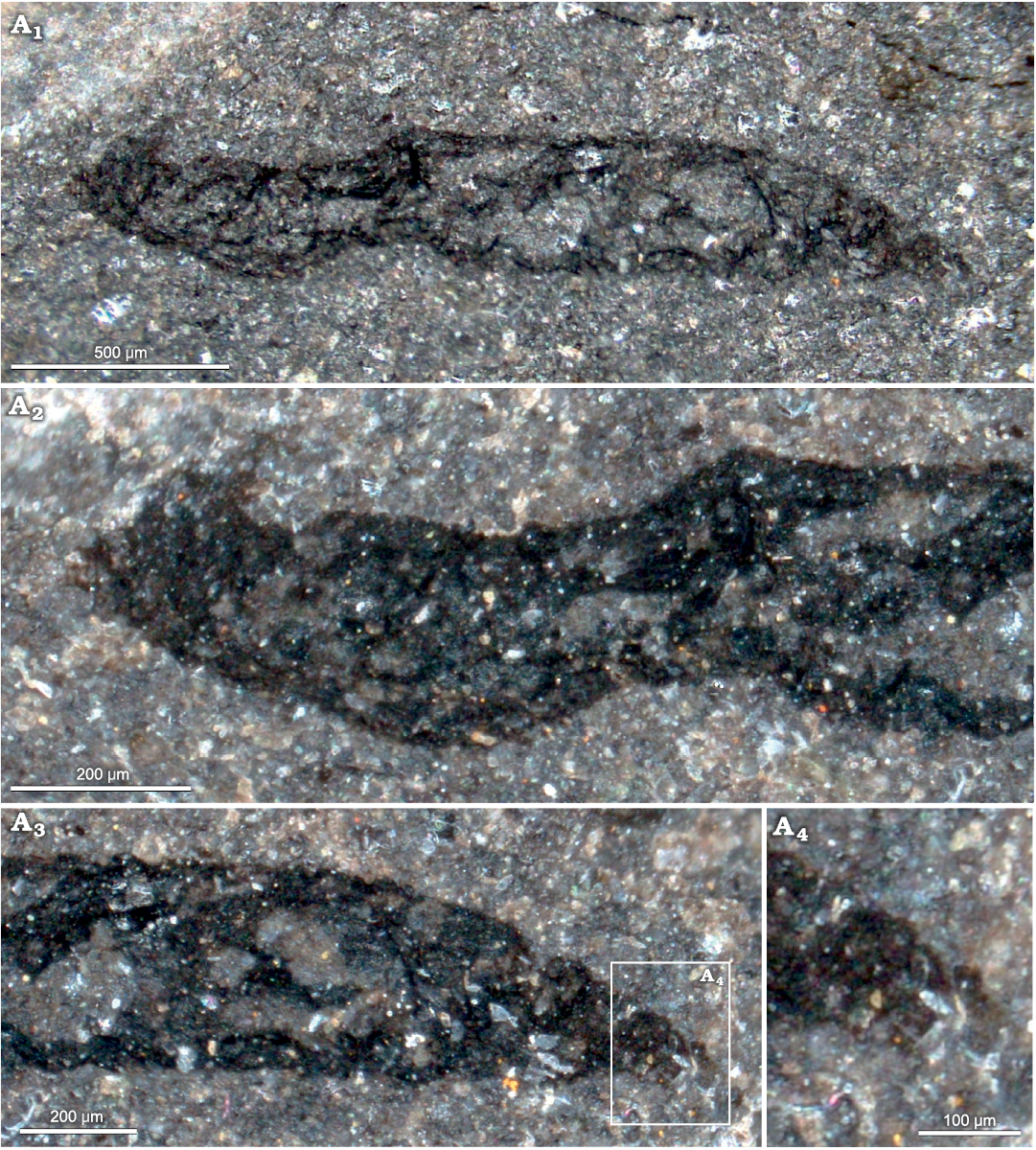
Fig. 6. Echiura gen. et sp. indet. from the Darriwilian (Middle Ordovician) of Castle Bank, Wales, UK, NMW.2021.3G.102. A1, overall view; A2, proboscis; A3, caudal region; A4, close up showing projections from anal region. A1 in plane-polarised light, A2–A4 in cross-polarised light.
Remarks.—No categorically diagnostic echiuran characters are visible in this specimen, but it shows close similarity to Llwygarua suzannae gen. et sp. nov. in most features, including the preservation, body shape and reticulation of the proboscis, and is presumed to be closely related to that species. Nonetheless, it differs in several key features. In particular, the proboscis is proportionally much longer and more sinuous. This is a fundamental difference and may well indicate its status as a separate species. The projections in the anal region also appear to differ significantly from Llwygarua suzannae gen. et sp. nov., but this would need to be confirmed from additional specimens.
Aside from being difficult to describe as a new species based on only a single specimen, the small size of this worm compared with the material of Llwygarua suzannae gen. et sp. nov. makes possible an interpretation as a juvenile of the latter. This interpretation would require allometric growth of the proboscis and trunk, with the proboscis being relatively long in juveniles. Such changes can occur in the related sipunculans (Schulze and Rice 2009), so this hypothesis is not unreasonable; however, the reticulation of the proboscis is on a similar scale to (and slightly coarser than) that in the much larger Llwygarua suzannae gen. et sp. nov. specimens, and this is difficult to reconcile with a growth difference. The anal structures, if confirmed, would also be compelling evidence of this being a distinct species.
Discussion
Taxonomic affinity and implications for echiuran diversification.—The Castle Bank echiurans are the oldest yet found, pre-dating the Carboniferous Coprinoscolex ellogimus by around 150 Myr. Although the echiuran fossil record is extremely sparse (no other body fossils have been recorded), this is an unexpectedly early date for this major group of annelids, and it might be assumed that the fossils represent an early-branching member of the echiuran clade. However, the morphology of the proboscis and the preserved musculature are diagnostic of the extant family Thalassematidae, and particularly close to the large genus Ochetostoma. This family was recovered by Goto et al. (2013) as topologically the most derived family of echiurans, although the Bonellidae and Ikedidae had longer branches leading to them. Within the Thalassematidae, Ochetostoma was recovered as the most derived genus by Goto et al. (2013), but genera in the family were not recovered as monophyletic by Goto et al. (2020).
The primary diagnostic features of Ochetostoma are the strongly bundled longitudinal trunk muscles, combined with finer but also fasciculated oblique muscles (Stephen and Edmonds 1972). Both muscle types are visible in the paratype, the longitudinal muscle bands being prominent, and the fasciculated oblique muscles fainter but locally visible. The non-monophyletic status of the genus recovered by Goto et al. (2020), however, makes this character potentially unreliable for phylogenetic classification, and the morphological characters needed to define genera may need to be reassessed. The number of longitudinal muscle bands is a species-level diagnostic character, and in living taxa ranges from seven to twenty-two (Stephen and Edmonds 1972). The exact number of longitudinal muscle bands in the new species is unclear due to superimposition and obscuration of the two surfaces, but the observable spacing suggests at least 8–10 bands around the circumference, and therefore falling within the lower end of the modern range.
The morphology of the proboscis is also strongly reminiscent of some species assigned to the extant Ochetostoma, including the broad, bilobed form with limited incurvature at the margins, the paired anterior projections either side of the midline, and the slightly scalloped, lobate lateral margins (Figs. 2B3, 3A3). Other echiuran groups have a wide diversity of proboscis morphology, ranging from strongly bifurcating to highly elongate, or strongly enrolled to become partly tubular. The morphology of the proboscis appears to be evolutionarily flexible, but the close similarity to some modern thalassematids is nonetheless remarkable.
The combination of proboscis morphology with the diagnostic trunk musculature requires an assignment not only to the Echiura, but also to the Thalassematidae, with an apparently close affinity to species that have been assigned to Ochetostoma. The taxonomic unreliability of the morphological characters, however, precludes an assignment to any genus within the family. Based on the sparse previous record (including their absence from other Burgess Shale-type faunas), this is a remarkable and unexpected finding, which if we accept the current molecular phylogenies, suggests that much of echiuran diversification had already occurred by the Middle Ordovician. There is little published work predicting the timing of origin of echiurans from molecular clocks, with one relatively unconstrained polychotomy in Parry et al. (2014) indicating an origin of the group after the beginning of the Ordovician. There is, however, increasing evidence for diversification of the annelid crown group within the early Cambrian (Chen et al. 2020; Zhang et al. 2023; Zhao et al. 2023). Given the position of echiurans + capitellids as being sister to Terebellida + Arenicolida (Kobayashi et al. 2022), and the apparent presence of a relatively derived, cirratuliform terebellid in the early Cambrian (Zhang et al. 2023), the presence of echiurans in the Middle Ordovician is not unexpected.
Preservation of muscles.—Interpretation of soft-bodied fossils is dependent on the preservational biases affecting their visible morphology, and the ability to distinguish real morphological features from taphonomic artefacts and mineralogical overprints. Different tissue types and organs often preserve very differently within and between deposits, and therefore knowledge of the typical preservation in the deposit in question is crucial to palaeobiological interpretation. Investigations into the taphonomy of Castle Bank are, however, in their very early stages, with only some generalisations yet being available. Initial results indicate the presence of a wide range of tissue types (Pates et al. 2022; Botting et al. 2023b), from sclerotised to entirely soft and delicate structures such as food-gathering tentacles. Internal organs include at least gut preservation and structures resembling neural tissue (Botting et al. 2023b), indicating at least the potential for other internal tissue types.
Preservation at Castle Bank is almost entirely as carbon films, with localised pyrite framboids (Botting et al. 2023b). In appearance, the unweathered fossils closely resemble those of the Burgess Shale, being composed largely of thin, reflective films and darker brown-to-black carbonaceous remains. Castle Bank has not been significantly metamorphosed or weathered, so the fossils lack substantial secondary clay minerals or oxides, and are less compressed (often preserved with a degree of wrinkling). Differentiation of the tissue types within the fossils has not yet been established in detail, but softer, more labile tissues often appear darker, rather than as dense reflective films that represent more sclerotized material. In the present paper there are structures interpreted as muscle bands based on their morphology and position. Muscles have not yet been recognised in other Castle Bank fossils, and here are much clearer in one specimen than the other.
In Cambrian Burgess Shale-type faunas, muscle-related structures are preserved commonly in chordates, but are also known or suspected from other groups such as the problematic Atalotaenia adela Garcia-Bellido Capdevila and Conway Morris, 1999, from the lower Cambrian Kinzers Formation of Pennsylvania, and possible muscle-associated collagenous tissues in the problematic, possible ctenophore Siphusauctum gregarium O’Brien and Caron, 2012 from the middle Cambrian Burgess Shale of British Columbia. Muscles themselves do not appear to be preserved within the Burgess Shale (Butterfield 2003; Gaines et al. 2012), consistent with taphonomic experiments that indicate loss of labile internal organs at a similar speed to loss of muscles, with significant decay within a few days (e.g., Sansom 2016). However, this pattern is contradicted by work on chordates, where myomeres apparently decay very slowly (Sansom et al. 2010), and are a diagnostic feature of the fossils (Conway Morris 2008). In the Chengjiang Biota, certain structures have been interpreted as muscles in, for example, arthropods (Fu and Zhang 2011), lobopodians (Vannier and Martin 2017), and cnidarians (Hou et al. 2005). Butterfield (2003) proposed that muscles are not preservable even in the Burgess Shale itself due to their labile nature, and emphasised that such labile structures cannot be recognised on the basis of morphology alone. However, neural tissue is now widely recognised across several Cambrian deposits, including the Burgess Shale, in arthropods, priapulids, annelids and ctenophores (Ortega-Hernández et al. 2019; Parry et al. 2021); such labile tissues may have been preserved preferentially relative to other tissues through specific chemical processes (Saleh et al. 2022). Preservation of labile tissues such as muscles therefore requires explanation, but cannot be assessed purely on the basis of a simple taphonomic gradient.
The preservation of muscle bands in the Ordovician Castle Bank echiurans suggests some taphonomic difference from the Cambrian Burgess Shale and equivalents. However, in this case the muscles are strong morphological features, with discrete and identifiable morphology, and the group is not present in the Burgess Shale. Sipunculans (a related group with similarly substantial muscles associated with the proboscis) are present in the Chengjiang Biota (Huang et al. 2004), but rarely, and muscles do not appear to be preserved in those specimens. In the case of the Castle Bank echiurans, longitudinal muscle bands in modern forms are sometimes associated with colour changes visible on the exterior (Stephen and Edmonds 1972; Fig. 2A), and it is possible that differential pigmentation or other cuticular characters could be exaggerating a preservational difference.
Given the known taphonomy of the Castle Bank fauna, and comparison with muscle preservation in the Chengjiang Biota, muscle preservation should not be unexpected. The presence of muscles may, however, suggest a slight difference in taphonomy from the Burgess Shale itself, with some aspect of the preservational history making muscle preservation more likely.
Conclusions
A new species of fossil echiuran worm from the Darriwilian, Middle Ordovician, Castle Bank Biota is not only the oldest known by some 150 million years, but also appears to be morphologically extremely derived, and assignable to the extant family Thalassematidae. This discovery indicates a very early cryptic diversification of the echiurans, supporting recent discoveries of other relatively derived annelids in early Cambrian Burgess Shale-type faunas (Chen et al. 2020; Zhang et al. 2023).
Acknowledgements
We thank Luke Parry (University of Oxford, UK), for suggesting features to look for in these fossils, and Olev Vinn (University of Tartu, Estonia) and Zhifei Zhang (Northwest University, Xian, China) for supportive reviews. Arthur Anker (Universidade Federal de Pelotas, Rio Grande do Sul, Brazil) generously provided the image of a live thalassematid in Fig. 2A. We appreciate the support of the landowners and their wider family throughout this project, and assistance on fieldwork from Xenia Warren and Berwyn Powell (Llandrindod, UK). The microscope equipment was purchased through crowdfunding, including a Holloway Bursary of the Warwickshire Geological Conservation Society. No other funding was received for this paper.
References
Botting, J.P. 2021. Hexactins in the “protomonaxonid” sponge Choiaella and proposal of Ascospongiae (class nov.) as a formal replacement for the Protomonaxonida. Bulletin of Geosciences 96: 265–277. Crossref
Botting, J.P. and Ma, J.Y. 2022. A probable hyalonematid sponge (Hexactinellida: Amphidiscophora) from the Middle Ordovician of the Builth Inlier, Wales. Palaeoworld 31: 621–632. Crossref
Botting, J.P. and Muir, L.A. 2008. Unravelling causal components of the Ordovician Radiation: the Builth Inlier (central Wales) as a case study. Lethaia 41: 111–125. Crossref
Botting, J.P., Muir, L.A., and Ma, J. 2023a. Teganium (Porifera, Hexactinellida) from the Middle Ordovician Castle Bank fauna of Avalonia (Wales, UK). Palaeontologia Electronica 26: 1–17. Crossref
Botting, J.P., Muir, L.A., Pates, S., McCobb, L.M., Wallet, E., Willman, S., Zhang, Y., and Ma, J. 2023b. A Middle Ordovician Burgess Shale-type fauna from Castle Bank, Wales (UK). Nature Ecology & Evolution 7: 1–9. Crossref
Butterfield, N.J. 2003. Exceptional fossil preservation and the Cambrian Explosion. Integrative and Comparative Biology 43: 166–177. Crossref
Chen, H., Parry, L.A., Vinther, J., Zhai, D., Hou, X., and Ma, X. 2020. A Cambrian crown annelid reconciles phylogenomics and the fossil record. Nature 583: 249–252. Crossref
Conway Morris, S. 2008. A redescription of a rare chordate, Metaspriggina walcotti Simonetta and Insom, from the Burgess shale (middle Cambrian), British Columbia, Canada. Journal of Paleontology 82: 424–430. Crossref
Forbes, E. and Goodsir, J. 1841. On the natural history and anatomy of Thalassema and Echiurus. Edinburgh New Philosophical Journal 30: 369–378.
Fu, D. and Zhang, X. 2011. A new arthropod Jugatacaris agilis n. gen. n. sp. from the Early Cambrian Chengjiang Biota, South China. Journal of Paleontology 85: 567–586. Crossref
Gaines, R.R., Hammarlund, E.U., Hou, X., Qi, C., Gabbott, S.E., Zhao, Y., Peng, J., and Canfield, D.E. 2012. Reply to Butterfield: Low-sulfate and early cements inhibit decay and promote Burgess Shale-type preservation. Proceedings of the National Academy of Sciences of the United States of America 109 (28): E1902. Crossref
Garcia-Bellido Capdevila, D. and Conway Morris, S. 1999. New fossil worms from the Lower Cambrian of the Kinzers Formation, Pennsylvania, with some comments on Burgess Shale-type preservation. Journal of Paleontology 73: 394–402. Crossref
Goto, R., Monnington, J., Sciberras, M., Hirabayashi, I., and Rouse, G.W. 2020. Phylogeny of Echiura updated, with a revised taxonomy to reflect their placement in Annelida as sister group to Capitellidae. Invertebrate Systematics 34: 101–111. Crossref
Goto, R., Okamoto, T., Ishikawa, H., Hamamura, Y., and Kato, M. 2013. Molecular phylogeny of echiuran worms (Phylum: Annelida) reveals evolutionary pattern of feeding mode and sexual dimorphism. PLoS One 8: e56809. Crossref
Grube, A.E. 1850. Die Familien der Anneliden. Archiv für Naturgeschichte, Berlin 16: 249–364.
Hou, X.G., Stanley, G., Zhao, J., and Ma, X.Y. 2005. Cambrian anemones with preserved soft tissue from the Chengjiang biota, China. Lethaia 38: 193–203. Crossref
Hu, Y., Knaust, D., Liang, Y., Holmer, L.E., and Zhang, Z. 2021. Burrows filled with faecal pellets from the Cambrian (Stage 4) Guanshan biota of South China and their palaeoecological implications. Palaeogeography, Palaeoclimatology, Palaeoecology 567: 110249. Crossref
Huang, D.Y., Chen, J.Y., Vannier, J., and Saiz Salinas, J.I. 2004. Early Cambrian sipunculan worms from southwest China. Proceedings of the Royal Society of London. Series B: Biological Sciences 271: 1671–1676. Crossref
Izumi, K. and Yoshizawa, K. 2016. Star-shaped trace fossil and Phymatoderma from Neogene deep-sea deposits in central Japan: probable echiuran feeding and fecal traces. Journal of Paleontology 90: 1169–1180. Crossref
Jones, D. and Thompson, I.D.A. 1977. Echiura from the Pennsylvanian Essex fauna of northern Illinois. Lethaia 10: 317–325. Crossref
Kobayashi, G., Itoh, H., and Nakajima, N. 2022. First mitochondrial genomes of Capitellidae and Opheliidae (Annelida) and their phylogenetic placement. Mitochondrial DNA Part B 7: 577–579. Crossref
Kotake, N. 1992. Deep‐sea echiurans: possible producers of Zoophycos. Lethaia 25: 311–316. Crossref
Lamarck, J.-B. de 1802. Discours d’Ouverture, Prononcé le 27 floréal An 10, au Muséum d’Histoire naturelle. Recherches sur l’organisation des corps vivans. Bulletin Scientifique de la France et de la Belgique, 5th series 40: 483–517.
Ma, X., Hou, X., and Baines, D. 2010. Phylogeny and evolutionary significance of vermiform animals from the Early Cambrian Chengjiang Lagerstätte. Science China Earth Sciences 53: 1774–1783. Crossref
Maiorova, A.S. and Adrianov, A.V. 2018. Deep-sea spoon worms (Echiura) from the Sea of Okhotsk and the adjacent slope of the Kuril-Kamchatka Trench. Deep Sea Research Part II: Topical Studies in Oceanography 154: 177–186. Crossref
O’Brien, L.J. and Caron, J.B. 2012. A new stalked filter-feeder from the middle Cambrian Burgess Shale, British Columbia, Canada. PLoS One 7: e29233. Crossref
Ohta, S. 1984. Star-shaped feeding traces produced by echiuran worms on the deep-sea floor of the Bay of Bengal. Deep Sea Research Part A. Oceanographic Research Papers 31: 1415–1432. Crossref
Orłowski, S. and Żylińska, A. 1996. Non-arthropod burrows from the middle and late Cambrian of the Holy Cross Mountains, Poland. Acta Palaeontologica Polonica 41: 385–409.
Ortega-Hernández, J., Lerosey-Aubril, R., and Pates, S. 2019. Proclivity of nervous system preservation in Cambrian Burgess Shale-type deposits. Proceedings of the Royal Society B 286: 20192370. Crossref
Parry, L.A., Edgecombe, G.D., Eibye-Jacobsen, D., and Vinther, J. 2016. The impact of fossil data on annelid phylogeny inferred from discrete morphological characters. Proceedings of the Royal Society B: Biological Sciences 283: 20161378. Crossref
Parry, L.A., Lerosey-Aubril, R., Weaver, J.C., and Ortega-Hernández, J. 2021. Cambrian comb jellies from Utah illuminate the early evolution of nervous and sensory systems in ctenophores. iScience 24 (9): 102943. Crossref
Parry, L.A., Tanner, A., and Vinther, J. 2014. The origin of annelids. Palaeontology 57: 1091–1103. Crossref
Pates, S., Botting, J.P., Muir, L.A., and Wolfe, J.M. 2022. Ordovician opabiniid-like animals and the role of the proboscis in euarthropod head evolution. Nature Communications 13: 6969. Crossref
Rüppell, E. and Leuckart, F.S. 1828. Neue wirbellose Thiere des rothen Meers. In: E. Rüppell (ed.), Atlas zu der Reise im nördlichen Afrika, 1–47, plates 1–12. H.L. Brönner, Frankfurt am Main.
Sansom, R.S. 2016. Preservation and phylogeny of Cambrian ecdysozoans tested by experimental decay of Priapulus. Scientific Reports 6: 1–12. Crossref
Sansom, R.S., Gabbott, S.E., and Purnell, M.A. 2010. Non-random decay of chordate characters causes bias in fossil interpretation. Nature 463: 797–800. Crossref
Saleh, F., Ma, X., Guenser, P., Mángano, M.G., Buatois, L.A., and Antcliffe, J.B. 2022. Probability-based preservational variations within the early Cambrian Chengjiang biota (China). PeerJ 10: e13869. Crossref
Schofield, D.I., Davies, J.R., Waters, R.A., Wilby, P.R., Williams, M., and Wilson, D. 2004. Geology of the Builth Wells District—a Brief Explanation of the Geological Map Sheet 196 Builth Wells. British Geological Survey, Keyworth.
Schulze, A. and Rice, M.E. 2009. Musculature in sipunculan worms: ontogeny and ancestral states. Evolution & Development 11: 97–108. Crossref
Sedgwick, A.1898. A Student’s Textbook of Zoology. 630 pp. Swan Sonnenschein & Co. Ltd., London.
Stephen, A. and Edmonds, S. 1972. The Phyla Sipuncula and Echiura. 269 pp. Trustees of the British Museum (Natural History), London.
Vannier, J. and Martin, E.L. 2017. Worm-lobopodian assemblages from the early Cambrian Chengjiang biota: insight into the “pre-arthropodan ecology”? Palaeogeography, Palaeoclimatology, Palaeoecology 468: 373–387. Crossref
Zhang, Z., Smith, M.R., and Ren, X. 2023. The Cambrian cirratuliform Iotuba denotes an early annelid radiation. Proceedings of the Royal Society B 290: 20222014. Crossref
Zhao, J., Li, Y., and Selden, P.A. 2023. A new primitive polychaete with eyes from the lower Cambrian Guanshan biota of Yunnan Province, China. Frontiers in Ecology and Evolution 11: 1128070. Crossref
Acta Palaeontol. Pol. 68 (4): 571–581, 2023
https://doi.org/10.4202/app.01107.2023