

Early Miocene aequipectininin bivalves of the Pirabas Formation of the Pará State, northeastern Brazil
MARÍA BELÉN SANTELLI, CLAUDIA J. DEL RÍO, VLADIMIR DE ARAÚJO TÁVORA, and MARIA INÊS FEIJÓ RAMOS
Santelli, M.B., Del Río, C.J., Araújo Távora, V. De, and Feijó Ramos, M.I. 2024. Early Miocene aequipectininin bivalves of the Pirabas Formation of the Pará State, northeastern Brazil. Acta Palaeontologica Polonica 69 (2): 281–302.
A taxonomic revision of the Tribe Aequipectini from the upper Burdigalian, Lower Miocene Pirabas Formation in Brazil) resulted in an identification of Leptopecten daideleus comb. nov., Perapecten tetristriatus and description of Iemanjavola monlafertei gen. et sp. nov. The genus Perapecten is re-validated, P. tetristriatus being the first fossil representative of the genus, extending it back to the Early Miocene of the western Atlantic Ocean and Middle Miocene–Pliocene Perapecten scabrellus from the Mediterranean Sea/Central Paratethys is included into Perapecten. We also reported the oldest record of Leptopecten, which along with Perapecten, seem to have originated in the Early Miocene tropical region of the western Atlantic Ocean. Leptopecten dispersed during the Middle or Late Miocene to the eastern Pacific through the Central American Seaway, while Perapecten spread across the North Atlantic into Europe via the Circumtropical Current and the Gulf Stream/North Atlantic Drift.
Key words: Bivalvia, Pectinidae, Leptopecten, Perapecten, Iemanjavola, Central American Seaway, ocean currents, Miocene, Pirabas Formation, Brazil.
María Belén Santelli [mbsantelli@gmail.com; ORCID: https://orcid.org/0000-0003-3077-4384 ] and Claudia J. del Río [claudiajdelrio@gmail.com; ORCID: https://orcid.org/0000-0001-5167-9656 ], División Paleoeinvertebrados, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN-CONICET), Av. Ángel Gallardo 470, C1405DJR, Ciudad Autónoma de Buenos Aires, Argentina.
Vladimir de Araújo Távora [vladimir@ufpa.br; ORCID: https://orcid.org/0000-0001-5053-1422 ], Instituto de Geociências (IG), Universidade Federal do Pará (UFPA), Av. Augusto Correa 01, Caixa Postal 1611, CEP 66075-110 Belém, Pará, Brasil.
Maria Inês Feijó Ramos [mramos@museu-goeldi.br; ORCID: https://orcid.org/0000-0003-0276-0575 ], Museu Paraense Emílio Goeldi (MPEG), Av. Perimetral 1901, CEP 66077-830 Belém, Pará, Brasil.
Received 18 December 2023, accepted 18 April 2024, published online 14 June 2024.
Copyright © 2024 M.B. Santelli et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The tribe Aequipectinini Nordsieck, 1969 (subfamily Pectininae Rafinesque, 1815) is well documented in the Lower Miocene Pirabas Formation exposed in northeastern Brazil, where it is associated with Amusium Röding, 1798 (Tribe Amusiini Ridewood, 1903) and Chlamys Röding, 1798 (Tribe Chlamydini Teppner, 1922) (Ferreira 1960; Fernandes and Távora 1989). Aequipectinini representatives were among the first fossils collected in the Pirabas Formation by Maury (1925b), who described five pectinid species. Subsequent authors recognized the presence of up to seven nominal species, placing them in Chlamys (Argopecten) Monterosato, 1889, and Chlamys (Leptopecten) Verrill, 1897 (Ferreira 1960; Ferreira and Cassab 1985; Fernandes and Távora 1989). The only modern mention of this group was by Waller (2011), who transferred Argopecten pirabensis Ferreira, 1960, and Leptopecten cf. L. latiauratus (Conrad, 1837) to Paraleptopecten Waller, 2011, a genus synonymized with Leptopecten by Huber (2015). In the light of the valuable microsculptural patterns proposed by Waller (1972) and detailed by Hayami and Okamoto (1986), the current systematic classification of the Aequipectinini of the Pirabas Formation is outdated and requires a comprehensive revision.
Institutional abbreviations.—DGM, Divisão de Museu de Ciências da Terra, Departamento Nacional de Produção Mineral, Rio de Janeiro, Brazil; MACN-IN, Colección Invertebrados, Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Buenos Aires, Argentina; MACN-Pi, Colección Paleoinvertebrados, Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Buenos Aires, Argentina; MG, Museu de Geociências da Universidade Federal do Pará, Pará, Brazil; MNHN-IM, Molluscs, Muséum national d’Histoire naturelle, Paris, France; MNHN.F.J, MNHN.F.R, MNHN.F.A, Invertebrates Paleontology, Muséum national d’Histoire naturelle, Paris, France; MNRJ, Paleoinvertebrates Collection, Museu Nacional da Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; MNRJmoll, Mollusc collection, Museu Nacional da Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; MPEG, Museu Paraense Emílio Goeldi, Pará, Brazil; NMR, Mollusca, Natural History Museum Rotterdam, Rotterdam, Netherlands; NMSA, Mollusca Collection, KwaZulu-Natal Museum, Pietermaritzburg, South Africa; PRI, Paleontological Research Institution; Ithaca, New York; RBINS-MT, Virtual collections, Mollusca Collection, Royal Belgian Institute of Natural Sciences, Brussels, Belgium; UCMP, Invertebrate Paleontology, University of California Museum of Paleontology, (Berkeley Natural History Museums), Berkeley, California, USA; USNM (IZ, Invertebrate Zoology, MO, Fossil Bivalvia), Department of Paleobiology, Smithsonian Institute, National Museum of Natural History, Washington DC, USA.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in Zoobank: urn:lsid:zoobank.org:pub:D59BEC02-211D-4482-9068-005C77DFDC93
Geological setting
The Paribas Formation in Para-Maranhao Basin (Antonioli et al. 2015) is exposed along the equatorial margin of northeastern Brazil and contains some of the most significant Cenozoic marine deposits in the country (Távora et al. 2010). Isolated occurrences comprise outcrops up to 20 m thick, mostly in quarries in the Capanema District and along the Atlantic littoral in the surroundings of Salinópolis (Salinópolis District) and Ilha de Fortaleza (São João de Pirabas District) (Fig. 1). This unit grades vertically upwards, and laterally into the siliciclastic Barreiras Formation and covers unconformably the Precambrian basement to the south of the Bragantina Platform or the Cretaceous–lower Paleogene? Ipixuna Formation. The Pirabas Formation consists of a carbonate succession of intercalated massive grey to yellowish limestones, greenish laminated mudstones and calcareous sandstones. Rossetti (2001) recognized a carbonate and a mixed carbonate-siliciclastic facies association. The carbonate facies association is composed of four vertically intercalated lithofacies that record deposition in an open marine shelf and consists of massive grainstones/packstones and stratified wackestones/packstones characterized by a highly abundant and diverse invertebrate and vertebrate fauna, and plant remains. The mixed carbonate-siliciclastic facies represents deposition in restricted environments (mainly lagoons) and although less abundant and diverse than in the carbonate facies, fossil remains are present.
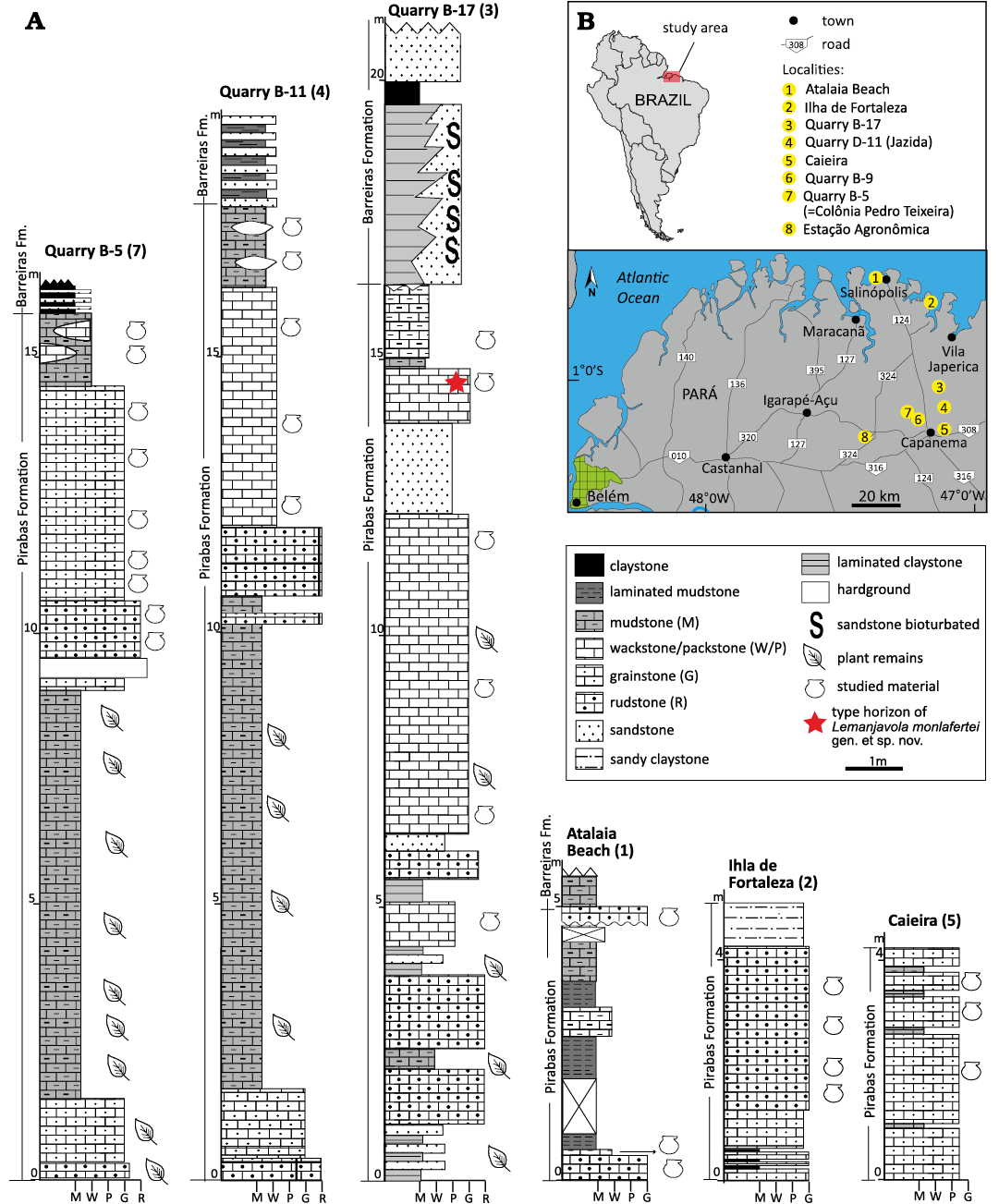
Fig. 1. Geographic and stratigraphic provenance overview of the examined material in this study. A. Stratigraphic sections of the Pirabas Formation from which pectinid material was collected. Caieira section constructed by VdAT; Quarry B-11 sections modified from Rossetti (2001); Atalaia Beach section modified from Aguilera et al. (2013) and Aguilera et al. (2017); Quarry B-5, Quarry B-17, and Ilha de Fortaleza sections modified from Góes et al. (1990). B. Map of northeastern Brazil showing the locations of cited fossiliferous sites of the Pirabas Formation.
The presence of the gastropod Orthaulax pugnax Heilprin, 1887, and the foram Globigerinoides Cushman, 1927, in the exposure at Atalaia indicate an Early Miocene age (Ferreira 1967, 1980; Keller 1981; Ferreira and Francisco 1988; Távora and Fernandes 1999; Rossetti et al. 2013). Rossetti (2001) proposed a late Oligocene–Early Miocene age for this unit, while planktonic foraminiferal zones N4 and N5 (Távora and Fernandes 1999) and the palynozone T13 (Antonioli et al. 2015) restrict the age to the Early Miocene or latest Early Miocene.
Sr-isotope data from pectinids at Quarries B-5, B-11 and Ilha de Fortaleza restricts the age to 17.3–16 Ma (late Burdigalian, Early Miocene) (Martínez et al. 2017). Based on the palynozone T15 defined by the occurrence of the palynomorphs Crassoretitriletes vanradshoovenii, Psilastephanocolporites tesseroporus, and Malvacipolloides maristellaefrom, Aguilera et al. (2020) proposed a Middle Miocene age for the top of the unit exposed at Atalaia Beach.
Material and methods
The material studied includes specimens collected by one of the authors (VT) at Quarry B-17 and Atalaia Beach, by Sergio Martínez at Atalaia Beach, and the types of collections made by Paulino de Carvalho, Hans Baumann (studied by Carlotta Maury [1925a]), Fritz L. Ackermann, and Cândido Ferreira. The geographic provenance of this material is listed in the SOM (Supplementary Online Material available at http://app.pan.pl/SOM/app69-Santelli_etal_SOM.pdf). The stratigraphic and geographic provenance of this material are displayed in Fig. 1. A significant portion of the material originates from currently abandoned and infilled quarries.
The MNRJ material fortunately was recovered and identified by the MNRJ staff after the tragic fire that affected the institution during 2017 (personal communication Sandro Scheffler and Luiz Felipe Lima Ferreira).
Pre-radial stages of the left beaks and detailed microsculpture features of both valves were examined. Twenty-one valves were treated with a metalizer (Quorum Technologies SC7620) and coated with a gold/palladium alloy (40:60) to obtain scanning electron microscope (SEM) images with a PhilipsXL 30 SEM. The morphological characters of the studied taxa were observed and photographed under a Zeiss Discovery V20 stereoscopic microscope. Terminology follows Waller (1969, 1991, 2011).
Systematic palaeontology
Phylum Mollusca Cuvier, 1797
Class Bivalvia Linnaeus, 1758
Order Pectinida Gray, 1854
Family Pectinidae Rafinesque, 1815
Subfamily Pectininae Rafinesque, 1815
Tribe Aequipectinini Nordsieck, 1969
Emended diagnosis.—Aequipectinoid shell-shape, usually prosocline, orbicular, equilateral or longer than high, auricles tending to be nearly equal in length, with shallow to moderately deep byssal notch and sinus; left valve usually convex, flat to slightly concave. Both valves sculptured with 5–24 evenly spaced primary radial costae or plicae, with a constant number throughout ontogeny; interstitial commarginal lamellae usually present; many genera with commarginal lamellae developing ventrally curved arcs on plicae flanks, at least in early ontogeny. Microsculpture of prominent, smooth pits in the pre-radial stage; internal rib carinae prominent; hinge dentition dominated by enlarged resilial teeth, in many taxa tending to extend anteriorly and posteriorly, nearly paralleling dorsal teeth.
Remarks.—Mesozoic mentions of Aequipectinini correspond to misclassified, poorly preserved and non-illustrated species (Woods 1902; Tamura 1959; Jefferies 1962; Riccardi 1977). According to Waller (1991), the earliest occurrence of this tribe is in the Eocene of Belgium, when it was represented by A. walleri (Glibert, 1975) (Lutetian; middle Eocene) and Aequipecten honi (Vincent, 1928) (Bartonian; middle Eocene).
Members of this tribe mainly inhabit tropical and subtropical regions, having its highest fossil and Recent diversity in the warm-temperate coasts of the American continent (Huber 2010), where it apparently first occurred in the eastern North Pacific during the late Oligocene (Addicott 1973; Moore 1984; Waller 2011). Later, it would have spread into the central-southeastern and western Atlantic Ocean (Maury 1917, 1920, 1925a, b; Grant and Gale 1931; Olsson 1932, 1961; Grau 1959; Ferreira 1960; Woodring 1982; Moore 1984, Petuch 1995; Coan et al. 2000; Waller 2011, 2018; Coan and Valentich-Scott 2012) and into the Indo-West Pacific (Hayami 1984; Dijkstra and Beu 2018).
According to the MolluscaBase, the tribe Aequipectinini has the highest specific diversity (49) within the subfamily Pectininae. Although the validity of some genera (Paraleptopecten Waller, 2011, Perapecten Wagner, 1985, and Lindapecten Petuch, 1995) and the inclusion of Serratovola Habe, 1951, and Volachlamys Iredale, 1939, were questioned (Huber 2015; Dijkstra and Kilburn 2001; Dijkstra 2013; Dijkstra and Beu 2018), it is mostly accepted that the tribe Aequipectinini unites 15 genera, occurring from Neogene to Recent times (Table 1).
Table 1. Geographic and stratigraphic distribution of the Aequipectinini genera. * according to Waller (2011) the first occurrences of Leptopecten are in the late Oligocene; ** in this paper, Leptopecten agronomica and Leptopecten pirabensis are included in Leptopecten, so the early occurrence of Paraleptopecten differs from Waller (2011); †, extinct.
|
Genus |
Stratigraphic range |
Geographic distribution |
References |
|
Aequipecten Fischer, 1886 |
Eocene–Recent |
eastern Atlantic
(Iceland-Angola), Mediterranean Sea, |
this paper |
|
eastern Atlantic (Iceland-South Africa), and Indian oceans of South Africa |
Glibert (1936); Waller (1991); Dijkstra and Kilburn (2001); Huber (2010) |
||
|
Cryptopecten Dall et al., 1938 |
Early Miocene– |
Indo-West Pacific and tropical western Atlantic |
Dijkstra and Beu (2018) |
|
Volachlamys Iredale, 1939 |
Miocene(?)–Recent |
Tropical Indo-West Pacific |
Dijkstra and Beu (2018) |
|
Serratovola Habe, 1951 |
Pliocene–Recent |
Indo-West Pacific |
Dijkstra and Beu (2018) |
|
Haumea Dall et al., 1938 |
Pliocene (?); |
Indo-West Pacific |
Dijkstra and Beu (2018) |
|
Argopecten Monterosato, 1889 |
Middle Miocene– |
western Atlantic
(Massachusetts-Brazil), and eastern |
Waller (2011) |
|
Leptopecten Verrill, 1897 |
Early Miocene (this paper)–Recent |
Tropical-subtropical eastern
Pacific (Washington†, |
Waller (2011)* |
|
Paraleptopecten Waller, 2011 |
Late Miocene–Recent |
Caribbean, tropical western Atlantic, and eastern Pacific (southern California-Ecuador) |
Waller (2011)** |
|
Lindapecten Petuch, 1995 |
Early Miocene– |
Tropical to temperate western
Atlantic (North Carolina- |
Waller (2011) |
|
Chesapecten Ward & Blackwelder, 1975 |
Miocene–Pliocene |
eastern North America |
|
|
Carolinapecten Ward & Blackwelder, 1975 |
Late Miocene– |
northwestern Atlantic (Virginia
to Florida) |
Waller (2018) |
|
Gurabopecten Waller, 2011 |
Late Miocene– |
Caribbean Sea (Dominican Republic) |
Waller (2011) |
|
Chagrepecten Waller, 2011 |
Late Miocene– |
Caribbean Sea, and northeastern
Pacific |
Waller (2011) |
|
Perapecten Wagner, 1985 |
Early Miocene of WA (this paper), Laghian–Recent (eastern Atlantic and Mediterranean) |
western tropical Atlantic†,
Mediterranean Sea, |
Dijkstra and Kilburn (2001) |
|
Flexopecten Sacco, 1897 |
Eocene(?), |
Mediterranean Sea and adjacent northeastern Atlantic |
Dijkstra and Goud (2002); Schneider et al. (2009); Cossmann (1922) |
|
Iemanjavola gen. et sp. nov. |
late Burdigalian |
western tropical Atlantic |
this paper |
The tribe was transferred to the subfamily Pectininae by Waller (2006, 2011), as supported by recent comprehensive phylogenies (Alejandrino et al. 2011; Sherratt et al. 2016). However, Carter et al. (2011) assigned it to the Pedinae Bronn, 1862 (= Chlamydinae Teppner, 1922), which was followed by Dijkstra and Beu (2018). We agree with its inclusion into Pectininae because of the presence of shared characters, such as: (i) a circular outline of the disc (aequipectinoid, pectinoid, amussioid shell-shape); (ii) relatively symmetrical auricles; (iii) a shallow to moderately shallow byssal notch with reduced or obsolete ctenolium in adults; (iv) commarginal lamellae present; (v) a constant number of primary radial sculpture throughout ontogeny; (vi) prominent internal rib carinae near the ventral margin, (vii) well-developed teeth, tending to be elongated and prominent, and (viii) ligamental area sculptured by deep ridges. Aequipectinini differ from the Chlamydinae (= Pedinae) by: (i) shells longer than high or equilateral, (ii) a shallower byssal notch, (iii) commarginal lamellae as main microsculpture on discs, lacking the shagreen microsculpture, (iv) a fixed number of primary ribs or plicae throughout ontogeny, without bifurcations and intercalations of the primary sculpture of Chlamydinae, (v) microsculpture of smooth, oval pits in the pre-radial stage of the umbonal area (while Chlamydinae has antimarginal ridges) and commarginal lamellae in early ontogeny, (vi) more prominent dentition, with resilial teeth tending to be elongated laterally, and (vii) prominent internal rib carinae near the ventral margin.
The first Aequipectinini species from the Pirabas Formation were documented in the pioneering work of Maury (1925b). Subsequently, Ferreira (1960) increased the taxonomic diversity of this group by introducing five new species, which he differentiated by slight sculptural differences. This group has not undergone comprehensive review for the past six decades, except for the brief insights provided by Waller (2011), in which a close relationship between some of these species and Paraleptopecten Waller, 2011, was proposed. However, the lack of diagnostic characters to support affinity among the species of the Pirabas Formation and Paraleptopecten proposed by Waller (2011), together with the outdated classification of the former, argues for the need for further studies.
Genus Leptopecten Verrill, 1897
Type species: Pecten monotimeris Conrad, 1837, by original designation, =Pecten latiauratus Conrad, 1837 (Bernard 1983); Recent, California, USA.
Emended diagnosis.—Medium-sized genus Aequipectinini, laterally compressed, biconvex valves, usually left-convex, occasionally right-convex, prosocline, orbicular to oblique. Auricles large compared to disc size, with sigmoidal to concave free margins inclined posteriorly; end of anterior auricles extended nearly to the antero-dorsal end of the disc; posterior auricles longer than anterior ones; posterior ones sculptured with well-spaced, thin costellae, sometimes vanishing distally; byssal notch deep for the tribe; functional ctenolium with 4–5 denticles. Discs sculptured with 9–20 narrow, evenly spaced radial plicae, of uniform height and width, usually lacking superimposed ribs; commarginal lamellae thin, straight or slightly curved on interspaces, sometimes dorsally curved arcs on plicae flanks in early ontogeny. Early microsculpture of large, oblong or subrounded, smooth, close-set (sometimes scattered on type species, L. palmeri) pits in the pre-radial and early radial stages. Hinge plate thin with well-developed resilial teeth ventrally elongated tending to be parallel to dorsal teeth, and very narrow dorsal teeth.
Remarks.—Olsson (1961) described Pacipecten (late Oligocene–Recent, eastern Pacific from middle California to Paita, Peru), designating to Pecten tumbezensis d’Orbigny, 1846 (Recent, eastern Pacific from Gulf of California, Mexico to Paita, Peru [Grau 1959]; Pliocene, Baja California Sur, Mexico [Moore 1984]; Late Pliocene?, lower Pleistocene–Recent, Tumbez, Peru [DeVries 1986]) as its type species. Olsson (1961) distinguished Pacipecten from Leptopecten by having more equilateral shells with large auricles. It was synonymized with Leptopecten by Waller (2011), based on the consideration of the characters listed by Olsson (1961) as transitional with those found in Leptopecten. The presence of shorter anterior auricles in the type species P. tumbezensis is here considered not enough to deserve a distinct generic rank.
The highest diversity of Leptopecten occurred during the Late Miocene and Early Pliocene (Smith and Jackson 2009), and the oldest occurrence corresponds to the “Leptopecten andersoni group” during the late Oligocene–Early Miocene in the eastern North Pacific (Waller 2011). This group was first documented by two fragmentary valves described by Addicott (1972, 1973) as L. subandersoni (Loel and Corey, 1932) (UCMP 31741, Vaqueros Formation, dated as 25 Ma by Keller et al. 1995; Fig. 2P) and Leptopecten? sp. (USNM MO 646784, lower Santos Shale Member, and USNM MO 646527, Wygal Sandstone Member, Temblor Formation, dated as 29–20 Ma and 30–29 Ma respectively by Johnson and Graham 2007; Fig. 2Q). The other taxa included in this group by Waller (2011) are subspecies of L. andersoni (Arnold, 1906), ranging from the Lower Miocene to the Middle Miocene, previously included in Pacipecten by Moore (1984). We accept as the oldest species of Leptopecten sensu stricto in the eastern Pacific those of the Late Miocene age listed by Waller (2011: 138).
The lack of diagnostic characters of the two valves of Leptopecten? sp. such as dimensions, shape and inclination of shells and auricles, and microsculpture and macrosculpture patterns, make uncertain their inclusion in Leptopecten. Moreover, the species included in the “Leptopecten andersoni group” are herein considered different from Leptopecten sensu stricto by having acline, fan-like shells, sculptured with flatter plicae, shorter and lower auricles, a shallower byssal notch, and free margins of the posterior auricles forming an obtuse or 90° angle with the hinge dorsal margin.
Waller (2011) erected Paraleptopecten (type species: Aequipecten bavayi Dautzenberg, 1900; Recent, Caribbean Sea southwards to Uruguay) but Coan et al. (2012) and Huber (2015) synonymized it with Leptopecten. Paraleptopecten is considered herein as a valid taxon, distinguishable from Leptopecten by the presence of higher and more elongated auricles and by its sculptural pattern on the left valve (e.g., Pecten bavayi, MNRJmoll 41788, RBINS-MT 734), with major and early plicae regularly separated by minor (late) plicae and by the development of coarser commarginal lamellae with ventrally curved arcs on plicae flanks. While secondary costellae are usually present in interspaces of Paraleptopecten, they are rarely developed in Leptopecten.
Stratigraphic and geographic range.—Lower Miocene to Recent, western tropical Atlantic and Caribbean Sea; Middle Miocene to Recent eastern Pacific from Peru to southeastern Washington State. Recent occurrences comprise the eastern Pacific from Point Reyes, California (USA) to Paita (Peru), and western tropical Atlantic of the Gulf of Mexico and Caribbean Sea from Honduras and the Bahamas to Surinam (Dijkstra 1996; Waller 2011).
Leptopecten daideleus (Maury, 1925b) comb. nov.
Figs. 2A–O, R–U, 3A–D.
1925 Pecten daideleus sp. nov.; Maury 1925b: 239–240, pl. 14: 8.
1925 Pecten agronomicus sp. nov.; Maury 1925b: 355, pl. 24: 6.
1960 Chlamys (Argopecten) daideleus (Maury, 1925b); Ferreira 1960: 146, 147, non pl. 3: 2, 2a.
1960 Chlamys (Argopecten) agronomica (Maury, 1925b); Ferreira 1960: 147–149 non pl. 4: 1, 1a.
1960 Chlamys (Argopecten) coopericellus sp. nov.; Ferreira 1960: 150, 151, pl. 2: 4.
1960 Chlamys (Argopecten) capanemensis sp. nov.; Ferreira 1960: 151, 152, pl. 3: 3, 3a.
1960 Chlamys (Leptopecten) cf. L. latiaurata (Conrad); Ferreira 1960: 152, 153, 154, pl. 4: 3, 3a, 3b.
1960 Chlamys (Leptopecten) pirabensis sp. nov.; Ferreira 1960: 154, 155, pl. 4: 2, 2a.
1989 Chlamys (Argopecten) agronomica (Maury, 1925b); Fernandes and Távora 1989: 106, pl. 2: 6.
1989 Chlamys (Argopecten) capanemensis Ferreira, 1960; Fernandes and Távora 1989: 106, pl. 2: 7.
1989 Chlamys (Argopecten) coopericellus Ferreira, 1960; Fernandes and Távora 1989: 107, pl. 2: 8.
1989 Chlamys (Argopecten) daidela (Maury, 1925b); Fernandes and Távora 1989: 107, pl. 2: 9.
1989 Chlamys (Leptopecten) latiaurata (Conrad); Fernandes and Távora 1989: 108, pl. 2: 11.
2011 Paraleptopecten pirabensis (Ferreira, 1960); Waller 2011: 91, 140.
2011 Paraleptopecten cf. L. latiauratus (Conrad) (Ferreira, 1960); Waller 2011: 91, 140.
Holotype: DGM 712, a right valve.
Type locality: Ponta de Pirabas, Ilha de Fortaleza, Brazil.
Type horizon: Upper Burdigalian (Lower Miocene).
Material.—Type material and DGM 895, a right valve, holotype of Pecten agronomicus, currently lost (Rodrigo Da Rocha Machado, personal communication 2017); DGM 896, a fragment of right valve, neotype of Pecten agronomicus designated here; DGM 897, three internal moulds and a fragment of shell, paratypes of P. agronomicus, all from the Estação Agronômica. MNRJ 4804-I, a left valve, holotype of Argopecten coopericellus, and MNRJ 4805-I, 4806-I, a pair of matching valves, holotype and paratype of L. pirabensis, respectively, from Fazenda, Ilha de Fortaleza. DGM 4611, 4612, four right and eight left valves and a fragmentary left valve, identified as Leptopecten cf. L. latiauratus by Ferreira (1960), from Igarapé Xibé, Colônia Pedro Teixeira, Capanema. MACN-PI 6500, MPEG 1504-I, 1508-I, 1589-I, 1808-I, two left and four right valves and an articulated specimen, from Atalaia Beach, Salinópolis. MG-1489-I, 1586-I, 1588-I, and 3628-I, two left and two right valves, from Quarry B-9, Capanema. MG-1583-I, 1587-I, a right and left valves, from Ilha de Fortaleza. MG-8624-I–8655-I, MPEG 1851-I, 1891-I, 2195-I, 2251-I, 2377-I, 2406-I, 2433-I, 2435-I, 2437-I, 2438-I, 32 left and 24 right valves, from Quarry B-17, Capanema. MPEG 802-I, 804-I, two left and three right valves, from Caieira, Capanema municipality. MPEG 922-I, 926-I, 942-I, 1053–1055-I, 1590-I, an articulated specimen, eight right and three left valves, from Jazida B-11, Capanema. MPEG 1577-I–1580-I, 1584-I–1585-I, four right and two left valves, from Quarry B-5, Capanema. All from Lower Miocene, Brazil. For details see SOM.
Dimensions.—See Table 2.
Emended diagnosis.—Leptopecten species with thick, orbicular to oblique shells, strongly prosocline in large specimens; subrectangular byssal notch. Discs sculptured with 16 to 19 subrounded to trigonal, scaly plicae and thin, evenly spaced commarginal lamellae on interspaces, varying from slightly ventrally convex curves to V-shaped. Early microsculpture of subrounded, smooth, close-set pits.
Description.—Shell medium-sized, up to 39 mm in length, moderately thick, orbicular to oblique, moderate to strongly prosocline, equilateral or longer than high, equiconvex to slightly left-convex; dorsal margins of disc moderately inclined; ventral margin frequently serrate. Umbonal angle 100–113°. Hinge dorsal margin straight, hinge length 75–83% of total disc length. Auricles elongated and large compared to disc size; posterior auricles slightly longer than anterior ones; anterior auricles nearly extended to end of antero-dorsal margin of disc in early ontogeny but shorter in mature specimens; free margins of auricles forming acute angles with dorsal margin, sigmoidal to concave on left anterior and posterior auricles; right anterior auricle sculptured with 5 coarse costellae, left anterior auricle with 6–8 thin costellae, posterior auricles with 3–8 costellae, weaker and more spaced than on anterior auricles, and sometimes vanishing distally; commarginal lamellae moderately coarse on anterior auricles and weak on right posterior auricle; deep byssal sinus; byssal notch subrectangular, deep, high, with functional ctenolium having 4–5 weak denticles. Disc sculptured with 16–19 broad, low, subrounded to trigonal, occasionally subquadrate, evenly spaced plicae, of uniform width on both valves, with a weak medial keel on the crests in some specimens; plicae wider than interspaces, sometimes with small, low, blunt, ventrally convex, widely spaced scales at 4–7 mm shell height on crests; commarginal lamellae usually present on rib interspaces of both valves throughout ontogeny and constituted by very thin, evenly spaced, commencing at 3–12 mm valve height, forming gentle curves with convexity towards ventral margin or occasionally deep V-shaped and rarely developing arcuate loops with convexity towards umbo on plicae flanks on early shell. Pre-radial stage of left valve with pitted microsculpture of moderately deep, subrounded pits. Interior of disc with auricular buttresses moderately coarse, and extended beyond mid-length of posterior auricles; left anterior auricle with an additional dorsal buttress; denticles prominent. Internal rib carinae prominent. Hinge plate very thin, crenulated with prominent vertical ridges and grooves; resilifer shallow, triangular, inclined posteriorly; dorsal teeth narrow and prominent; resilial teeth elongated parallel to dorsal teeth, and right anterior resilial tooth higher than the posterior resilial one.
Remarks.—Leptopecten daideleus (Maury, 1925b) is the most abundant aequipectininin species in the Pirabas Formation. It was placed in Argopecten Monterosato, 1889, by Ferreira (1960), but this species develops thinner shell thickness, more compressed than in species of Argopecten, which are sculptured with less, flatter and more widely spaced plicae, having larger and more elongated auricles with a deeper byssal notch, and a ctenolium that persists throughout ontogeny. It also has thinner hinge teeth and less elongated resilial teeth, precluding its inclusion in Argopecten.
Pecten daideleus is included in Leptopecten because it has compressed, prosocline, orbicular to oblique shells sculptured with uniform, evenly and widely spaced radial plicae, elongated and large auricles, the anterior ones nearly as long as the antero-dorsal end of the disc, with free margins of auricles forming acute angles with the dorsal margin (Fig. 2D–E, H–L, N–O, R–U), deep byssal notch with an active ctenolium, thin and widely spaced costellae on posterior auricles, elongate resilal teeth, and narrow dorsal teeth.

Fig. 2. Aequipectinin bivalves from the upper Oligocene of California, USA (P, Q) and the Pirabas Formation, upper Burdigalian (Lower Miocene) of Pará state, Brazil (A–O, R–U). A–O, R–U. Leptopecten daideleus (Maury, 1925b) comb. nov. A. DGM 712, an internal mould of a right valve, holotype of Pecten daideleus Maury, 1925b; Ponta de Pirabas, Ihla de Fortaleza. B. DGM 896, a fragment of a right valve in external view with internal mould of the rest of the valve, neotype of Pecten agronomicus Maury, 1925b; Estação Agronômica. C. DGM 897, fragment of shell in internal view, paratype of Pecten agronomicus Maury, 1925b; Estação Agronômica. D. DGM 4611, a right valve in external view, originally classified as L. cf. L. latiauratus (Conrad, 1837) (= L. daideleus [Maury, 1925b]) by Ferreira (1960); Colônia Pedro Teixeira. E. DGM 4612, a left valve in external view, originally classified as L. cf. L. latiauratus (Conrad, 1837) (= L. daideleus [Maury, 1925b]) by Ferreira (1960); Colônia Pedro Teixeira. F. MNRJ 4804-I, a left valve in internal (F1) and external (F2) views, holotype of Argopecten coopericellus Ferreira, 1960 (= L. daideleus [Maury, 1925b]); Fazenda, Ihla de Fortaleza. G. MPEG 1508-I, a left valve in external view; Atalaia Beach. H. MPEG 1587-I, a left valve in external (H1) and internal (H2) views; Ilha de Fortaleza. I. MPEG 922-I, a right valve in external (I1) and internal (I2) views; Quarry B-11. J. DGM 4613, a left valve in external (J1) and internal (J2) views, holotype of Argopecten capanemensis Ferreira, 1960 (= L. daideleus [Maury, 1925b]); Colônia Pedro Teixeira. K. DGM 4614, a right valve in external view, paratype of Argopecten capanemensis Ferreira, 1960; Colônia Pedro Teixeira. L. MNRJ 4805-I, a left valve in external view, holotype of Leptopecten pirabensis Ferreira (= L. daideleus [Maury, 1925b]); Fazenda, Ihla de Fortaleza. M. MNRJ 4806-I, a fragmented right valve in external view, paratype of Leptopecten pirabensis Ferreira, 1960 (= L. daideleus [Maury, 1925b]); Fazenda, Ihla de Fortaleza. N. DGM 4612, a left valve in external view, originally classified as L. cf. L. latiauratus (Conrad, 1837) (= L. daideleus [Maury, 1925b]) by Ferreira (1960); Colônia Pedro Teixeira. O. MPEG 1585-I, a left valve in external view; Quarry B-5. R. MPEG 802-I, a left valve in external (R1) and internal (R2) views; Caieira. S. MPEG 2195-I, a right valve in external view; Quarry B-17, Caieira. T. MACN-Pi 6500, a left valve in external view; Atalaia Beach. U. DGM 4612, a left valve in external view, originally classified as L. cf. L. latiauratus (Conrad, 1837) (= L. daideleus [Maury, 1925b]) by Ferreira (1960); Colônia Pedro Teixeira. P, Q. Aequipectinini bivalve “Leptopecten” Verrill, 1897. P. UCMP 31741, a right valve in external view, holotype ?Leptopecten subandersoni (Loel and Corey, 1932); Vaqueros Formation. Q. USNM MO 646784, a fragment of left valve in external view, Leptopecten? sp.; Temblor Formation.
Pecten daideleus from Rio Pirabas and P. agronomicus from Estação Agronômica were the first aequipectininin species described from the Pirabas Formation (Maury 1925b). Ferreira (1960) assigned them to Chlamys (Argopecten) and described five taxa: Chlamys (Argopecten) tetristriata Ferreira, 1960, C. (A.) coopericellus Ferreira, 1960, C. (A.) capanemensis Ferreira, 1960, C. (L.) pirabensis Ferreira, 1960, and C. (Leptopecten) cf. L. latiauratus (Conrad, 1837) differing from each other by sculptural variations (Ferreira 1960; Fernandes and Távora 1989). Waller (2011) transferred C. (Leptopecten) pirabensis and C. (L.) cf. L. latiauratus to Paraleptopecten and proposed P. agronomicus as an ancestor of Paraleptopecten.
Argopecten agronomicus and A. pirabensis are considered herein as junior synonyms of L. daideleus. They were included within Paraleptopecten by Waller (2011), who mentioned transitional characters without diagnostic significance such as the development of angular ribs (also present in species of Leptopecten) and the densely costate auricles. Leptopecten agronomicus and L. pirabensis develop smaller auricles, with anterior auricles shorter than in Paraleptopecten species and discs sculptured with plicae of uniform width that emerge synchronously in early ontogeny and are separated by smooth interspaces.
As the type specimen of L. daideleus (Fig. 2A) Maury (1925b) designated a right internal mould sculptured with 16 plicae. Pecten agronomicus (Fig. 2B, C) was typified on a series of syntypes comprising a left valve (DGM 895-I, illustrated in the original description), casts and fragmentary specimens (DGM 896, 897) (Maury 1925b), with DGM 895-I subsequently designated as its holotype by Ferreira (1960). However, the latter specimen is reported as lost in the Coleção de Paleontologia do Museu de Ciências da Terra (Rodrigo Da Rocha Machado, personal communication 2017). Comparison of type material allows us to conclude that they are conspecific (see Table 3). Leptopecten daideleus is chosen as the valid taxon name according to the Principle of Priority of the ICZN (1999: article 23.1). DGM 896 (Fig. 2B) is designated as a neotype of P. agronomicus according to ICZN (1999: article 72.4.5, recommendation 75A) because it is the specimen that best characterizes the sculptural pattern of the species. Some specimens identified as P. daideleus and P. agronomicus by Ferreira (1960) (ex MNRJ 4800-I and 4801-I) are herein assigned to two other taxa (see following discussion).
Ferreira (1960) described Argopecten coopericellus (Fig. 2F), Leptopecten pirabensis (Fig. 2L, M), L. cf. L. latiauratus (Fig. 2D–E, N) and A. capanemensis (Fig. 2J, K) and distinguished them from L. daideleus based on slight morphological differences that are within the range of variation of L. daideleus and thus we consider as part of this nominal species (see Table 3). All these taxa have anterior auricles extending as far or nearly as far as the dorsal margin of the disk (Fig. 2I, J–L, N, R–U, see Table 2).
Table 2. Morphologic traits of the studied taxa. Abbreviations: br, broken; c, covered; H, holotype; HL, hinge length; LAA, left anterior auricle; LPA, left posterior auricle; lv/rv, left/right valve; P, paratype; Pl, plesiotype; PLC, number of plicae; RAA, right anterior auricle; RPA, right posterior auricle; SH, shell height; SL, shell length; UA, umbonal angle; Taxa: A., Argopecten; I., Iemanjavola; L., Leptopecten; P., Perapecten.
|
Collection number |
Original |
This paper |
Material |
Valve |
SH (mm) |
SL (mm) |
HL (mm) |
HL/SL |
SL/SH |
PLC |
Number of ribs |
UA (º) |
|||
|
RAA |
RPA |
LAA |
LPA |
||||||||||||
|
DGM 712 |
Pecten daideleus |
L. daideleus |
H |
1 rv |
13.2 |
13.7 |
br |
|
1.04 |
17 |
|
|
|
|
111 |
|
MNRJ 4804-I |
A. coopericellus |
L. daideleus |
H |
1 lv |
25 |
26 |
br |
|
1.04 |
19 |
|
|
9 |
8 |
101 |
|
MNRJ 4805-I |
L. pirabensis |
L. daideleus |
H |
1 lv |
14.2 |
14.8 |
br |
|
1.04 |
19 |
|
|
10 |
8 |
99 |
|
MNRJ 4806-I |
L. pirabensis |
L. daideleus |
P |
1 rv |
13.5 |
14.6 |
br |
|
1.08 |
19 |
br |
7 |
|
|
98 |
|
DGM 4611 |
L. cf. L. latiauratus |
L. daideleus |
H |
1 rv |
24 |
26.9 |
18.72 |
0.70 |
1.12 |
17 |
5 |
3 |
|
|
113 |
|
DGM 4612 |
L. cf. L. latiauratus |
L. daideleus |
P |
1 lv |
17 |
18 |
13.68 |
0.76 |
1.06 |
17 |
|
|
8 |
4 |
107 |
|
DGM 4612 |
L. cf. L. latiauratus |
L. daideleus |
P |
1 lv |
16.6 |
17.8 |
br |
|
1.07 |
18 |
|
|
6 |
6 |
103 |
|
DGM 4612 |
L. cf. L. latiauratus |
L. daideleus |
P |
1 lv |
16.6 |
17.8 |
br |
|
1.07 |
18 |
|
|
6 |
6 |
104 |
|
DGM 4612 |
L. cf. L. latiauratus |
L. daideleus |
P |
1 lv |
15.2 |
15 |
br |
|
0.99 |
19 |
|
|
|
5 |
106 |
|
DGM 4613 |
A. capanamensis |
L. daideleus |
H |
1 lv |
14.9 |
15 |
10.6 |
0.71 |
1.01 |
18 |
|
|
8 |
6 |
97 |
|
DGM 4613 |
A. capanamensis |
L. daideleus |
H |
1 rv |
15 |
15 |
10.76 |
0.72 |
1.00 |
19 |
5 |
7 |
|
|
96 |
|
DGM 4614 |
A. capanamensis |
L. daideleus |
P |
1 rv |
14.3 |
14.9 |
br |
|
1.04 |
18 |
5 |
6 |
|
|
98 |
|
DGM 4614 |
A. capanamensis |
L. daideleus |
P |
1 rv |
8.9 |
8.7 |
6.6 |
0.76 |
0.98 |
17 |
4 |
|
|
|
91 |
|
DGM 4614 |
A. capanamensis |
L. daideleus |
P |
1 rv |
10.2 |
10.2 |
7.55 |
0.74 |
1.00 |
17 |
5 |
5 |
|
|
95 |
|
DGM 4614 |
A. capanamensis |
L. daideleus |
P |
1 rv |
14.6 |
15 |
10.74 |
0.72 |
1.03 |
19 |
5 |
7 |
|
|
95 |
|
DGM 4614 |
A. capanamensis |
L. daideleus |
P |
1 rv |
17.7 |
19.7 |
12.38 |
0.63 |
1.11 |
19 |
c |
4 |
|
|
101 |
|
DGM 4614 |
A. capanamensis |
L. daideleus |
P |
1 rv |
19.8 |
|
br |
|
0.00 |
19 |
5 |
7 |
|
|
102 |
|
DGM 4614 |
A. capanamensis |
L. daideleus |
P |
1 lv |
18.8 |
19.7 |
br |
|
1.05 |
18 |
|
|
|
5 |
101 |
|
DGM 4614 |
A. capanamensis |
L. daideleus |
P |
1 lv |
16.5 |
16.2 |
10.97 |
0.68 |
0.98 |
18 |
|
|
7 |
4 |
93 |
|
DGM 4614 |
A. capanamensis |
L. daideleus |
P |
1 lv |
6.1 |
6 |
4.93 |
0.82 |
0.98 |
16 |
|
|
3 |
|
89 |
|
MNRJ 12065-I |
A. daideleus |
P. tetristriatus |
Pl |
1 rv |
11.65 |
11.7 |
6.7 |
0.57 |
1.00 |
20 |
5 |
5 |
|
|
99 |
|
MNRJ 12065-I |
A. daideleus |
P. tetristriatus |
Pl |
1 lv |
12.04 |
12.37 |
br |
|
1.03 |
22 |
|
|
|
|
104 |
|
MNRJ 4802-I |
A. tetristriatus |
P. tetristriatus |
H |
1 rv |
15.4 |
16.3 |
7.7 |
0.47 |
1.06 |
24 |
5 |
7 |
|
|
107 |
|
MNRJ 4803-I |
A. tetristriatus |
P. tetristriatus |
P |
1 lv |
15.5 |
16.2 |
7.6 |
0.47 |
1.05 |
23 |
|
|
5 |
6 |
107 |
|
MNRJ 4800-I |
A. daideleus |
I. monlafertei |
Pl |
1 rv |
12.3 |
12.4 |
8.73 |
0.70 |
1.01 |
16 |
|
|
|
|
103 |
|
MNRJ 4801-I |
A. agronomicus |
I. monlafertei |
Pl |
1 lv |
7.4 |
7.6 |
5.7 |
0.75 |
1.03 |
15 |
|
|
|
|
94 |
|
MNRJ 4801-I |
A. agronomicus |
I. monlafertei |
Pl |
1 rv |
8.3 |
8.8 |
5.7 |
0.65 |
1.06 |
16 |
|
|
5 |
3 |
107 |
|
MACN-Pi 6501 |
|
I. monlafertei |
|
1 lv |
11.9 |
13.3 |
8.42 |
0.63 |
1.12 |
16 |
|
|
4 |
3 |
105 |
|
MG 8622-I |
|
I. monlafertei |
H |
1 lv |
10.82 |
11.78 |
7.7 |
0.654 |
1.09 |
15 |
|
|
0 |
0 |
104 |
|
MG 8623-I |
|
I. monlafertei |
H |
1 rv |
10.98 |
11.35 |
7.95 |
0.7 |
1.03 |
16 |
2 |
|
|
|
103 |
Although L. daideleus commonly has non-costellae plicae and interspaces, a few specimens exhibit a single thin, scaly costella on some interspaces at shell heights of 10–20 mm (MPEG 1577-I, 2437-I, 1054-I, MNRJ 4804-I, Fig. 2F), while others are more densely sculptured with flanks of plicae and interspaces bearing one or two secondary costellae (MPEG 1508-I and 1589-I, Fig. 2G) near the ventral margin.
Sculptural variations are also documented in ecomorphs of L. latiauratus, varying from smooth shells like in L. latiauratus monotimeris (Conrad, 1837) to densely sculptured with thin commarginal lamellae like in L. latiauratus latiauratus (Clark 1971; Coan et al. 2000; Waller 2011). Leptopecten daideleus differs from L. latiauratus (= Pecten monotimeris Conrad, 1837) (MACN-IN 2409, 2410, 29171; PRI 73267, 73729) by having thicker, less oblique shells and anterior auricles a little shorter and lower (see Table 3). The pre-radial stage is fairly well preserved in four left beaks of L. daideleus (Fig. 3A–C), with more rounded pits, closer set than in L. latiauratus (Fig. 3E).
The number of shell plicae, size of auricles, and thickness of the commarginal lamellae in L. daideleus are comparable to those found in the living Atlantic L. linki (Dall, 1926) (Recent, Caribbean Sea; Dijkstra 1996: figs. 1–4) but L. daideleus has a more prosocline shell and coarser costellae on auricles.

Fig. 3. SEM photographs of the umbonal area of the aequipectinin bivalve Leptopecten Verrill, 1897, from the Pirabas Formation, upper Burdigalian (Lower Miocene) of Pará state, Brazil (A–D) and Recent of the northeastern Pacific Ocean (E). A–D. Sculpture details of Leptopecten daideleus (Maury, 1925b). A. MG 8627-I, detail of the umbonal area of left valve showing the preserved pitted microsculpture in the pre-radial stage and commarginal lamellae in “V” (A1) and commarginal microsculpture on disc and auricles (A2); Quarry B-17. B. MPEG 942-I, weakly preserved pitted microsculpture on the left beak; Quarry B-11. C. MPEG 2251-I, detail of the umbonal area of a left valve showing the preserved commarginal lamellae (C1) and pitted microsculpture in the pre-radial stage (C2); Quarry B-11. D. MPEG 2437-I, detail of a left hinge; Quarry B-17. E. MACN-IN 2409, sculpture details of the umbonal area of a left valve of Leptopecten latiauratus (Conrad, 1837) from San Diego, California, USA, showing the pitted microsculpture and commarginal lamellae (E1), and more detail on pitted microsculpture in the pre-radial stage (E2).
Table 3. Comparisions among the taxa synonymized under Leptopecten daideleus (Maury, 1925b) comb. nov., the latter and the type species of Leptopecten. Abbreviations: AAA, angle between the free margin of anterior aurciles and the hinge dorsal margin; APA, angle between the free margin of posterior aurciles and the hinge dorsal margin; BN, byssal notch; BS, byssal sinus; CM, commarginal lamellae; HL, hinge lenght; SH, shell height; SL, shell length.
|
Taxon |
Shell shape |
Shell |
SL/ |
AAA/ |
BS/ |
Auricles dimension |
Free margin of posterior auricle |
Plicae number |
UA |
CM on |
|
Pecten |
orbicular |
prosocline |
>1 |
fragmented |
fragmented |
fragmented |
fragmented |
17 |
111 |
thin |
|
Pecten |
orbicular |
prosocline |
>1 |
acute |
BS: deep (left valve) |
elongated (partially F) |
sigmoidal |
16 |
101 |
no preserved |
|
Argopecten |
orbicular |
prosocline |
>1 |
acute |
deep |
elongated |
sigmoidal |
16–19 |
96.2 |
thin, moderately spaced |
|
Argopecten |
anteriorly oblique |
prosocline |
>1 |
fragmented |
fragmented |
fragmented |
fragmented |
19 |
101 |
thin, moderately spaced |
|
Leptopecten pirabensis |
orbicular |
prosocline |
>1 |
AAA: acute, APA: F |
BS: deep |
fragmented |
sigmoidal |
19 |
98.6 |
thin, moderately spaced |
|
Leptopecten cf. |
orbicular–anteriorly oblique |
highly prosocline |
>1 |
acute |
deep |
elongated |
sigmoidal |
17–19 |
106.8 |
thin, moderately spaced |
|
Leptopecten latiauratus |
obliquely subovate–orbicular subovate |
highly prosocline |
≥1 |
acute |
deep |
HL/SL = 0.83–0.85 |
concave to sigmoidal |
9–16 |
97.5 |
smooth to thin, widely to closely spaced |
Leptopecten daideleus differs from L. cracens (Olsson, 1964) (Angostura Formation, Upper Miocene, Ecuador; type material, USNM MO 643860–643861) by having larger shells with radial sculpture emerging earlier on the right valve, smaller scales on plicae, weaker commarginal lamellae, and more closely spaced costellae on auricles.
Leptopecten daideleus is distinguished from L. ecnomius Woodring, 1982 (Gatun Formation, uppermost Middle–lowermost Upper Miocene, Panama; Collins and Coates 1999; type material, USNM MO 647128–647130; PRI 68520), in having larger shells with more prominent commarginal lamellae and the entire surface of posterior auricles covered with costellae, while they are entirely or ventrally smooth in L. ecnomius.
Leptopecten daideleus is distinguished from L. thompsoni (Maury, 1917) (Cercado Formation, Upper Miocene, Dominican Republic; syntypes, PRI 28966–28967, USNM 540981–540984) by having larger, less prosocline shells, more convex right valves, a shallower byssal notch, right auricles with straight dorsal margins, not projecting upwards, and posterior auricles longer than the anterior ones.
Leptopecten daideleus differs from L. maturensis (Maury, 1925a) (Talparo Formation, Lower Pleistocene, Matura, Trinidad; syntypes, PRI 900, 901) because it has more elongated anterior auricles, and valves sculptured with well-marked plicae covered by coarser commarginal lamellae, while L. maturensis has very low, barely distinguishable undulations on the left valve.
Leptopecten daideleus is similar to Leptopecten tabaquita (Maury, 1925a) (Tabaquite Limestone, Guaracara Limestone Member, Middle Miocene, Trinidad; Wilson et al. 2010; Wilson 2012; holotype, PRI 898) in size and number of plicae, but differs in having less inclined dorsal margins of the disc, higher plicae, a lower and longer right anterior auricle, and a subrectangular byssal notch.
Leptopecten daideleus is distinguishable from L. gilbertharrisi (Hodson in Hodson et al., 1927) (Cují, Socorro Formation, lower Middle Miocene, Venezuela; holotype PRI 21967, paratype PRI 21958) by having shells sculptured with more plicae lacking commarginal lamellae on their crests, more widely spaced commarginal lamellae on interspaces, and posterior auricles covered by coarser costellae.
Leptopecten daideleus and L. coderensis (Harris in Hodson et al., 1927) (Codore Formation, Upper Miocene–Middle Pliocene, Venezuela; Smith et al. 2010; type material, PRI 21953, 21955, 21956) are differentiated because the first has a shorter postero-dorsal margin, lower right anterior auricle with dorsal margin less projected upwards, narrower left interspaces, scaly plicae, more widely spaced commarginal lamellae on interspaces, and posterior auricles covered by coarser costellae.
Stratigraphic and geographic range.—Upper Burdigalian (Lower Miocene), Pirabas Formation; Ilha de Fortaleza, Atalaia beach, Estação Agronômica, Colônia Pedro Teixeira, Caieira, Quarry B-11, Quarry B-9, Quarry B-5, Quarry B-17; northeastern Pará state, Brazil.
Genus Perapecten Wagner, 1985
Type species: Pecten commutatus Monterosato, 1875 (= Pecten philippii Récluz, 1853 [non Pecten philippii Michelotti, 1839]) by original designation, (= Pecten atlanticus Smith, 1890, junior synonym); Recent, Mediterranean Sea and eastern Atlantic from Portugal to South Africa (Dijkstra and Kilburn 2001; Huber 2010).
Species included: Recent, Aequipecten commutatus peripheralis Dijkstra and Kilburn, 2001, Indian Ocean, KwaZulu-Natal and Transkei, South Africa (Dijkstra and Kilburn 2001); upper Burdigalian (Lower Miocene), Chlamys (Argopecten) tetristriata Ferreira, 1960, Pirabas Formation, Brazil; Langhian (Middle Miocene)–Pliocene, Pecten scabrellus Lamarck, 1819 (= Pecten seniensis Lamarck, 1819) (Benyoucef et al. 2021; Talmat et al. 2022), Mediterranean Sea and central Paratethys.
Emended diagnosis.—Small to moderate-sized Aequipectinini, orbicular or subovate, globose, right-convex. Auricles low and very short; posterior shorter than anterior ones, and with free margins strongly inclined anteriorly; byssal sinus and notch very shallow; notch angulate; byssal fasciole and ctenolium obsolete; right anterior auricle sculptured with coarse costae. Shell with 16–24 subrectangular plicae with three or four costellae of equal width on crests near ventral margin; interspaces with 1–3 costellae; commarginal lamellae very coarse and closely arranged, forming convex (towards hinge margin) loops on plicae flanks; plicae flanks and interspaces sometimes covered with delicate scales that imbricate like roof-tiles, enclosing hollow chambers and covering costellae. Pre-radial microsculpture of deep, subrounded, small, close-set pits. Right valve with intermediate and resilial teeth thin, intermediate teeth elongated to nearly the middle of the auricles.
Remarks.—Perapecten Wagner, 1985, was described as a subgenus of Aequipecten Fischer, 1886 (Table 1) to include A. commutatus (Monterosato, 1875) (MNHN IM 2000-24361, 2000-24362, Fig. 4G, H) and A. flabellum (Gmelin, 1791) (Recent, Mauritania to Angola; Wagner 1985), and was subsequently raised to full genus status by Wagner (1991) based on unspecified microsculpture characters. Grau (1959) erroneously had considered Pecten commutatus as the type species of Argopecten, but Waller (1969: 34–34) separated it from that genus, stating that Argopecten is an American taxon. Dijkstra and Kilburn (2001) erected Aequipecten commutatus peripheralis (NMSA E8964) and considered Perapecten as a junior synonym of Aequipecten.
Perapecten belongs in the tribe Aequipectinini because it has longer than high, prosocline shells, with auricles nearly equal in length, valves sculptured with a uniform number of undivided radial plicae throughout ontogeny, and commarginal lamellae frequently developed on interspaces. The inner ventral margin is strongly carinate and the hinge dentition is characterized by prominent resilial, dorsal and infradorsal teeth, as occur in this tribe.
We consider Perapecten as a valid genus, distinguished from Aequipecten by the development of more globose, orbicular, right convex shells, sculptured with subrectangular plicae with sharply demarcated flanks and crests covered with three, occasionally four costellae of uniform width and with coarse commarginal lamellae on both valves. The auricles are shorter than in Aequipecten, with free margins highly inclined anteriorly, and the right anterior one covered by coarser and fewer costellae. The rudimentary byssal sinus and notch, obsolete byssal fasciole, and ctenolium with 1–3 tiny denticles, distinguish it from Aequipecten.
Wagner (1985) assigned to Aequipecten flabellum of the tropical southeastern Atlantic to belong within Perapecten but it does not belong in this genus because it has larger, acline, left to right-convex shells, noticeably more elongated auricles, with free margins of the posterior ones nearly vertical, a moderately deep byssal sinus and notch, a ctenolium with 4–5 stronger denticles, and plicae and interspaces bearing more superimposed costae.
Perapecten was previously known only from the Recent species P. commutatus commutatus, which is distributed in the Mediterranean Sea and the eastern Atlantic, and P. commutatus peripheralis, found in the Indian Ocean. The present work includes two species to Perapecten: Pecten scabrellus Lamarck, 1819, from the Middle Miocene of Europe, and Argopecten tetristriatus Ferreira, 1960, extending range of Perapecten back to the Early Miocene in the tropical western Atlantic.
Perapecten scabrellus (Langhian, Middle Miocene–Pliocene, Mediterranean Sea and Paratethys; stratigraphic distribution sensu Studencka 1986; Aguirre et al. 1996; Studencka et al. 1998; Lacour et al. 2002; Rico-García 2008; Jiménez et al. 2009; Talmat et al. 2022) has been considered a near relative of P. commutatus (Sacco 1897; Talmat et al. 2002; Rico-García 2008). Perapecten scabrellus is herein considered to belong in Perapecten because of its inflated, right-convex shells, with short auricles, posterior auricles with free margins strongly inclined anteriorly and shorter than the anterior ones, rudimentary byssal sinus and notch, an obsolete ctenolium, plicae and interspaces with three or four equal-sized costellae arising near the ventral margin, and coarse commarginal lamellae that persist throughout ontogeny. Specimens of P. scabrellus are herein illustrated for comparison (syntype MNHN.F.A 50227, 50229, MNHN.F.R 63425, 07288, 07290; Fig. 4E, F).
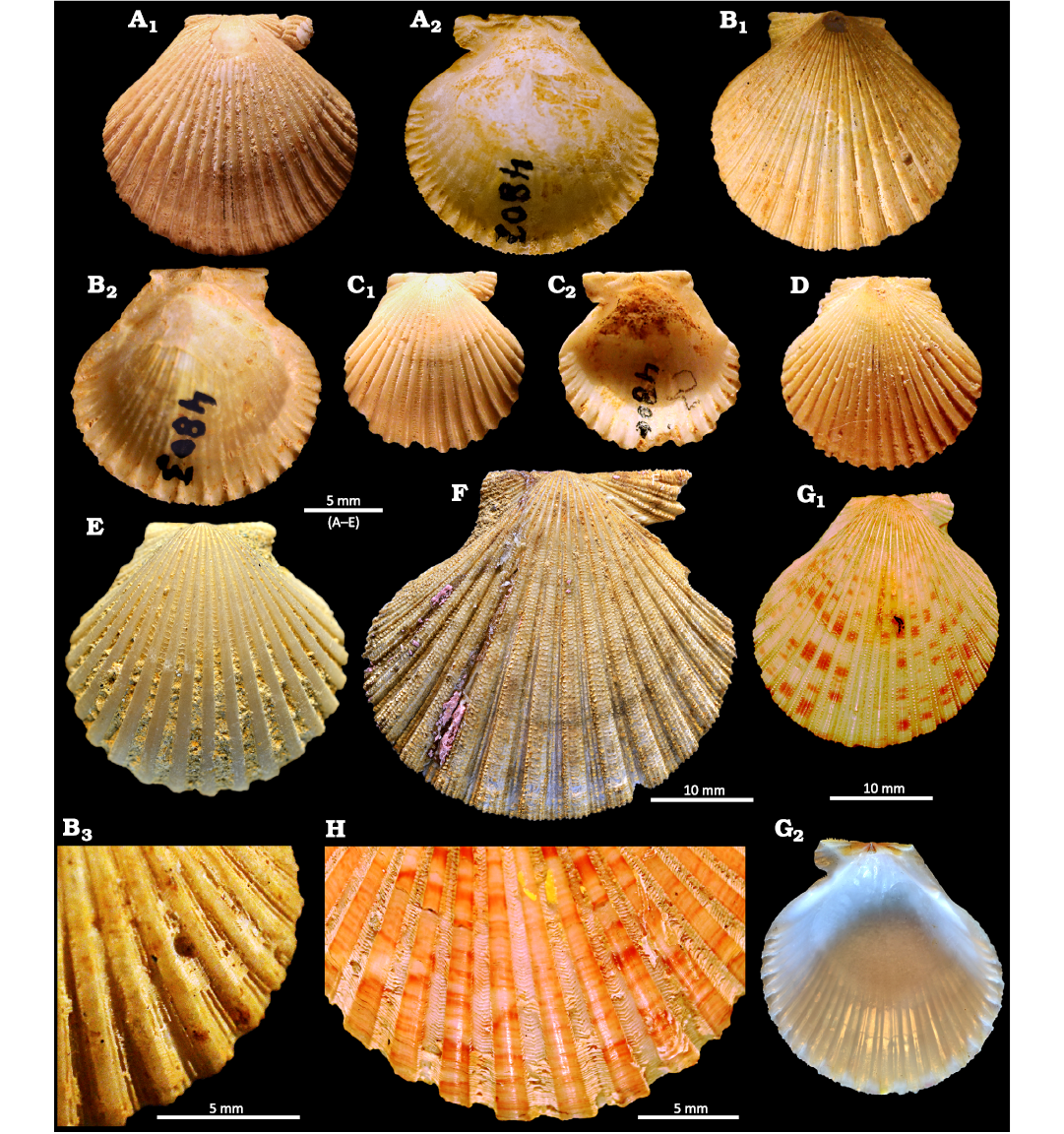
Fig. 4. Aequipectinin bivalve Perapecten Wagner, 1985, from the Pirabas Formation, upper Burdigalian (Lower Miocene) of Pará state, Brazil (A–D, H), Messinian (Upper Miocene) of Spain (E), the Pliocene of Italy (F), and Recent of Italy (G, I). A–D. Perapecten tetristriatus (Ferreira, 1960). A. MNRJ 4802-I, a right valve in external (A1) and internal (A2) views, holotype of Argopecten tetristriatus Ferreira, 1960; Fazenda, Ihla de Fortaleza. B. MNRJ 4803-I, a left valve in external (B1) and internal (B2) views, paratype of A. tetristriatus Ferreira, 1960, detail of imbricated scales that enclose hollow chambers on interspaces in external view of a left valve (B3); Fazenda, Ihla de Fortaleza. C, D. MN12065-I (ex MNRJ 4800-I 2-3). C. A right valve in external (C1) and internal (C2) views. D. A left valve in external view. originally classified as Leptopecten daideleus (Maury, 1925b) by Ferreira (1960); Fazenda, Ihla de Fortaleza. . E, F. Perapecten scabrellus (Lamarck, 1819). E. MNHN.F.R 63425, a right valve in external view; Cerro Mandras, Sorbas. F. MNHN.F.A 50227, a right valve in external view, syntype of Pecten scabrellus Lamarck, 1819; unknown locality. G, H. Perapecten commutatus Monterosato, 1875; Sicily. G. MNHN-IM-2000-24362, a right valve in external (G1) and internal (G2) views, paratype of Pecten philippii Récluz, 1853 (name replaced by Pecten commutatus Monterosato, 1875). H. MNHN-IM-2000-24361, detail of sculpture showing imbricated scales that enclose hollow chambers on interspaces on a left valve, holotype of Pecten philippii Récluz, 1853.
In this contribution, we propose the inclusion of P. scabrellus in the genus Perapecten. The oldest records of P. scabrellus are of Langhian and early Serravalian (Middle Miocene) age from Central Paratethys (Studencka 1986; Studencka et al. 1998; age after Kováč et al. 2007 and Harzhauser et al. 2002). Records of this species in circum-Mediterranean Sea area are from the Upper Miocene (Messinian) of Algeria and Spain (Freneix et al. 1987; Lacour et al. 2002) where it extends into the Pliocene (Talmat et al. 2002; Jiménez et al. 2009; Rico-García 2008; Benyoucef et al. 2021; Spadini 2022).
Dollfus and Dautzenberg (1920) and Studencka (1986) regarded Aequipecten liberata Cossmann and Peyrot, 1914 (MNHN.F.J 06455–06458) from the Lower Miocene of the Aquitaine Basin, France, to be a synonym of P. scabrellus, extending its stratigraphic range to the Lower Miocene. However, we recognize these as distinct species based on several morphological differences. Aequipecten liberata differs from P. scabrellus by its more compressed, acline to opisthocline shells, a less globose umbo, longer auricles with a deeper byssal notch, free margins of the posterior auricles inclined posteriorly, and shells sculptured with a greater number of non-costellate plicae.
Perapecten differs from Leptopecten in having more inflated shells, shorter and smaller auricles, posterior ones with free margins sharply inclined anteriorly, shallower byssal sinus and notch, an obsolete ctenolium (1–3 active, very weak denticles compared to 4–5 strong denticles in Leptopecten) and coarser hinge teeth. The auricles are sculptured with coarser costellae than in Leptopecten, and the discs with more closely spaced plicae with costellae on the crests.
Perapecten is distinguished from Argopecten (Table 1) in having less inflated shells, posterior auricles shorter than the anterior ones, with free margins strongly inclined anteriorly, a shallower byssal sinus and notch, plicae and interspaces with three or four uniformly sized costellae near the ventral margin, coarse commarginal lamellae persistent throughout ontogeny, and thinner, less elongated resilial teeth.
Ferreira (1960) compared Chlamys (Argopecten) tetristriatus with Chlamys corymbiata Hedley, 1909 (= Cryptopecten nux [Reeve, 1853]) (Upper Pleistocene–Recent, Indo-West Pacific; Dijkstra and Beu 2018; USNM IZ 764159); however, the latter was later included into Cryptopecten Dall et al., 1938 (Dijkstra and Beu 2018). Perapecten differs from Cryptopecten (Table 1) in having more inflated shells, weaker ctenolial teeth, smaller auricles, shorter anterior auricles, and posterior auricles with the free margin straight and inclined anteriorly. Perapecten sometimes have interspaces and flanks of the plicae covered with imbricated scales that enclose small hollow chambers in their junction with the radial ornamentation (Fig. 4B3, H), but this sculpture pattern is much more frequent in Cryptopecten, with the scales reaching the crests of plicae (Hayami 1984).
Stratigraphic and geographic range.—Upper Burdigalian (Lower Miocene): northeastern Brazil, tropical western Atlantic. Middle Miocene–Pliocene: Europe and North Africa. Recent: tropical-subtropical eastern Atlantic and Mediterranean Sea, from Portugal to South Africa, and Indian Ocean of South Africa.
Perapecten tetristriatus (Ferreria, 1960) comb. nov.
Fig. 4A–D.
1960 Chlamys (Argopecten) tetristriata sp. nov.; Ferreira 1960: 149, 150, pl. 3: 1, 1a.
1960 Chlamys (Argopecten) daideleus (Maury, 1925b) Ferreira 1960: pl. 3: 2, 2a.
1989 Chlamys (Argopecten) tetristriata Ferreira, 1960; Fernandes and Távora 1989: 107, 108, pl. 2: 10.
Type material: MNRJ 4802-I, 4803-I, a right and left valve, holotype and paratype of Chlamys (Argopecten) tetristriata, respectively.
Type locality: Fazenda Ilha de Fortaleza, Brazil.
Type horizon: Upper Burdigalian (Lower Miocene).
Material.—MNRJ 12065-I (ex MNRJ 4800-I 2, 3) a left and right valves, from Fazenda Ilha de Fortaleza. For details see SOM.
Dimensions.—See Table 2.
Emended diagnosis.—Small size, rudimentary byssal notch and obsolete byssal fasciole, posterior auricles very inclined anteriorly. Radial plicae ranging from 20 to 24, closely arranged, with very narrow interspaces; plicae and interspaces bearing up to three thin costellae near ventral margin.
Description.—Shell small, up to 20 mm in length, globose, thick, orbicular, prosocline, slightly longer than high, right-convex; dorsal margins of disc very short, ventral margin entire. Umbonal angle 100–107°. Hinge dorsal margin straight; hinge length ca. 45–57% of total disc length. Auricles small, short, anterior ones slightly longer than posterior ones; free margins of posterior auricles straight and strongly inclined anteriorly; right anterior auricle with free margin rounded and slightly scrolled dorsally; right anterior auricle sculptured with 5 coarse, evenly thick, closely-packed radial costae, left anterior and posterior auricles with 5–7 costellae; commarginal lamellae well-developed, close-set on auricles, coarse on anterior ones; byssal notch and sinus rudimentary, byssal fasciole obsolete, byssal notch angulate; ctenolium with one denticle throughout ontogeny, or obsolete. Disc sculptured with 20–24, subrectangular, low, evenly spaced plicae, wider than interspaces, both bearing up to three superimposed thin, equal-width radial costellae that emerge at the same growth stage, near ventral margin; plicae sometimes bearing a central secondary thin costella developed in earlier stages; interspaces on right valve very narrow; commarginal lamellae coarse, evenly spaced and very close-set on both valves, commencing at initial radial stage and persisting throughout ontogeny on interspaces, only weakly projected over crests of plicae in umbonal zone, slightly ventrally arcuate on right umbonal zone, vanishing ventrally; plicae flanks and interspaces sometimes covered with imbricated “roof-tile” scales that enclose hollow chambers on the intersection with commarginal lamellae. Pre-radial stage of left valve not preserved. Interior of disc with moderately well-developed pustular auricular buttresses. Internal imprint of external costae dorsally obliterated by the inner calcite layer, rib carinae prominent and short on ventral margin with dish edge-like shape. Hinge plate low, with prominent vertical ridges and grooves; coarse infradorsal and dorsal teeth, right valve with intermediate and resilial teeth thin, intermediate elongated to nearly the middle of the auricles.
Remarks.—Chlamys (Argopecten) tetristriatus Ferreira, 1960, is transferred to Perapecten for its distinctive globose and right-convex shells, with subrectangular plicae sculptured with three costellae of uniform width on crests near the ventral margin, and thick, closely arranged commarginal lamellae and occasionally patches of delicate scales imbricated like roof-tiles forming hollow chambers (Fig. 4B3). Size and shape of auricles, byssal sinus, notch and fasciole, the poorly developed ctenolium and the coarse hinge teeth with the resilial ones extended ventrally also support inclusion in Perapecten.
The specimens previously classified as L. daideleus (MN12065-I, ex MNRJ 4800-I; Fig. 4C, D) by Ferreira (1960) are placed herein in P. tetristriatus. This reassignment is because they have very short auricles, a rudimentary byssal notch, sinus and fasciole, an obsolete ctenolium, free margins of the posterior auricles strongly inclined anteriorly, a similar number of plicae, interspaces crossed by closely spaced commarginal lamellae of the same thickness, and coarse hinge teeth on both valves.
Perapecten tetristriatus is comparable to the type species of Perapecten, P. commutatus (e.g., of consulted material of the latter species, MNHN-IM-2000-24361, 24362, Fig. 4G, H; NMR 38887, 38890, USNM IZ 764279, 764284, 764286), in various aspects. These include shell outline, convexity, inclination of the small auricles, development of a rudimentary byssal notch and fasciole, obsolete ctenolium, very short posterior auricles, and the presence of up to three secondary costellae of even width usually developed on interspaces and crests of plicae. However, P. tetristriatus has smaller, less prosocline shells sculptured with 20–24 radial plicae while P. commutatus has 17–20 plicae separated by wider interspaces.
Perapecten tetristriatus differs from P. commutatus peripheralis (MNHN-IM-2014-10184, 10185; NMSA E8964, V8730a) because of its smaller, orbicular, longer than high shells, with a wider umbonal angle and a ctenolium with only one denticle (three in P. commutatus peripheralis).
Small specimens of P. scabrellus (MNHN.F.R 63425, Messinian, Upper Miocene, Cerro Mandras, Spain; Lacour et al. 2002) are very similar to P. tetristriatus in shell-shape, relative dimensions and the shape of auricles. However, large specimens differ from P. tetristriatus in developing more prosocline and larger shells, with longer anterior auricles and a deeper byssal notch. The less inclined free margins of the posterior auricles and the lower number of radial plicae bearing up to four costellae are further characteristics that distinguish P. scabrellus from P. tetristriatus.
Stratigraphic and geographic range.—Upper Burdigalian (Lower Miocene), Pirabas Formation, Ilha de Fortaleza, northeastern Pará state, northeastern Brazil.
Genus Iemanjavola nov.
Zoobank LSID: urn:lsid:zoobank.org:act:3C961310-9754-4200-95D6-06A319F0753C
Etymology: From the Goddess Iemanja of the sea waters revered in northern Brazil, and vola from the common stem used for many Pectininae taxa, such as Serratovola Habe, 1951, based on close morphological resemblance to this genus.
Type species: Iemanjavola monlafertei gen. et sp. nov., by monotypy; see below.
Remarks.—Iemanjavola gen. nov. is placed in the tribe Aequipectinini because of its fan-like shells with inner ventral carinae, sculptured with undivided even-width plicae with no intercalation throughout ontogeny, and with commarginal lamellae well developed on interspaces. Resilial, dorsal and infradorsal teeth are much thinner than in any other Aequipectinini. The microsculpture of the pre-radial stage of this new taxon is unknown because the umbo of the available left valves is lacking or poorly preserved.
Iemanjavola gen. nov. differs from Leptopecten by having a small, less prosocline, fan-like, flat left valve and more convex right one, a shorter hinge dorsal margin that forms an obtuse angle with the free convex margins of the auricles, shallower byssal notch and sinus, obsolete ctenolium, and auricles that are smooth or sculptured with weaker costellae that fade out distally.
Iemanjavola gen. nov. is distinguished from Perapecten by having fan-like, inequiconvex shells with a more globose umbo on the right valve, a weaker hinge dentition, fewer plicae sculptured with small, widely spaced, not imbricated scales, and thinner and more spaced commarginal lamellae on interspaces. The free margins of auricles are convex, the posterior auricles are longer than the anterior ones, and the auricles may be smooth or sculptured with weaker costellae, with the right anterior one bearing higher and thicker ventral costae.
Unlike Argopecten, Iemanjavola gen. nov. has small, less prosocline, fan-like and inequiconvex shells, with a shallower byssal notch. The auricles are smooth or crossed by weaker costellae, the free margins are convex and posteriorly inclined in the anterior ones, while in Argopecten they are concave or sigmoidal and anteriorly inclined.
Iemanjavola gen. nov. resembles the genus Serratovola (Pliocene–Recent, Indo-West Pacific area; Dijkstra and Beu 2018) by sharing small to medium-sized, fan-like and inequiconvex shells, characterized by globose right valves, and flat (in both) to slightly concave left ones (in Serratovola). Left auricles are sculptured with very weak costellae, and resilial and dorsal teeth are weakly developed in both genera. Iemanjavola gen. nov. differs from Serratovola in having no costellate plicae (while Serratovola has tripartite ones with three superimposed costellae on the crests), with no imbricated scales, lacking hollow chambers enclosed by the imbricated scales, and by having a smaller and shorter right anterior auricle, and a higher and shallower, subrounded, byssal notch.
Iemanjavola gen. nov. can be separated from Minnivola Iredale, 1939, from the Miocene–Recent of the Indo-West Pacific area (Hayami 1989) by having a flat left valve sculptured with entire plicae of uniform width with no pseudo-shagreen microsculpture, and lateral margins of the left disc entirely sculptured with radial costae, and located in the same plane as the central area of the disc.
Stratigraphic and geographic range.—Upper Burdigalian (Lower Miocene), Pirabas Formation, northeastern Pará state, northeastern Brazil.
Iemanjavola monlafertei sp. nov.
Fig. 5.
1960 Chlamys (Argopecten) agronomica (Maury, 1925b); Ferreira 1960: pl. 2: 1, 1a (non Pecten agronomicus Maury, 1925b).
Zoobank LSID: urn:lsid:zoobank.org:act:E98BA031-B102-4875-B9AE -25ADD35462FA
Etymology: Named after Norma Monserrat Bustamante Laferte (born 1983), multi-awarded Chilean-Mexican singer-songwriter artistically known as Mon Laferte. She is recognized for her musical versatility and powerful voice.
Holotype: MG-8622-I and 8623-I, a left and right well-preserved valve (with the right valve internally covered with carbonate rock and bearing encrusting serpulid worm tubes in low abundance).
Type localitiy: Quarry B-17, Capanema, Brazil.
Type horizon: Wackestone/packstone bed from the upper section of the Pirabas Formation (Fig. 1A), Upper Burdigalian (Lower Miocene).
Material.—Type material and MNRJ 4800-I, a right valve, classified as Argopecten daideleus (Maury, 1925b) by Ferreira (1960), from Fazenda, Ilha de Fortaleza. MNRJ 4801-I, a right and left valves, identified as Argopecten agronomica (Maury, 1925b) by Ferreira (1960), from Caieira, Capanema. MACN-Pi 6501, a left valve, from Atalaia Beach. All from Lower Miocene, Brazil. For details see SOM.
Dimensions.—See Table 2.
Diagnosis.—Aequipectinini with small fan-like shell, strongly inequiconvex, right valve globose with very convex, curved umbo and left valve barely inflated in umbonal area and ventrally flat. Auricles short, posterior slightly longer than anterior ones with free margins convex and forming acute or right-angle with the hinge margin; smooth or sculptured with very weak, widely spaced costellae, vanishing distally, the two ventral-most on the right anterior auricle more prominent than the dorsal ones, and persisting throughout ontogeny. Byssal notch very shallow, ctenolium obsolete, byssal sinus absent. Shell with 15–16 non-costellate plicae.
Description.—Shell small for the Aequipectinini tribe, attaining up to 14 mm in length, moderately thick, flaring, prosocline, longer than high, strongly inequiconvex, right valve inflated with umbo strongly convex, left valve flat with a barely convex umbo; dorsal margins of disc very short; ventral margin slightly serrated. Umbonal angle 102–107°. Hinge dorsal margin straight, hinge length ca. 64–75% of total disc length. Auricles elongate, large compared to size of disc, posterior auricles longer than anterior ones; free margins convex and forming an acute or right-angle with the dorsal margin; left auricles smooth or bearing up to 5 very thin costellae vanishing distally, right auricles dorsally smooth and ventrally with three or four radial costae vanishing towards free margin, the two ventral-most costae on the right anterior one remaining coarse through ontogeny and coarser and more elevated than the others; left auricles with commarginal lamellae, on the anterior one developed only on ventral area; byssal notch rounded, very shallow and ctenolium with two or three very weak denticles; byssal sinus absent. Disc sculptured with 15–16 subrounded, smooth, evenly spaced radial plicae, narrower than interspaces on right valves and equal in width or narrower than interspaces on the left; costae absent on interspaces; right plicae may have very low scales on crests near ventral margin; commarginal lamellae on both valves non-sinuous, evenly and widely spaced, very thin in interspaces and weakly projecting on plicae flanks, persistent throughout ontogeny or restricted to umbonal area, and ventrally replaced by antimarginal ridgelets; crests of left plicae without microsculpture or bearing very fine antimarginal ridgelets. Pre-radial stage unknown; radial stage commencing at ca. 0.3 mm. Interior of disc with auricular buttresses, posterior stronger than anterior ones, elongated on left and right posterior auricles, ending in moderately strong denticle; rib carinae prominent. Hinge plate narrow, crossed by prominent vertical ridges and grooves; resilifer shallow, very short, triangular, posteriorly inclined; dorsal and infradorsal teeth very narrow and low, resilial tooth extremely weak, intermediate teeth absent.
Remarks.—The material originally assigned to Chlamys (Argopecten) daideleus (Maury, 1925b) (MNRJ 4800-I) and Chlamys (Argopecten) agronomicus (Maury, 1925b) (MNRJ 4801-I, Fig. 5D, E) by Ferreira (1960) is herein referred to Iemanjavola monlafertei gen. et sp. nov. These specimens differ from L. daideleus by having fan-like shells; more convex right valves and flat left ones, sculptured with fewer plicae; smaller and shorter anterior auricles with a shallower byssal notch and no byssal sinus; and fewer and weaker ctenolium denticles.
This new taxon is rare at Caieira, Quarry B-17, (Capanema), Fazenda (Ilha de Fortaleza) and Atalaia Beach.
Stratigraphic and geographic range.—Upper Burdigalian (Lower Miocene); Pirabas Formation, northeastern Pará state, northeastern Brazil.
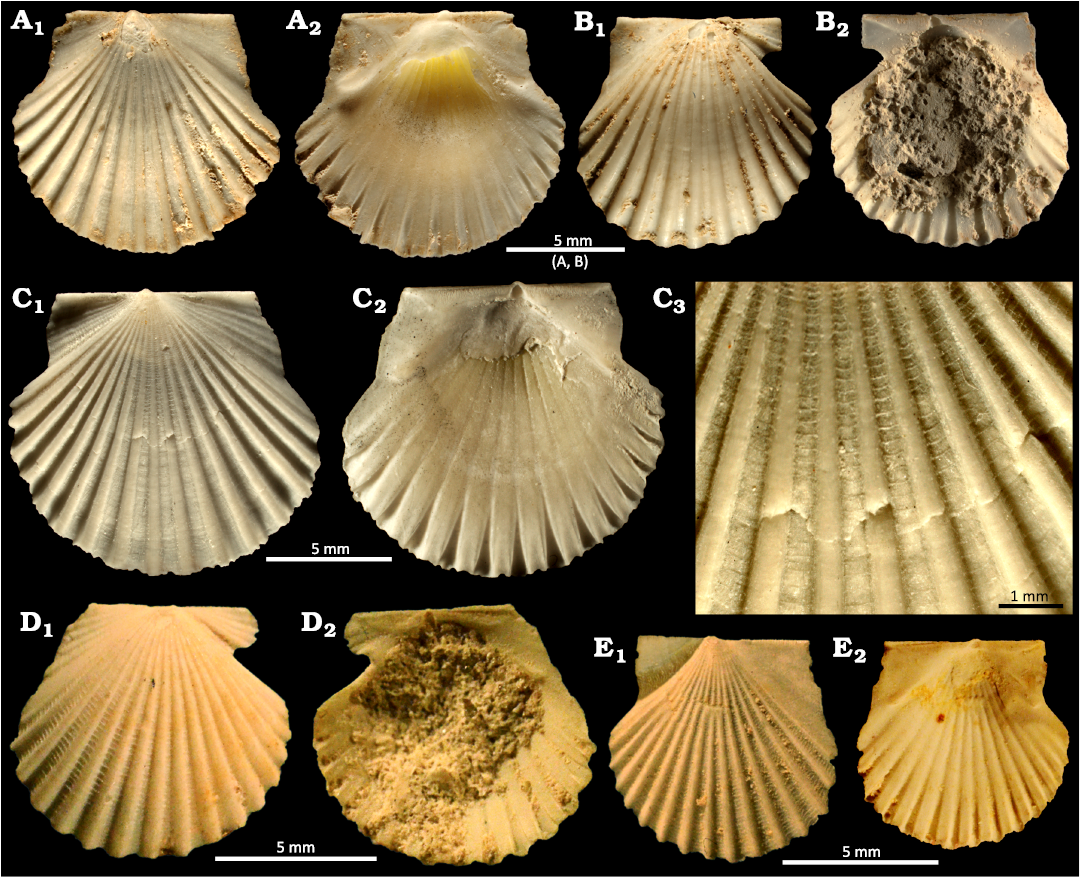
Fig. 5. Aequipectinin bivalve Iemanjavola monlafertei gen. et sp. nov. from the Pirabas Formation, upper Burdigalian (Lower Miocene) of Pará state, Brazil. A. MG-8622-I, a left valve in external (A1) and internal (A2) views; Quarry B-17. B. MG-8623-I, a right valve in external (B1) and internal (B2) views; Quarry B-17. C. MACN-PI 6501, left valve in external (C1) and internal (C2) views, close up showing detail of commarginal lamellae (C3); Atalaia Beach. D, E. MNRJ 4801-I, originally classified as Argopecten agronomicus (Maury, 1925b) by Ferreira (1960); Caieira. D. Right valve in external (D1) and internal (D2) views. E. Left valve in external (E1) and internal (E2) views.
Discussion
Maury (1925b) proposed Brazil as the center of the origin and dispersal of the Cenozoic Caribbean molluscan fauna. Ferreira (1960) identified morphological similarities between the Caribbean Leptopecten cf. L. latiauratus and the northeastern Pacific L. latiauratus, suggesting that L. cf. L. latiauratus would have first appeared in the Atlantic Ocean and spread to the eastern Pacific during the Pliocene. Waller (2011) stated that Leptopecten evolved from the late Oligocene–Early Miocene “Leptopecten andersoni group” (eastern North Pacific), migrating to the western Atlantic in the Middle Miocene.
As discussed above, and according to the present analysis the late Oligocene species included in the “L. andersoni group” do not belong in Leptopecten and undoubted Leptopecten species in the eastern Pacific are of Late Miocene age. These latter are L. praevalidus (Jordan and Hertlein, 1926) (Almejas Formation, Mexico), L. bellilamellatus (Arnold, 1906) (San Diego, Almejas, and Purisima formations, middle California to Baja California Sur) (Carreño and Smith 2007; Powell et al. 2007), L. cracens (Olsson, 1964) (Angostura Formation, Ecuador) (Brichau et al. 2021), and L. nelsoni (Olsson, 1932) (Tumbes Formation, Peru) (Fernández et al. 2005).
We propose the tropical western Atlantic as the origin region of Leptopecten, where the earliest representatives are L. daideleus (Early Miocene), L. gilbertharrisi and L. tabaquita (Early and early Middle Miocene), followed by L. ecnomius (latest Middle–earliest Late Miocene) and L. coderensis (Late Miocene).
During Miocene times a communication pathway, the so-called Central American Seaway (CAS) existed between the western Atlantic and the Pacific oceans. The westward surface Circumtropical Current allowed faunal interchange between both oceans before the uplift of the Central American Isthmus (Vermeij and Rosenberg 1993; Droxler et al. 1998; Iturralde-Vinent 2003, 2006; Schweitzer et al. 2006). This has been documented by molluscan dispersions through the CAS (Woodring 1982; Del Río 1990; Beu 2001; Waller 2007, 2011). Leptopecten sensu stricto was first recorded in the Upper Miocene of northeastern Brazil, and would have dispersed northwards via the Guiana Current, spreading during the Middle Miocene in the Caribbean Sea and from this area to the eastern Pacific by means of the westwards Circumtropical Current in the Late Miocene. This dispersal mechanism is supported by other pectinid groups such as Euvola Dall, 1898, Amusium Röding, 1798, Spatochlamys Waller, 1993, and Paraleptopecten (Waller 1993, 2007, 2011). Following Late Miocene restriction of flow between the Caribbean and eastern Pacific (Kirillova et al. 2019), Leptopecten could have dispersed to the north (Baja California and California) and to the south (Ecuador, Peru) via branches of the Pacific Equatorial Counter Current.
The presence of Perapecten Wagner, 1985, in the Early Miocene of Brazil constitutes its oldest occurrence of the genus and extends its distribution to the western Atlantic region, where it became locally extinct some time after the late Burdigalian, Early Miocene. Perapecten continued to exist in the Middle Miocene of Europe and North Africa (Perapecten scabrellus) and persisted until Recent times in that area, as well as elsewhere in the tropical eastern Atlantic, and in a small area of the Indian Ocean (P. commutatus commutatus and P. commutatus peripheralis).
As happened with some gastropods and decapods (Bernasconi and Robba 1982; Dolin 1991; Schweitzer et al. 2006), Perapecten would have spread to Europe via the South Equatorial Current, the Gulf Stream, and the North Atlantic Drift during the latest Early Miocene–Middle Miocene, and the planktotrophic larvae in pectinids would have facilitated that dispersal. This is coincident with the exchange peak of gastropod fauna during the Burdigalian suggested by Harzhauser et al. (2002). Landau et al. (2015) also proposed a similar dispersal mechanism for some Caribbean gastropods such as Chauvetia as late as the Tortonian, Late Miocene, when the Gulf Stream and the North Atlantic Current would have become stronger (Kameo and Sato 2000).
Perapecten would have arrived in European seas in the Middle Miocene, when the Mediterranean Sea and Central Paratethys were connected (Popov et al. 2004; Kováč et al. 2017), being first recorded in the Central Paratethys, but likely passing first through the Mediterranean Sea from eastern Atlantic waters.
Conclusions
The tribe Aequipectinini constituted a moderately diverse group during the Early Miocene in northeastern Brazil, being represented in the Pirabas Formation by Leptopecten daideleus (Maury, 1925b), Perapecten tetristriatus (Ferreira, 1960), and Iemanjavola monlafertei gen. et sp. nov., revealing a lower diversity than today. The genus Perapecten Wagner, 1985, is re-validated, its occurrence in the Pirabas Formation predating that of Perapecten scabrellus Lamarck, 1819 (Middle Miocene–Pliocene) in Europe.
As the occurrences of Leptopecten Verrill, 1897, and Perapecten Wagner, 1985, in the Pirabas Formation are the oldest known, these tropical genera could have originated in the western Atlantic. Leptopecten dispersed northwards during the Early Miocene and westwards to Venezuela and Panama during the Middle Miocene through the Circumtropical Current, ultimately reaching the eastern Pacific via the CAS. Perapecten dispersed eastwards through the Gulf Stream and North Atlantic Drift during the latest Early–Middle Miocene, reaching Europe and North Africa, where P. scabrellus is recorded. Perapecten became extinct in the western Atlantic but survived until the Recent times in the Mediterranean Sea and subtropical eastern Atlantic and Indian Ocean, where it is represented by two subspecies of P. commutatus.
Acknowledgements
The authors are thankful to the curators and technicians: Rodrigo Da Rocha Machado (DGM), Sandro Scheffler and Alexandre Dias Pimenta (both MN-UFRJ), Alejandro Tablado (MACN), Philippe Bouchet, Philippe Maestrati, Virginie Héros, Didier Merle, Jean-Michel Pacaud, and Géraldine Toutirais (all MNHN), Leslie Skibinski and Vicky Wang (both PRI), Stewart Edie and Nicholas Drew (both Department of Paleobiology, USNM), John Pfeiffer and Yolanda Villacampa (both Invertebrate Zoology, USNM) for access to respective collections. The following curators provided high quality images of the material used for comparison: Igor Muratov (NMSA), Ashley Dineen (UCMP), Yves Samyn and Florence Trus (both RBINS). Fabián Tricárico (MACN) took the SEM images, Diego Urteaga and Guido Pastorino (both MACN) helped with the preparation of material and Sergio Martínez (Universidad de la República, Montevideo, Uruguay) provided material from Praia do Atalaia. This research received support from the SYNTHESYS+ Project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the H2020 Integrating Activities Programme, Project number 8238272023, Sepkoski Grant of the Paleontological Society International Research Program (PalSIRP), John W. Wells Grants-in-Aid of Research Program of the Paleontological Research Institution, and the PICT 2021-00277 of the ANPCyT to MBS, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico-Brazil (307424/2019-7) to MIFR. We thank the three anonymous reviewers, Andrew Johnson (University of Derby, UK), and the editors whose valuable comments and suggestions have significantly improved the quality and language of the manuscript.
Authors’ contributions
MBS: conceptualization, investigation, writing, original draft preparation, review and editing, figures. CJdR: stratigraphy, writing, review and editing. VAT: provision of study materials, stratigraphy. MIFR: provision of study materials.
References
Addicott, W.O. 1972. Provincial middle and late Tertiary molluscan stages, Temblor Range, California. In: E.H. Stinemeyer and C.C. Church (eds.), Proceedings, 47th Annual Convention: Pacific Coast Miocene Biostratigraphic Symposium, Pacific Section S.E.P.M., 1–26. Society of Economic Paleontologists and Mineralogists, Bakersfield
Addicott, W.O. 1973. Oligocene molluscan biostratigraphy and paleontology of the lower part of the type Temblor Formation, California. United States. Geological Survey Professional Paper 791: 1–48. Crossref
Aguilera, O.A., Moraes-Santos, H., Costa, S., Ohe, F., Jaramillo, C., and Nogueira, A. 2013. Ariid sea catfishes from the coeval Pirabas (Northeastern Brazil), Cantaure, Castillo (Northwestern Venezuela), and Castilletes (North Colombia) formations (Early Miocene), with description of three new species. Swiss Journal of Palaeontology 13: 45–68. Crossref
Aguilera, O., Luz, Z., Carrillo-Briceño, J.D., Kocsis, L., Vennemann, T.W., de Toledo, P.M., Nogueira, A., Borges Amorim, K., Moraes-Santos, H., Polck, M.R., Ruivo, M.L., Linhares, A.P., and Monteiro-Neto, C. 2017. Neogene sharks and rays from the Brazilian “Blue Amazon”. PLoS One 12 (8): e0182740. Crossref
Aguilera, O., de Araújo, O.M.O., Hendy, A., Nogueira, A.A., Nogueira, A.C., Maurity, C.W., Kutter, V.T., Martins, M.V.A., Coletti, G., Dias, B.B., da Silva-Caminha, S.A., Jaramillo C., Bencomo, K., and Lopes R.T. 2020. Palaeontological framework from Pirabas Formation (North Brazil) used as potential model for equatorial carbonate platform. Marine Micropaleontology 154: 101813. Crossref
Aguirre, J., Braga, J.C., Jiménez, A.P., and Rivas, P. 1996. Substrate-related changes in pectinid fossil assemblages. Palaeogeography, Palaeoclimatology, Palaeoecology 126: 291–308. Crossref
Alejandrino, A., Puslednik, L., and Serb, J.M. 2011. Convergent and parallel evolution in life habit of the scallops (Bivalvia: Pectinidae). BMC Evolutionary Biology 11: 1–164. Crossref
Antonioli, L., Távora, V.A., and Dino, R. 2015. Palynology of carcinolites and limestones from the Baunilha Grande Ecofacies of the Pirabas Formation (Miocene of Pará state, northeastern Brazil). Journal of South American Earth Sciences 62: 134–147. Crossref
Arnold, R. 1906. Tertiary and Quaternary pectens of California. United States Geological Survey Professional Paper 47: 1–264. Crossref
Benyoucef, M., Bendella, M., Brunetti, M., Ferré, B., Koci, T., Bouchemla, I., Slamif, R., and Ghenim, A.F. 2021. Upper Pliocene bivalve shell concentrations from the Lower Chelif basin (NW Algeria): Systematics, sedimentologic and taphonomic framework. Annales de Paléontologie 107: 102509. Crossref
Bernard, F.R. 1983. Catalogue of the living Bivalvia of the eastern Pacific Ocean: Bering Strait to Cape Horn. Canadian Special Publication of Fisheries and Aquatic Science Publication Spéciale Canadienne des Sciences Halieutiques et Aquatiques 61: 1–102.
Bernasconi, M.P. and Robba, E. 1982. The thecosomatous pteropods: a contribution toward the Cenozoic Tethyan paleobiogeography. Bollettino della Società Paleontologico Italiana 21: 211–222.
Beu, A.G. 2001. Gradual Miocene to Pleistocene uplift of the Central American Isthmus: evidence from tropical American tonnoidean gastropods. Journal of Paleontology 75: 706–720. Crossref
Brichau, S., Reyes, P., Gautheron, C., Hernández, M.J., Michaud, F., Leisen, M., Vacherat, A., Saillard, M., Proust, J.N., and O’Sullivan, P. 2021. First timing constraints on the Ecuadorian Coastal Cordillera exhumation: Geodynamic implications. Journal of South American Earth Sciences 105: 103007. Crossref
Bronn, H.G. 1862. Die Klassen und Ordnungen der Weichtiere (Malacozoa), wissenschaftlich dargestellt in Wort und Bild, Band 3, Abt. 1. Kopflose Weichthiere (Malacozoa Acephala). 518 pp. Winter’sche Verlagshandlung, Leipzig. Crossref
Carreño, A.L. and Smith, J.T. 2007. Stratigraphy and correlation for the ancient Gulf of California and Baja California Peninsula, Mexico. Bulletins of American Paleontology 371: 1–146.
Carter, J.G., Altaba, C.R., Anderson, L.C., Araujo, R., Biakov, A.S., Bogan, A.E., Campbell D.C., Campbell, M., Chen, J.-H., Cope, J.C.W., Delvene, G., Dijkstra, H.H., Fang, Z.-J., Gardner, R.N., Gavrilova, V.A., Harries, P.J., Hartman, J.H., Hautmann, M., Hoeh, W.R., Hylleberg, J., Jiang, B.-Y., Johnston, P., Kirkendale, L., Kleeman, K., Koppka, J., Kříž, J., Machado, D., Malchus, N., Márquez-Aliaga, A., Masse, J.-P., McRoberts, C.A., Middelfart, P.U., Mitchell, S., Nevesskaya, L.A., Özer, S., Pojeta, J., Polubotko, I.V., Pons, J.M., Popov, S., Sánchez, T., Sartori, A.F., Scott, R.W., Sey, I.I., Signorelli, J.H., Silantiev, V.V., Skelton, P.W., Steuber, T., Waterhouse, J.B., Wingard, G.L., and Yancey, T. 2011. A synoptical classification of the Bivalvia. Paleontological Contribution, Paleontological Institute, University of Kansas 4: 1–47.
Clark, G.R. 1971. The influence of water temperature on the morphology of Leptopecten latiauratus (Conrad, 1837). The Veliger 13: 269–272.
Coan, E.V. and Valentich-Scott, P. 2012. Bivalve Seashells of Tropical West America: Marine Bivalve Mollusks from Baja California to Northern Peru. 1258 pp. Museum of Natural History, Santa Barbara.
Coan, E.V., Valentich-Scott, P.H., and Bernard, F.R. 2000. Bivalves sea shells of Western North America. Marine bivalve mollusks from Arctic Alaska to Baja California. 764 pp. Santa Barbara Museum of Natural History, Santa Barbara.
Collins, L.S. and Coates., A.G. 1999. A paleobiotic survey of Caribbean faunas from the Neogene of the Isthmus of Panama. Bulletins of American Paleontology 357: 5–13.
Conrad, T.A. 1837. Description of new marine shells, from Upper California. Collected by Thomas Nuttall, Esq. Journal of the Academy of Natural Sciences, Philadelphia 7: 227–268.
Cossmann M. and Peyrot A. 1914. Conchologie néogénique de l’Aquitaine. Actes de la Société Linnéenne de Bordeaux 68 (1–2): 5–210.
Cushman, J.A. 1927. An outline of a reclassification of the foraminifera. Contributions from the Cushman Laboratory for Foraminiferal Research 3: 1–105.
Cuvier, G.L.C.F.C. 1797. Tableau Élémentaire de l’Histoire Naturelle des Animaux. 710 pp. Impreimeur Baudouin, Paris. Crossref
Dall, W.H. 1926. A new Pecten from Colombia. Proceedings of the Biological Society of Washington 39: 61.
Dall, W.H. 1898. Contributions to the Tertiary fauna of Florida with especial reference to the Miocene silex-beds of Tampa and the Pliocene beds of the Caloosahatchie River. Part IV. I. Prionodesmacea: Nucula to Julia. II. Teleodesmacea: Teredo to Ervilia. Transactions of the Wagner Free Institute of Science 3: 571–948.
Dall, W.H., Bartsch, P., and Rehder, H.A. 1938. A manual of the Recent and fossil marine pelecypod mollusks of the Hawaiian Islands. Bulletin of the Bernice P. Bishop Museum 153: 1–233.
Dautzenberg, P. 1900. Croisières du yacht Chazalie dans l’Atlantique: Mollusques. Mémoires de la Société Zoologique de France 13: 145–265.
Del Río, C.J. 1990. Composición, origen y significado paleoclimático de la malacofauna “Entrerriense” (Mioceno medio) de la Argentina. Anales de la Academia Nacional de Ciencias Exactas, Físicas y Naturales 42: 207–226.
DeVries T.J. 1986. Geology and Paleontology of Tablazos in Northwest Peru. Unpublished Ph.D. Dissertation. 964 pp. Ohio State University, Columbus.
Dijkstra, H.H. 1996. Notes on taxonomy and nomenclature of Pectinidae (Mollusca: Bivalvia). On the identity of Pecten pulchellus Reeve, 1853. Basteria 60 (1–3): 41–44.
Dijkstra, H.H. 2013. Pectinoidea (Bivalvia: Propeamussiidae and Pectinidae) from the Panglao region, Philippine Islands. Vita Malacologica 10: 1–108.
Dijkstra, H.H. and Beu, A.G. 2018. Living scallops of Australia and adjacent waters (Mollusca: Bivalvia: Pectinoidea: Propeamussiidae, Cyclochlamydidae and Pectinidae). Records of the Australian Museum 70 (2): 113–330. Crossref
Dijkstra, H.H. and Goud, J. 2002. Pectinoidea (Bivalvia, Propeamussiidae & Pectinidae) collected during the Dutch Cancap and Mauritania expeditions in the south-eastern region of the North Atlantic Ocean. Basteria 66: 31–82.
Dijkstra, H.H. and Kilburn, R.N. 2001. The family Pectinidae in South Africa and Mozambique (Mollusca: Bivalvia: Pectinoidea). African Invertebrates 42: 263–321.
Dolin, L. 1991. Cypraeoidea and Lamellarioidea (Mollusca: Gastropoda), from the Chipola Formation (late Early Miocene) of northwestern Florida. Tulane Studies in Geology and Paleontology 24 (1–2): 1–60.
Dollfus, G.F. and Dautzenberg, P. 1920. Conchyliologie du Miocène moyen du bassin de la Loire. I: Pelecypodes. Mémoires de la Société géologique de France. Paléontologie 27: 379–500.
d’Orbigny, A.D. 1846. Voyage dans l’Amérique méridionale (le Brésil, la république orientale de l’Uruguay, la République argentine, la Patagonie, la république du Chili, la république de Bolivia, la république du Pérou), exécuté pendant les années 1826, 1827, 1828, 1829, 1830, 1831, 1832 et 1833. Tome 5 (3) Mollusques. 489–758. A. Bertrand, Paris. Crossref
Droxler, A.W., Burke, K., Cunningham, A.D., Hine, A.C., Rosencrantz, E., Duncan, D. S., Hallock, P., and Robinson, E. 1998, Caribbean constraints on circulation between Atlantic and Pacific oceans over the past 40 million years. In: T. Crowley and K. Burke (eds.), Tectonic Boundary Conditions For Climate Reconstruction, 160–191. Oxford University Press, Oxford. Crossref
Fernández, J., Martínez, E., Calderón, Y., Hermoza, W., and Galdos, C. 2005. Tumbes and Talara Basins Hydrocarbon Evaluation. 290 pp. Perupetro S.A., Lima [available online, https://www.scribd.com/document/144666876/Tumbes-Talara-Basins-Report-Perupetro-2005]
Fernandes, J.M.G. and Távora, V.A. 1989. Estudo sistemático da subclasse Pteriomorphia Beurlen, 1944 (Mollusca–Bivalvia) na Formação Pirabas (Mioceno Inferior) do acervo do Museu de Geociências, UFPA. Boletim do Museu Paraense Emílio Goeldi (Ciências Da Terra) 1: 91–118.
Ferreira, C.S. 1960. Contribuiçao á paleontologia do estado do Pará. Revisao da família Pectinidae da formaçao Pirabas (Mioceno inferior), com a discriçao de novas espécies. VI. Mollusca-Pelecypoda. Arquivos do Museu Nacional (Brazil) 50: 135–165.
Ferreira, C.S. 1967. Contribuição à Paleontologia do Estado do Pará. O gênero Orthaulax Gabb, 1872 na Formação Pirabas. In: H. Lent (ed.), Atas do Simpósio sobre a Biota Amazónica, Belem, Brazil 1, 169–181. Conselho Nacional de pesquisa CNPQ, Rio de Janeiro.
Ferreira, C.S. 1980. Correlação da Formação Pirabas (Mioceno Inferior), N e NE do Brasil, com as Formações Chipola e Tampa da península da Flórida, USA. Anales de Congreso Argentino de Paleontología y Biostratigrafía, 1 Congreso Latinoamericano de Paleontologia 1 (3): 49–55. Asociación Paleontológica Argentina, Buenos Aires.
Ferreira, C.S. and Cassab, R.C.T. 1985. Implicações faciológicas da família Pectinidae (Mollusca-Bivalvia) da Formação Pirabas, Oligo-Mioceno do norte e nordeste do Brasil. In: D.A Campos, C.S. Ferreira, I.M. Brito, and C.F. Viana (eds.), Coletânea de Trabalhos Paleontológicos. Série Geologia, MME-DNPM, Seção de Paleontologia e Estratigrafia, Brasília 27: 205–209.
Ferreira, C.S. and Francisco, B.H.R. 1988. As relações da Formação Pirabas (Oligoceno–Mioceno), com as formações continentais terciárias no NE do Pará. In: Anais do XXXV Congresso Brasileiro de Geologia 2: 761–764. Sociedade Brasileira de Geologia, Belém.
Fischer, P.H. 1886. Manuel de conchyliologie et de paléontologie conchyliologique, ou histoire naturelle des mollusques vivants et fossiles. Part 10, 897–1008. F. Savy, Paris. Crossref
Freneix, S., Saint Martin, J.P., and Moissette, P. 1987. Bivalves Ptériomorphes du Messinien d’Oranie (Algérie occidentale). Bulletin du Muséum National d’Histoire Naturelle (4ème série), Paris, Section C1 9: 3–61.
Góes, A.M., Rossetti, D.D.F., Nogueira, A.C.R., and Toledo, P.M.D. 1990. Modelo deposicional preliminar da Formação Pirabas no nordeste do Estado do Pará. Boletim do Museu Paraense Emílio Goeldi, Série Ciências da Terra 2: 3–15.
Glibert, M. 1936. Faune malacologique des sables de Wemmel. I Pelecypodes. Memoires du Musee Royal d’Histoire Naturelle de Belgique 78: 1–190.
Glibert, M. 1975. Les Bivalvia du Ledien (Eocene Moyen Supérieur) de la Belgique premiere note: Palaeotaxodonta, Cryptodonta, Pteriomorphia. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique 51: 1–61.
Gmelin, J.F. 1791. Vermes. In: J.F. Gmelin (ed.), Caroli a Linnaei Systema Naturae per Regna Tria Naturae, Ed. 13. Tome 1 (6), 3021–3910. G.E. Beer, Lipsiae [Leipzig].
Grant, U.S. and Gale, H.R., 1931. Catalogue of the marine Pliocene and Pleistocene Mollusca of California and adjacent regions: with notes on their morphology, classification, and nomenclature and a special treatment of the Pectinidae and the Turridae (including a few Miocene and Recent species) together with a summary of the stratigraphic relations of the formations involved. Memoirs of the San Diego Society of Natural History 1: 1–1036.
Grau, G. 1959. Pectinidae of the eastern Pacific. Allan Hancock Pacific Expeditions 23: 1–308.
Gray, J.E. 1854. II.—Additions and corrections to the arrangement of the families of Bivalve shells. Annals and Magazine of Natural History 14 (79): 21–28. Crossref
Habe, T. 1951. Genera of Japanese Shells. Pelecypoda (I). 96 pp. Kairui bunken kankô-kai, Kyoto.
Harzhauser, M., Piller, W.E., and Steininger, F.F. 2002. Circum-Mediterranean Oligo-Miocene biogeographic evolution—the gastropods’ point of view. Palaeogeography, Palaeoclimatology, Palaeoecology 183: 103–133. Crossref
Harzhauser, M., Kranner, M., Mandic, O., Strauss, P., Siedl, W., and Piller, W.E. 2020. Miocene lithostratigraphy of the northern and central Vienna Basin (Austria). Austrian Journal of Earth Sciences 113: 169–199. Crossref
Hayami, I. 1984. Natural history and evolution of Cryptopecten (a Cenozoic–Recent pectinid genus). The University Museum and The University of Tokyo Bulletin 24: 1–149.
Hayami, I. 1989. Outlook on the post-Paleozoic historical biogeography of pectinids in the western Pacific region. In: H. Ohba, I. Hayami, and K. Mochizuki (eds.), Current Aspects of Biogeography in West Pacific and East Asian Regions, 3–25. The University Museum of the University of Tokyo, Tokyo.
Hayami, I. and Okamoto, T. 1986. Geometric regularity of some oblique structures in pectinid and other bivalves: recognition by computer simulations. Paleobiology 12: 433–449. Crossref
Hedley, C. 1909. Mollusca from the Hope Islands, North Queensland. Proceedings of the Linnean Society of New South Wales 34: 420–466.
Heilprin, A. 1887. Explorations on the West Coast of Florida and in the Okeechobee Wilderness. Transactions of the Wagner Free Institute of Science and Philosophy 1: 1–134.
Hodson, F., Hodson, H.K., and Harris, G.D. 1927. Some Venezualan and Caribbean Mollusks. Bulletins of America Paleontology 13 (49): 1–160.
Huber, M. 2010. Compendium of Bivalves. A Full-color Guide to 3.300 of the World’s Marine Bivalves. A Status on Bivalvia After 250 Years of Research. 901 pp. ConchBooks, Hackenheim.
Huber, M. 2015. Compendium of Bivalves 2. A Full-color Guide to the Remaining Seven Families. A Systematic Listing of 8’500 Bivalve Species and 10’500 Synonyms. 907 pp. ConchBooks, Harxheim.
ICZN 1999. International Code of Zoological Nomenclature. 4th Edition. The International Commission on Zoological Nomenclature [available online at https://www.iczn.org/the-code/the-code-online/]
Iturralde-Vinent, M. 2003. A brief account of the evolution of the Caribbean seaway: Jurassic to present. In: D. Prothero, L. Ivany, and E. Nesbitt (eds.), From Greenhouse to Icehouse: The Marine Eocene–Oligocene Transition. Proceedings of the Penrose Conference held 17–22 August 1999 in Olympia, Washington 22, 386–396. Columbia University Press, New York.
Iturralde-Vinent, M.A. 2006. Meso-Cenozoic Caribbean paleogeography: implications for the historical biogeography of the region. International Geology Review 48: 791–827. Crossref
Iredale, T. 1939. Mollusca. Part I. Scientific Reports of the Great Barrier Reef Expedition 1928–1929 5: 209–425.
Jefferies, R.P.S. 1962. The palaeoecology of the Actinocamax plenus subzone (lowest Turonian) in the Anglo-Paris Basin. Palaeontology 4: 609–647. Crossref
Jiménez, A.P., Aguirre, J., and Rivas, P. 2009. Taxonomic study of scallops (Pectinidae: Mollusca, Bivalvia) from Pliocene deposits (Almería, SE Spain). Revista Española de Paleontología 24: 1–30. Crossref
Johnson, C.L. and Graham, S.A. 2007. Middle Tertiary Stratigraphic Sequences of the San Joaquin Basin, California. In: A.H. Scheirer (ed.), Petroleum Systems and Geologic Assessment of Oil and Gas in the San Joaquin Basin Province, California 1713: 1–18. U.S. Geological Survey Professional Paper, California. Crossref
Jordan, E.K. and Hertlein, L.G. 1926. Contribution to the geology and paleontology of the Tertiary of Cedros Island and adjacent parts of Lower California. California Academy of Sciences Proceedings, ser. 4 15 (14): 409–464.
Kameo, K. and Sato, T. 2000. Biogeography of Neogene calcareous nannofossils in the Caribbean and the eastern equatorial Pacific-floral response to the emergence of the Isthmus of Panama. Marine Micropaleontology 39: 201–218. Crossref
Keller G. 1981. Origin and Evolution of the Genus Globigerinoides in the Early Miocene of the Northwestern Pacific, DSDP Site 292. Micropaleontology 27: 293–304. Crossref
Keller, M.A. Tennyson, M.E., and Denison R.E. 1995. Chapter P. Strontium isotope evidence for the age of the Vaqueros Formation and latest Oligocene marine transgression in the northern Santa Maria province, central California. In: M.A. Keller (ed.), Evolution of Sedimentary Basins/Onshore Oil and Gas Investigations—Santa Maria Province. US Geological Survey Bulletin 1995: P1–P8.
Kirillova, V., Osborne, A.H., Störling, T., and Frank, M. 2019. Miocene restriction of the Pacific-North Atlantic throughflow strengthened Atlantic overturning circulation. Nature Communications 10 (1): 4025. Crossref
Kováč, M., Andreyeva-Grigorovich, A., Bajraktarević, Z., Brzobohatý, R., Filipescu, S., Fodor, L., Harzhauser, M. Nagymarosy, A., Pavelić, D., Rögl, F., Saftić, B., Sliva, L., and Studencka, B. 2007. Badenian evolution of the Central Paratethys Sea: paleogeography, climate and eustatic sea-level changes. Geologica Carpathica 58: 579–606.
Kováč, M., Hudáčková, N., Halásová, E., Kováčová, M., Holcová, K., Oszczypko-Clowes, M., Báldi, K., Less, G., Nagymarosy, A., Ruman, A., Klučiar, T., and Jamrich, M. 2017. The Central Paratethys palaeoceanography: A water circulation model based on microfossil proxies, climate, and changes of depositional environment. Acta Geologica Slovaca 9: 75–114.
Lacour, D., Lauriat-Rage, A., Saint Martin, J.P., Videt, B., Neraudeau, D., Goubert, E., and Bongrain, M. 2002. Les associations de bivalves (Mollusca, Bivalvia) du Messinien du bassin de Sorbas (SE Espagne). Geodiversitas 24: 641–657.
Landau, B., da Silva, C.M., and Vermeij, G.J. 2015. First record of buccinid genus Chauvetia (Mollusca: Gastropoda) from the fossil record of the New World (Miocene, Venezuela) and its paleobiogeographic implications. Journal of Paleontology 89: 487–493. Crossref
Lamarck, J.B.P.A., de 1819. Histoire naturelle des animaux sans vertèbres: présentant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres, et la citation des principales espèces qui s’y rapportent et Supplément à divers types de gastéropodes et de Trachélipode comprenant l’indication des coquilles fossiles qui ne furent point citées dans leurs genres respectifs. Tome sixième. 232 pp. Verdière, Paris.
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Laurentius Salvius, Stockholm. Crossref
Loel, W. and Corey, W.H. 1932. The Vaqueros Formation, lower Miocene of California. I. Paleontology. University of California Publications, Bulletin of the Department of Geological Sciences 22 (3): 31–410.
Martinez, S., Ramos, M.I.F., McArthur, J.M., Del Río, C.J., and Thirlwall, M.F. 2017. Late Burdigalian (Miocene) age for pectinids (Mollusca-Bivalvia) from the Pirabas Formation (northern Brazil) derived from Sr-isotope (87Sr/86Sr) data. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 284: 57–64. Crossref
Maury, C.J. 1917. Santo Domingo type sections and fossils. Part 1. Mollusca. Bulletins of American Paleontology 29: 1–251.
Maury, C.J. 1920. Scientific survey of Porto Rico and the Virgin Islands. The New York Academy of Sciences 3: 1–77.
Maury, C.J. 1925a. A further contribution to the paleontology of Trinidad (Miocene horizons). Bulletins of American Paleontology 10: 1–250.
Maury, C.J. 1925b. Fosseis terciarios do Brasil com discripçao de novas formas cretaceas. Monographias do Serviço Geologico e Mineralogico do Brasil 4: 1–711.
Michelotti, G. 1839. Brevi cenni di alcuni resti delle Classi Brachiopodi ed Acefali, trovati fossili in Italia. Annali delle Scienze del Regno Lombardo-Veneto 9: 119–138, 157–174.
MolluscaBase 2024. Aequipectinini F. Nordsieck, 1969 [available at https://www.molluscabase.org/aphia.php?p=taxdetails&id=577989, accessed 29 May 2024].
Monterosato, T.A., di 1875. Poche note sulla Conchiologia Mediterranea. 15 pp. Tipografia del Giornale di Sicilia, Palermo, printed for the Author.
Monterosato, T.A. di 1889. Coquilles marines Marocaines. Journal de Conchyliologie 37: 20–40.
Moore, E.J. 1984. Tertiary marine pelecypods of California and Baja California: Propeamussiidae and Pectinidae. United States Geological Survey Professional Paper 1228B: 1–112. Crossref
Nordsieck, F. 1969. Die europäischen Meeresmuscheln (Bivalvia): Vom Eismeer bis Kapverden, Mittelmeer und schwarzes Meer. 256 pp. G. Fischer Verlag, Stuttgart.
Olsson, A.A. 1932. Contributions to the Tertiary paleontology of northern Peru: Part 5. The Peruvian Miocene. Bulletins of American Paleontology 68: 1–272.
Olsson, A.A. 1961. Mollusks of the Tropical Eastern Pacific. 574 pp. Paleontological Research Institute. Ithaca, New York.
Olsson, A.A. 1964. Neogene Mollusks from Northwestern Ecuador. 256 pp. Paleontological Research Institute. Ithaca, New York.
Petuch, E.J. 1995. Molluscan diversity in the Late Neogene of Florida: evidence for a two-staged mass extinction. Science 270: 275–277. Crossref
Popov S.V., Rögl F., Rozanov A.Y., Steininger F.F., Shcherba I.G., and Kováč M. 2004. Lithological-paleogeographic maps of Paratethys. 10 maps Late Eocene to Pliocene. Courier Forschungsinstitut Senckenberg 250: 1–46.
Powell, C., Barron, J., Sarna-Wojcicki, A., Clark, J.C., Perry, F.A., Brabb, E.E., and Fleck, R.J. 2007. Age, stratigraphy, and correlation of the Late Neogene Purisima Formation, central California Coast Ranges. United States Geological Survey Professional Paper 1740: 1–30. Crossref
Rafinesque, C.S. 1815, Analyse de la nature ou tableau de l’univers et des corps organisés. 225 pp. Jean Barravecchia, Palermo. Crossref
Récluz, C.A. 1853. Description de coquilles nouvelles. Journal de Conchyliologie 4: 49–54.
Reeve, L.A. 1853. Monograph of the genus Pecten. In: L. Reeve & Co. (eds.), Conchologia Iconica, or, Illustrations of the Shells of Molluscous Animals, Vol. 8, pls. 1–35 and unpaginated text. Reeve, London.
Riccardi, A.C. 1977. Berriasian invertebrate fauna from the Springhill Formation of Southern Patagonia. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 155: 216–252. Crossref
Rico-García, A.R. 2008. Pectínidos pliocenos de la Cuenca de Vejer (Cádiz, SO de España). Studia Geologica Salmanticensia 44: 91–140.
Ridewood, W.G. 1903. On the structure of the gills of the Lamellibranchia. Philosophical Transactions of the Royal Society B 195: 147–284. Crossref
Röding, P.F. 1798. Museum Boltenianum sive Catalogus cimeliorum e tribus regnis naturae quae olim collegerat Joa. Fried Bolten, M. D. p. d. per XL. annos proto physicus Pars secunda continens Conchylia sive Testacea univalvia, bivalvia & multivalvia. 199 pp. Johan Christi Trappii, Hamburg.
Rossetti, D.F. 2001. Late Cenozoic sedimentary evolution in northeastern Pará, Brazil, within the context of sea level changes. Journal of South American Earth Sciences 14: 77–89. Crossref
Rossetti, D.F., Bezerra, F.H., and Dominguez, J.M. 2013. Late Oligocene–Miocene transgressions along the equatorial and eastern margins of Brazil. Earth-Science Reviews 123: 87–112. Crossref
Sacco, F. 1897. I molluschi del terreni terziari del Piemonte e delle Liguria. Parte 24, 1–116. Carlo Clausen, Turino.
Schneider, S., Berning, B., Bitner, M. A., Carriol, R.P., Jäger, M., Kriwet, J., Kroh, A., and Werner, W. 2009. A parautochthonous shallow marine fauna from the Late Burdigalian (early Ottnangian) of Gurlarn (Lower Bavaria, SE Germany): macrofaunal inventory and paleoecology. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 254: 63–103. Crossref
Schweitzer, C.E., Iturralde-Vinent, M., Hetler, J.L., and Velez-Juarbe, J. 2006. Oligocene and Miocene decapods (Thalassinidea and Brachyura) from the Caribbean. Annals of Carnegie Museum 75: 111–136. Crossref
Sherratt, E., Alejandrino, A., Kraemer, A., Serb, J., and Adams, D.C. 2016. Trends in the sand: Directional evolution in the shell shape of recessing scallops (Bivalvia: Pectinidae). Evolution 70: 2061–2073. Crossref
Smith, E.A. 1890. Report on the marine molluscan fauna of the island of St. Helena. Proceedings of the Zoological Society of London 1890: 247–317.
Smith, J.T. and Jackson, J.B. 2009. Ecology of extreme faunal turnover of tropical American scallops. Paleobiology 35: 77–93. Crossref
Smith, C.J., Collins, L.S., Jaramillo, C., and Quiroz, L.I. 2010. Marine paleoenvironments of Miocene–Pliocene formations of north-central Falcón State, Venezuela. The Journal of Foraminiferal Research 40: 266–282. Crossref
Spadini, V. 2022. La famiglia Pectinidae nel Pliocene senese (Italia, Toscana). Società per gli Studi Naturalistici della Romagna 55: 67–136.
Studencka, B. 1986. Bivalves from the Badenian (Middle Miocene) marine sandy facies of southern Poland. Palaeontologia Polonica 47: 3–128.
Studencka, B., Gontsharova, I.A., and Popov, S.V. 1998. The bivalve faunas as a basis for reconstruction of the Middle Miocene history of the Paratethys. Acta Geologica Polonica 48: 285–342.
Talmat, S., Benyoucef, M., Ferré, B., Bouzeguella, S., and Ouchene, F.Z. 2022. Pliocene bivalves (Pteriomorphia) of Algiers Sahel (Algeria): Systematics and palaeoecology. Carnets de Geologie 22 (18): 795–824. Crossref
Tamura, M. 1959. Taxodonta and Isodonta from the Upper Jurassic Sakamoto Formation in central Kyushu, Japan. Transactions and proceedings of the Paleontological Society of Japan. New series 34: 53–65.
Távora, V.A. and Fernandes, J.M.G., 1999. Estudio de los foraminíferos de la Formación Pirabas (Mioceno Inferior), estado de Pará, Brasil y su correlación con faunas del Caribe. Revista Geológica de América Central 22: 63–74.
Távora, V.A., Santos, A.A.R.D., and Neto Araújo, R. 2010. Localidades fossilíferas da Formação Pirabas (Mioceno Inferior). Boletim do Museu Paraense Emílio Goeldi Ciências Naturais 5: 207–224. Crossref
Teppner, W., von 1922. Lamellibranchia Tertiaria, Anisomyaria II. In: C. Diener (ed.), Fossilium Catalogus, I. Animalia, Pars 15, 67–296. W. Junk, Berlin.
Vermeij, G.J. and Rosenberg, G. 1993. Giving and receiving: the tropical Atlantic as donor and recipient region for invading species. American Malacological Bulletin 10: 181–194.
Verrill, A.E. 1897. A study of the family Pectinidae, with a revision of the genera and subgenera. Transactions of the Connecticut Academy of Sciences 10: 41–96.
Vincent, E. 1928. Les Pecten des Sables de Wemmel. Annales de la Société royale Zoologique de Belgique 58: 89–95.
Wagner, H.P. 1985. Notes on type material of the family Pectinidae (Mollusca: Bivalvia). 3. On the identity of Pecten solidulus Reeve, 1853, and Pecten commutatus Monterosato, 1875. Basteria 49 (4–6): 81–84.
Wagner, H.P. 1991. Review of the European Pectinidae. Overzicht van de Europese Pectinidae (Mollusca: Bivalvia). Vita Marina 41: 1–48.
Waller, T.R. 1969. The evolution of the Argopecten gibbus stock (Mollusca: Bivalvia), with emphasis on the Tertiary and Quaternary species of eastern North America. Journal of Paleontology 43 (S3): 1–125. Crossref
Waller, T.R. 1972. The Pectinidae (Mollusca: Bivalvia) of Eniwetok Atoll, Marshall Islands. The Veliger 14: 221–264.
Waller, T.R. 1991. Evolutionary relationships among commercial scallops (Mollusca: Bivalvia: Pectinidae). In: S.E. Shumway (ed.), Scallops: Biology, Ecology and Aquaculture, 1–73. Elsevier, New York.
Waller, T.R. 1993. The evolution of “Chlamys” (Mollusca: Bivalvia: Pectinidae) in the tropical western Atlantic and eastern Pacific. American Malacological Bulletin 10 (2): 195–249.
Waller, T.R. 2006. New phylogenies of the Pectinidae (Mollusca: Bivalvia): reconciling morphological and molecular approaches. In: S.E. Shumway and G.J. Parsons (eds), Scallops: Biology, Ecology and Aquaculture. Ecology and Aquaculture 35: 1–44. Crossref
Waller, T.R. 2007. The evolutionary and biogeographic origins of the endemic Pectinidae (Mollusca: Bivalvia) of the Galápagos Islands. Journal of Paleontology 81: 929–950. Crossref
Waller, T.R. 2011. Neogene paleontology of the northern Dominican Republic, 24. Propeamussiidae and Pectinidae (Mollusca: Bivalvia: Pectinoidea) of the Cibao Valley. Bulletins of American Paleontology 381: 1–250.
Waller, T.R. 2018. Systematics and biostratigraphy of Chesapecten and Carolinapecten (Mollusca: Bivalvia: Pectinidae) in the upper Miocene and Pliocene “lower Tamiami Formation” of southwestern Florida. Bulletin of the Florida Museum of Natural History 56: 1–47. Crossref
Ward, L.W. and Blackwelder, B.W. 1975. Chesapecten, a new genus of Pectinidae (Mollusca: Bivalvia) from the Miocene and Pliocene of eastern North America. United States Geological Survey Professional Paper 861: 1–24. Crossref
Wilson, B., Jones, B., and Birjue, K. 2010, Palaeoenvironmental interpretations based on foraminiferal abundance biozones, Mayo Limestone, Trinidad, West Indies, including alpha and beta diversities: Palaios 25: 158–166. Crossref
Wilson, B. 2012. Planktonic Foraminifera in the Early to Middle Miocene “Lower Concord Calcareous Silt Member” at Mayo Quarry, Central Trinidad, and the invalidity of the Tamana Formation. Newsletters on Stratigraphy 45 (2): 105–114. Crossref
Woodring, W.P. 1982. Geology and paleontology of Canal Zone and adjoining parts of Panama. Description of Tertiary mollusks (pelecypods: Propeamussiidae to Cuspidariidae; additions to families covered in P306-E; additions to gastropods; cephalopods). United States Geological Survey Professional Paper 306: 541–759. Crossref
Woods, H. 1902. A Monograph of the Cretaceus Lamellibranchia of England. Vol. I. Part IV Pectinidae, 145–196. The Paleontographical Society, London. Crossref
Acta Palaeontol. Pol. 69 (2): 281–302, 2024
https://doi.org/10.4202/app.01129.2023