

First record of palaeopathologies in appendicular bones of the Triassic pseudosuchians Erpetosuchidae and Aetosauria based on microstructural approaches
DENIS A. PONCE, IGNACIO A. CERDA, and JULIA B. DESOJO
Ponce, D.A., Cerda, I.A., and Desojo, J.B. 2024. First record of palaeopathologies in appendicular bones of the Triassic pseudosuchians Erpetosuchidae and Aetosauria based on microstructural approaches. Acta Palaeontologica Polonica 69 (3): 395–402.
Pseudosuchians were the dominant group of archosaurs on continental ecosystems during the Triassic. However, studies that report palaeopathologies based on osteohistological evidence in this group are scarce. Here, two cases of palaeopathologies found in appendicular bones of two clades of pseudosuchians are presented: Aetosauria, a distal fragment of the fibula of Aetosauroides scagliai and Erpetosuchidae, the distal half of the tibia of Tarjadia ruthae from the Ischigualasto and Chañares formations, respectively (province of La Rioja, Argentina). The cortex in both specimens is composed of woven-fibred bone in the deepest part and by parallel-fibred bone in the subperiosteum. Towards the outermost portion of the cortex, a thin layer of periosteal bone with an irregular margin is recorded, mainly formed by a fibrolamellar bone vascularized with relatively wide and anastomosed radial canals. These features are compatible with a specific tissue recognized in pathological conditions, the radial fibrolamellar bone (RFB), generated by periosteal reactive bone. Additionally, a thin layer of parallel-fibred/lamellar bone crossed this structure in A. scagliai and surrounding the outermost portion in both specimens. The presence of RFB shows an abnormally accelerated bony overgrowth. However, due to the short thickness of this layer and the subsequent formation of parallel-fibred bone, it indicates a slowdown in its development and a possible recovery of the pathological condition. The configuration of the injury is compatible with periostitis and it constitutes the first record of this type of pathologies in non-crocodylomorph pseudosuchians. As the causes for this benign injury, it is inferred a non-traumatic stress followed by a pyogenic infection.
Key words: Aetosauria, Erpetosuchidae, bone histology, injury, Triassic, South America.
Denis A. Ponce [denispunrn@yahoo.com.ar; ORCID: https://orcid.org/0000-0002-3941-963X ] and Ignacio A. Cerda [ nachocerda6@yahoo.com; ORCID: https://orcid.org/0000-0001-6279-0392 ], Instituto de Investigación en Paleobiología y Geología (IIPG), Universidad Nacional de Río Negro-CONICET, Av. J.A. Roca 1242, 8332 General Roca, Río Negro, Argentina; Museo ‘Carlos Ameghino’, Belgrano 1700, Paraje Pichi Ruca (predio Marabunta), 8300 Cipolletti, Río Negro, Argentina.
Julia B. Desojo [julideso@fcnym.unlp.edu.ar; ORCID: https://orcid.org/0000-0002-2739-3276 ], CONICET; División Paleontología Vertebrados, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Paseo del Bosque s/n, B1900FWA La Plata, Buenos Aires, Argentina.
Received 13 February 2024, accepted 6 June 2024, published online 17 September 2024.
Copyright © 2024 D.A. Ponce et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The pseudosuchians reached a great variety of forms, sizes and habits during the Triassic, being the ruling archosaurs reptiles in continental environments during this period (Nesbitt et al. 2013; Pradelli et al. 2022). Among pseudosuchians, aetosaurs, and erpetosuchids were a common element in ecosystems from South America (Nesbitt et al. 2013; Pradelli et al. 2022). Aetosaurs were small to medium sized quadrupedal animals with an extensive dorsal and ventral armour of osteoderms and with an herbivorous to omnivorous feeding habit (Desojo et al. 2013; Reyes et al. 2020; Taborda et al. 2021). On the other hand, Erpetosuchidae were small to medium carnivorous quadrupedal pseudosuchians, they possessed a particularly ornamented robust skull and with several rows of dorsal and/or lateral, and appendicular osteoderms, depending on the species (Ezcurra et al. 2017; Nesbitt et al. 2017).
Studies that focused on the anatomy (e.g., Paes Neto et al. 2021; Desojo et al. 2024), systematics (e.g., Foffa et al. 2020; Reyes et al. 2024) and palaeobiology (e.g., Cerda et al. 2015; Parker et al. 2023) of aetosaurs and erpetosuchids have increased in recent years. Nevertheless, palaeopathologies topics are scarcely examined currently to date. Only Lucas (2000) inferred an osteoblastic reaction on paramedian osteoderms of Paratypothorax andressorum as study based on injuries. Disease and traumas in skeletal elements in the field of palaeontology are commonly reported (e.g., Rega et al. 2012; Hamm et al. 2020; Herbst et al. 2019; Hao et al. 2020; Baiano et al. 2024). The record of palaeopathologies can provide insights about diverse palaeobiological aspects of extinct taxa, such as, physiology, ecology and behaviour, among others (e.g., Tumarkin-Deratzian 2007; Rothschild et al. 2013; Benoit et al. 2021; Samathi et al. 2023). Our current knowledge about pathologies on Mesozoic archosaurs is mostly founded in studies performed on non-avian dinosaurs and in some crocodylomorphs. These studies employ several methods to examine the injuries, from the description of the external morphology (e.g., Bertozzo et al. 2021), to the observation of the microanatomy and histology in two or three dimensions (e.g., Barbosa et al. 2016). In recent years, analyses that examine the internal microstructure of bone injuries use CT scanners (e.g., Woodruff et al. 2022), synchrotron imaging (e.g., Anné et al. 2014), SEM (e.g., Rothschild and Depalma 2013) and thin sections (e.g., Redelstorff et al. 2014). Although the latter is a destructive technique, it remains as a reliable method to observe the bone histology and extract palaeobiological information (Chinsamy 2023).
In the present contribution, we provide evidence of osseous pathologies in two specimens of Aetosauria and Erpetosuchidae from South America based on histological and morphological features. A diagnosis and the possible causes of these pathologies are given. In addition, we also discuss the palaeobiological and phylogenetical implications of the presence of these lesions. This study represents the first report of osseous pathologies in appendicular bones for aetosaurs and erpetosuchids.
Institutional abbreviations.—CRILAR-Pv, Palaeovertebrates Collection of the Centro Regional de Investigaciones Científicas y Transferencia Tecnológica de La Rioja, Anillaco, La Rioja, Argentina.
Other abbreviations.—LAGs, lines of arrested growth; RFB, radial fibrolamellar bone.
Material and methods
The materials include a distal fragment of the fibula of the aetosaur Aetosauroides scagliai CRILAR-Pv 580 and approximately half of a distal fragment of the tibia of the erpetosuchid Tarjadia ruthae CRILAR-Pv 478. The specimens come from the Ischigualasto (Upper Triassic) and Chañares (Middle–Upper Triassic) formations respectively, in La Rioja province, at NW of Argentina. The materials were recovered by the Archosauriform Research Group in 2014 in Campo de Cordoba Norte locality in Chañares Formation and in 2017 in Hoyada del Cerro Las Lajas Locality in Ischigualasto Formation. The A. scagliai CRILAR-Pv 580 sample was previously identified by Desojo et al. (2020), while the T. ruthae CRILAR-Pv 478 specimen was described by Ezcurra et al. (2017). The phylogenetic relationships of the examined taxa were recently assessed by Ezcurra et al. (2023) and these are shown in Fig. 1. The specimens are housed at Centro Regional de Investigaciones Científicas y Transferencia Tecnológica de La Rioja (CRILAR) (Anillaco, La Rioja, Argentina). Thin sections were prepared in the “Laboratorio de Paleohistología” of the Museo Provincial Carlos Ameghino (MPCA) (Cipolletti, Río Negro, Argentina) according to standardized protocols (Chinsamy and Raath 1992; Cerda et al. 2020). In both cases, transversal sections were obtained from the diaphyseal region, when possible. The slides were analyzed with a petrographic microscope (Nikon E200) under plane and cross-polarized light. For the terminology of growth marks, we follow the nomenclature proposed by de Buffrénil and Quilhac (2021). It is important to indicate that the pathological nature of the samples here analyzed was revealed during a broad study of the growth dynamics of Triassic archosaurs from South America (Ponce 2024).
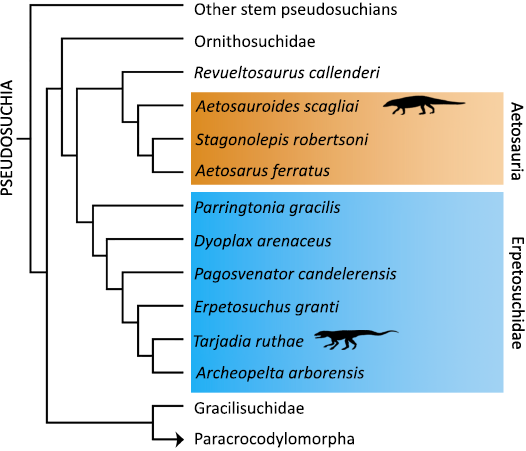
Fig. 1. Simplified cladogram (based on Ezcurra 2023) showing the phylogenetic positions of the studied taxa (black silhouettes).
Results
External morphology.—Externally, the features of both specimens are not diagnostic of the presence of pathologies, or their presence are ambiguous, at most. The distal fragment of the fibula of Aetosauroides scagliai CRILAR-Pv 580 (Fig. 2A) and the half of distal tibia of Tarjadia ruthae CRILAR-Pv 478 lack the characteristic bone callus (Fig. 2E). Plus, some portions of the shaft in both individuals are clearly missing, especially in the T. ruthae sample, likely indicative of an apparent erosive process (Fig. 2A, E). Additionally, their superficial appearance is slightly to moderately rugous, with some subrounded small pits present (Fig. 2A, E). This feature is more evident in the diaphyseal portion of the A. scagliai sample (Fig. 2A, E).
Histological descriptions.—Aetosauroides scagliai CRILAR -Pv 580: The transverse section was extracted from a distal portion of the diaphysis from the fibula (Fig. 2B). The sample has an elliptical shape in section (Fig. 2B). The medullary cavity is formed by fine cancellous bone composed of relatively wide intertrabecular spaces and long, thin trabeculae (Fig. 2B). A reduction in the size of the intertrabecular spaces and an increase in the thickness of the trabeculae is observed from the core to the outer area of the sample, which is formed by a thin layer of compact bone (Fig. 2B). This layer of compact tissue is formed by woven-fibred bone in its deepest portion and by parallel-fibred bone in its outermost portion (Fig. 2B). While the portion of parallel-fibred bone is mostly avascular (Fig. 2B), the layer of woven-fibred bone exhibits some longitudinal canals.
The outermost portion of the cortex, in the lateral and medial sides, exhibits an additional layer of bone tissue, recognized as a bony overgrowth, which shows an irregular appearance (Fig. 2C, D). This overgrowth is composed of two different types of bone tissue. The first type, the most extensively distributed, is formed by well vascularized woven-fibred bone, which contains wide and anastomosed radial vascular canals (Fig. 2C, D). This tissue includes large cavities with irregular edges (Fig. 2D). In addition, in some areas (mainly toward the subperiosteal cortex), this tissue becomes mostly avascular (Fig. 2D). The second tissue type is composed by avascular parallel-fibred bone. This is recognized as a thin layer located in the outermost portion of the overgrowth (i.e., it forms the external “edge” of the overgrowth) (Fig. 2C). The same tissue is also observed as a circumferential layer formed in the mid portion of the overgrowth at the medial side (Fig. 2C). Some lines of arrested growths (LAGs) are identified in these layers of parallel-fibred bone (Fig. 2C).
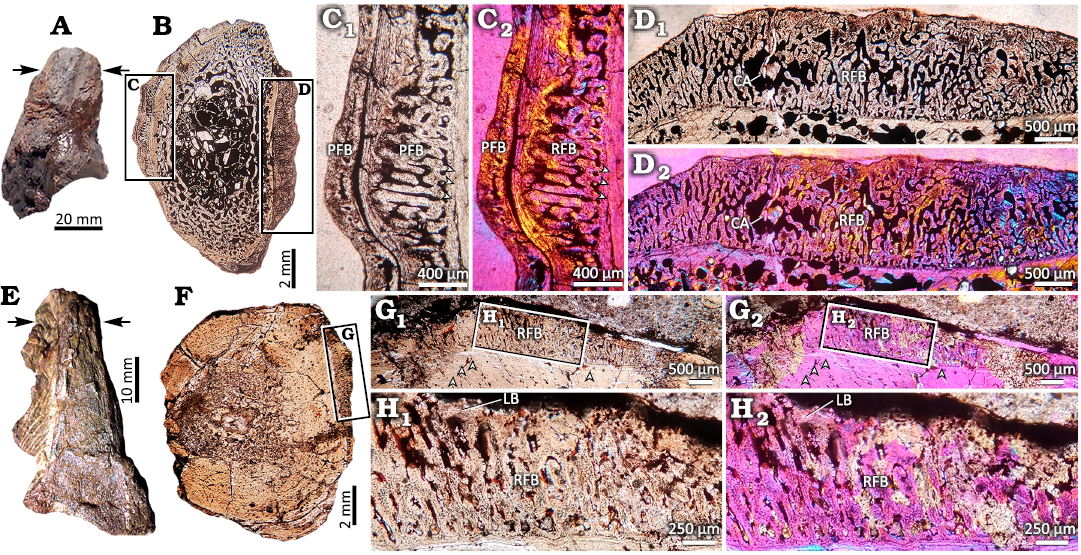
Fig. 2. Complete elements and thin sections of the aetosaur Aetosauroides scagliai CRILAR-Pv 580, Hoyada del Cerro Las Lajas, Ischigualasto Formation, Upper Triassic (A–F) and the erpetosuchid Tarjadia ruthae CRILAR-Pv 478, Campo Córdoba North, Chañares Formation, Middle–Upper Triassic (G–L) showing the pathologic bone tissues in detail. Inset boxes indicate the position of the detailed pictures in the figure. A. Distal fragment of the fibula of A. scagliai CRILAR-Pv 580. The arrows indicate the location of sectioning. B. Complete transverse section. Observe the presence of an irregular layer mainly on lateral and medial positions. C. View of the cortex in the medial portion of the shaft. Note the presence of radial fibrolamellar bone (RFB) intersperse with a thin layer of parallel-fibred bone (PFB). Also, another layer of PFB surrounds the most external portion of the cortex. Some LAGs are observed as well (arrowheads). D. Detail of the cortex in the lateral region, showing the presence of a continuous layer of RFB. The presence of an irregular large cavity indicates a possible cystic area (CA). E. Distal half of the tibia of T. ruthae CRILAR-Pv 478. The arrows indicate the location of sectioning. F. Complete transverse section. Observe the presence of an irregular layer on the lateral region of the section. G. View of the cortex in the lateral position. Note the abrupt change between the “normal” cortex and the layer of RFB. Some LAGs are marked as well (arrowheads). H. Detail of the RFB layer besides a thin layer of lamellar bone (LB) deposited in the outermost external cortex. A, B, C1, D1, E, F, H1: normal light; C2, D2, G2, H2: polarized light with lambda filter.
Tarjadia ruthae CRILAR-Pv 478: The transverse section was extracted from the mid-shaft portion of the diaphysis from approximately the half distal fragment of the tibia (Fig. 2E). Although the sample preserves its original shape in general, some portions of the cortex are missing (mainly in the medial margin), likely due to an erosive process (Fig. 2F). The medullary region exhibits a relatively small free medullary cavity encircled by a layer of fine cancellous bone, which gradually changes into a cortex of compact bone tissue (Fig. 2F). The compacta is mostly composed by woven-fibred bone, except in the subperiosteum, which is built by lamellar bone and it is crossed by up to 16 cyclical growth marks (LAGs/annuli) (Fig. 2G). The vascularization in the compact bone of the cortex is poor, formed by some longitudinal canals, and decreases toward the periosteum (Fig. 2G). Up to eight strongly grouped lines of arrested growth (LAGs) are recorded in the subperiosteal cortex. The last four of these LAGs are shown in the Fig. 2G.
The subperiosteal portion of the cortex at the medial side of the shaft exhibits an abnormal bony overgrowth (Fig. 2G, H). The features of this are, in general, the same as those observed in the fibula of Aetosauroides scagliai CRILAR-Pv 580. In this regard, the overgrowth is formed by two types of differentiated bone tissue (Fig. 2G, H). Thus, this is mostly composed by highly vascularized woven-fibred bone, which contains wide and anastomosed radial vascular canals (Fig. 2G, H). This tissue becomes avascular toward the subperiostal margin (Fig. 2H). The second tissue consists of a thin layer of lamellar bone located in the outermost portion of the overgrowth (Fig. 2G, H). Unfortunately, because of the fragmentary nature of the bone, it is not possible to assess the continuity of this irregular overgrowth through the whole cortex.
Discussion
Diagnosis of the injuries.—The irregular shape of the abnormal structure recorded on the appendicular section of both specimens, along the presence of woven-fibred bone matrix and anastomosed radial canals (that extends perpendicularly to the circumference of the section) are compatible with the occurrence of radial fibrolamellar bone (RFB) (Chinsamy and Tumarkin-Deratzian 2009; Shelton et al. 2019; Jentgen-Ceschino et al. 2020). Nevertheless, the presence of RFB by itself is not indicative of a disease. Erickson and Tumanova (2000) recognized a “highly porous radially vascularized bone” for the first time in three long bones of the ceratopsian dinosaur Psittacosaurus. These authors did not rule out a pathological origin of this tissue, but they did not find evidence for this. Later, Klein and Sander (2008) introduced the term RFB since the identification of an unusual bone tissue that consists of a fibrolamellar complex with a dense radial vascular pattern located in the exterior portion of the cortex in some specimens of the basal sauropodomorph dinosaur Plateosaurus. In this case, the RFB was not treated as pathological because both the bone surface as well as the general morphology of the affected bones were not altered. Hurum et al. (2006) and Chinsamy-Turan (2012) reported the occurrence of RFB in long bones of an unidentified Triassic dinosaur and a non-mammalian Permian therapsid respectively. Nevertheless, they did not provide an interpretation about the presence of this structure. Next, Hedrick et al. (2016) described RFB associated to a fracture callus in a Psittacosaurus fibula. Shelton and Sander (2017) proposed that the RFB recorded from early to advanced stages in the cortex of several individuals of the synapsid Ophiacodon is a particular characteristic of the growth strategies for this genus. Finally, Heckert et al. (2021) recognized a severely affected skeleton by osseous lesions in the phytosaur Smilosuchus gregorii. They considered that some of the exostoses recorded are compatible with RFB. Heckert et al. (2021) tentatively interpreted this pathological alteration as either osteomyelitis or hypertrophic osteopathy.
For this study case, it is clear that the RFB emerged at a relatively advanced ontogenetic stage for both individuals. Assuming that LAGs correspond to annual cycles, one can infer a minimal age of death of 16 and 8 years old for the sampled individuals of Aetosauroides scagliai and Tarjadia ruthae, respectively. The formation of the RFB late during the ontogeny is evidenced by the fact that these layers are restricted to the outermost portion of the cortex, mainly over the lateral and medial sides of the sections, showing an abrupt transition between the ‘normal’ bone and the RFB. In this sense, the nature of this abnormality reveals an extremely rapid periosteal bone formation in advanced stages of the ontogeny of the specimens. In medical literature, this kind of bone development is known as periosteal reactive bone (Rothschild and Martin 2006; Rothschild 2009; Weston 2011; Dutour 2022). In consequence, the identification of periosteal reactive bone allows to recognize the disease as periostitis in both specimens, which is the inflammation of the periosteum and the subjacent tissue (Rothschild and Martin 2006; Rothschild 2009; Weston 2011; Dutour 2022).
Aetiology.—Periostitis by itself suggests an inflammation of the periosteum, but this does not describe the origin of the condition. The causes for the development of this disease include traumas, collateral effects of an overlying pathology that affects the normal development of the metabolism or the physiology of the organism (like neoplasia, for example) or infections (Rothschild and Martin 1993, 2006; Rothschild 2009; Weston 2011; Cubo et al. 2015; Dutour 2022).
A traumatic cause (i.e., bone fracture) generates a bone callus after its healing, which is absent on the studied samples in a morphological description (Rothschild and Martin 1993; Lovell 1997). Alongside the bone callus, in transverse thin section, also some fracture lines would be visible in the case of fractures (Bishop et al. 2015; Chinzorig et al. 2022). Histologically, the affected area around the fracture generates: (i) cartilage mineralization, (ii) formation of woven-fibred bone (periosteal reaction is possible as well), and (iii) remodelling in the last stages of healing (formation of hard callus), whose are able to preserve in the fossil record (Gerstenfeld et al. 2007; Bucher et al. 2016). None of these features are present in the specimens here studied, thus a typical traumatic cause as the origin of the periostitis is unlikely.
The bone damaged by periostitis also can be affected by other overlying diseases. These involve genetical, metabolic and physiological anomalies (McWhinney et al. 2001; Cubo et al. 2015). Joint-related diseases, like arthrosis, are common causes for the development of periosteal reactive bone. In this case, the bone shows bone spurs (i.e., osteophytes) at diarthrodial joint margins (Moskowitz et al. 1984; Resnick 2002; Rothschild and Martin 2006), also visible in thin section. At morphological and microstructural view, the current samples do not show osteophytes and the injures are produced in diaphyseal portions, far away from metaphysis, where arthrosis lesions would be expected. Thus, a joint-related disease is excluded here. Likewise, neoplasia is another possible cause that generates periostitis. Although it is difficult to assess the presence of bone cancer on extinct taxa, some studies have reached this diagnostic, with a high grade of confidence, based on specific features (Barbosa et al. 2016; Haridy et al. 2019; Ekhtiari et al. 2020; Surmik et al. 2022). In this sense, the occurrence of a relatively large irregular mass with rugose appearance on the surface of bone on external view has been recognized as a possible indicative of bone cancer (Barbosa et al. 2016; Haridy et al. 2019; Ekhtiari et al. 2020; Surmik et al. 2022). At the histological level, bone cancer is usually related with a profuse formation of spicules of the periosteal reactive bone, lytic lesions and destruction of the bone cortex and the medulla, the presence of triangle of Codman and no transition zone between the “normal bone” and the pathological bone (Haridy et al. 2019; Ekhtiari et al. 2020; Surmik et al. 2022). The lesions in the studied samples are of relatively small size compared with other reported in the literature referring bone cancer inferences and possess a more or less smooth texture. Also, they do not report the microstructural features recorded for cancer development. A neoplastic origin for the periostitis should be dismissed, then.
An infectious process can produce periostitis as well (Rothschild and Martin 1993, 2006; Rothschild 2009; Weston 2011; Dutour 2022). During an infection, like osteomyelitis, the periosteal reactive bone can acquire different shapes (Rothschild and Martin 2006). However, the appearance of slightly spiculated matrix mixed with avascular compact bone of the periosteal reactive recorded in the specimens does not fit with the categories of periosteal reactive bones proposed by Rothschild and Martin (2006). Usually, the presence of involucrum and sinus drainage for the pus, which are evident at mesoscopic and microscopic scale, are a common feature of infections (Rothschild and Martin 2006; Waldron 2009; Rothschild 2010; Weston 2011; García et al. 2017; Gonzalez et al. 2017; Khurana 2019). Nevertheless, their absence does not rule out an infection. Likewise, cystic areas (cloacae in this case) are recognizable as signalling of infections (Fig. 2D1). Additionally, in cases of severe infections, like chronic osteomyelitis, a destruction of the bone tissue is produced, including the medullary cavity and the compact bone cortex due to sclerosis and bone necrosis (Shelton et al. 2019; Chinzorig et al. 2022). The specimens here studied possess a relatively small development of reactive periosteal bone formed on the cortex and show the presence of cystic areas (specially developed in Aetosauroides scagliai sample, Fig. 2D1). In this sense, a typical infectious disease is highly plausible.
As seen, the injuries registered on the fibula of Aetosauroides scagliai CRILAR-Pv 580 and in the tibia of Tarjadia ruthae CRILAR-Pv 478 do not fit accurately with the typical causes proposed for the periostitis. In this sense, based on the following features, we infer a non-traumatic and a pyogenic infection as the causes of the lesions (Rothschild 2010; Kato et al. 2020): (i) absence of any kind of irregular formations on the surface of the bone in general external view, (ii) location on the lateral and medial sides of the bones, (iii) development of radial fibrolamellar bone which indicates a periosteal reactive bone condition, (iv) appearance of weakly spiculated shape of the periosteal reactive bone along avascular compact bone, (v) abrupt transition between the ‘normal’ bone and the periosteal reactive bone, (vi) absence of fracture lines, involucrum and evidences of destruction of the cortex or medullary cavity, (vii) presence of cystic areas (likely cloacae). In this sense, we propose that the bones of both specimens (on lateral and medial margins) were subjected to a prolonged in time stress or to a striking shock, without reaching a fracture, and, in consequence, generated the condition of the periosteal reactive bone (Fig. 3). Afterwards, it is likely that this produced a short event of infection, evidenced by the presence of cystic areas (Fig. 3).
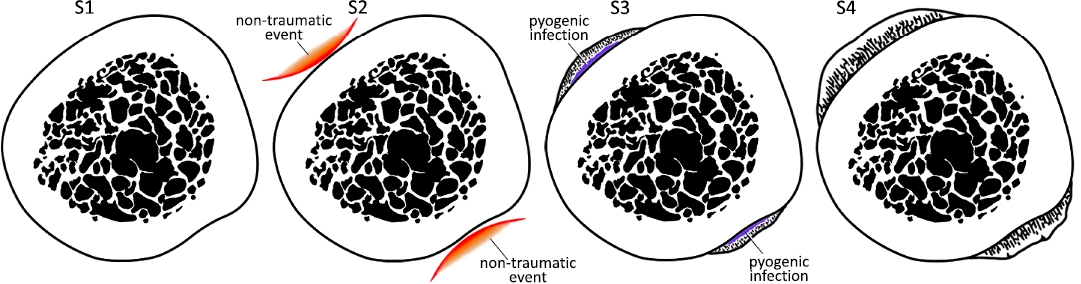
Fig. 3. The different inferred stages of progression of the injuries reported in the fibula of Aetosauroides scagliai CRILAR-Pv 580, Hoyada del Cerro Las Lajas, Ischigualasto Formation, Upper Triassic and in the tibia of Tarjadia ruthae CRILAR-Pv 478, Campo Córdoba North, Chañares Formation, Middle–Upper Triassic, at transverse histological section view. At first stage (S1), the bone is normal. A non-traumatic event affects the bone (mainly in lateral and medial margins) in stage two (S2), likely due to a prolonged in time stress or a striking shock, but not generating a fracture. At stage three (S3), the cortex reacts to the non-traumatic event developing the periosteal reactive bone and a short event of pyogenic infection is produced. Finally, at the final stage (S4), the bone has completely recovered. Not to scale.
Palaeobiological and phylogenetical implications.—The inferred disease constitutes a rather benign injury in relatively advanced stages of the ontogeny of both individuals, showing a rather rapid recovery of the lesions. This is marked by the small size of the pathologies and the presence of a thin layer of parallel-fibred/lamellar bone surrounding the layers of periosteal reactive bone.
According to the database of Kato et al. (2020), the periostitis condition is broadly distributed among extant and extinct taxa of tetrapods, including lepidosaurs (Rothschild 2010), crocodylomorph pseudosuchians (Rothschild 2010), avian and non-avian dinosaurs (Moodie 1923; Foth et al. 2015), non-mammalian synapsids and mammals (Weston 2011; Kato et al. 2020; Dutour 2022) and is a common lesion recorded in humans as well (e.g., van der Merwee et al. 2010). More specifically, the features observed in our samples are similar to that reported in varanids (Rothschild 2010) and gorgonopsids (Kato et al. 2020), although no haematoma is reported in the current study. The presence of periostitis in Aetosauria and Erpetosuchidae expands the phylogenetic distribution of this pathology, mainly in non-Crocodylomorpha Pseudosuchia. Besides, it fills the gap of the occurrence of this disease at the beginning of the Mesozoic, during the Middle–Late Triassic. Nevertheless, although Aetosauria is a relatively common taxon with a broad record registered in Upper Triassic deposits since late 19th century (Desojo et al. 2013), this kind of slight and non-deadly injuries had hitherto not been documented.
Conclusions
The microstructural characteristics of an abnormality on the bone tissue on the external margin of thin sections of a distal fragment of the fibula of the aetosaur Aetosauroides scagliai CRILAR-Pv 580 and half of a distal fragment of the tibia of the erpetosuchid Tarjadia ruthae CRILAR-Pv 478 are reported here. This irregular layer is barely visible in external morphology, and this is composed by radial fibrolamellar bone (RFB), suggesting a periosteal reactive bone. The diagnosis of the pathology is recognized as periostitis. As the causes of the development of this, a non-traumatic origin is inferred, followed by a short event of pyogenic infection, revealing a relatively rapid recovery based on the short extension of the RFB layer. The features of this kind of disease recorded on both specimens resemble those described in varanids and gorgonopsids. The periostitis here recognized is reported for the first time in Middle–Late Triassic taxa and for the first time in non-crocodylomorph pseudosuchians, showing a common occurrence of these pathologies among Tetrapoda. It must be noted that the diagnosis and the aetiology inferred are based only in histological and morphological observations only. In future studies, it is recommended to use several methods to explore the injuries on bones considering different scales and dimensions (CT scans, SEM, synchrotron, among others), to achieve more accurate inferences.
Acknowledgements
We are thankful to Lucas Fiorelli (CRILAR) for allowing us the access to the materials and preparing them into thin sections, and Torsten Scheyer (Universität Zürich, Zürich, Switzerland) for his comments on an early version of the manuscript. Also, we are thankful to the reviewers, Florian Witzmann (Museum für Naturkunde, Berlin, Germany) and Bruce Rothschild (Carnegie Museum of Natural History, Pittsburgh, USA) for their suggestions and corrections. Funding: PICT 2021-2704 (ANPCyT) to JBD, Subsidio APA-Bunge & Born 2022 and Sepkosky grant 2022 to DAP.
References
Anné, J., Edwards, N.P., Wogelius, R.A., Tumarkin-Deratzian, A.R., Sellers, W.I., Veelen, A. van, Bergmann, U., Sokaras, D., Alonso-Mori, R., Ignatyev, K., Egerton, V.M., and Manning, P.L. 2014. Synchrotron imaging reveals bone healing and remodelling strategies in extinct and extant vertebrates. Journal of the Royal Society Interface 11 (96): 20140277. Crossref
Baiano, M.A., Cerda, I.A., Bertozzo, F., and Pol, D. 2024. New information on paleopathologies in non-avian theropod dinosaurs: a case study on South American abelisaurids. BMC Ecology and Evolution 24 (1): article 6. Crossref
Barbosa, F.H.Z. de, Paulo, Costa Pereira, V.L.G. da, Bergqvist, L.P., and Rothschild, B.M. 2016. Multiple neoplasms in a single sauropod dinosaur from the Upper Cretaceous of Brazil. Cretaceous Research 62: 13–17. Crossref
Benoit, J., Browning, C., and Norton, L.A. 2021. The first healed bite mark and embedded tooth in the snout of a Middle Permian gorgonopsian (Synapsida: Therapsida). Frontiers in Ecology and Evolution 9: 699298. Crossref
Bertozzo, F., Manucci, F., Dempsey, M., Tanke, D.H., Evans, D.C., Ruffell, A., and Murphy, E. 2021. Description and etiology of paleopathological lesions in the type specimen of Parasaurolophus walkeri (Dinosauria: Hadrosauridae), with proposed reconstructions of the nuchal ligament. Journal of Anatomy 238: 1055–1069. Crossref
Bishop, P.J., Walmsley, C.W., Phillips, M.J., Quayle, M.R., Boisvert, C.A., and McHenry, C.R. 2015. Oldest pathology in a tetrapod bone illuminates the origin of terrestrial vertebrates. PLoS ONE 10(5): e0125723. Crossref
Bucher, C.H., Lei, H., Duda, G., Volk, H.-D., and Schmidt-Bleek, K. 2016. The role of immune reactivity in bone regeneration. In: A.M. Zorzi and J.B. de Miranda (eds.), Advanced Techniques in Bone Regeneration, 169–194. IntechOpen, Rijeka. Crossref
Buffrénil, V. de, Quilhac, A., and Castanet, J. 2021. Cyclical growth and skeletochronology. In: V. de Buffrénil, A.J. Ricqlès, L. Zylberberg, and K. Padian (eds.), Vertebrate Skeletal Histology and Paleohistology, 626–644. CRC Press, Boca Raton. Crossref
Cerda, I.A., Desojo, J.B., and Scheyer, T.M. 2015. Osteoderm histology of Proterochampsia and Doswelliidae (Reptilia: Archosauriformes) and their evolutionary and paleobiological implications. Journal of Morphology 276: 385–402. Crossref
Cerda I.A., Pereyra M.E., Garrone, M.C., Ponce D.A., Navarro, T.G., González R., Militello M., Luna, C., and Jannello, M.J. 2020. A basic guide for sampling and preparation of extant and fossil bones for histological studies. Publicación Electrónica de la Asociación Paleontológica Argentina 20: 15–28. Crossref
Chinsamy-Turan, A. 2012. The microstructure of bones and teeth of nonmammalian therapsids. In: A. Chinsamy-Turan (ed.), Forerunners of Mammals—Radiation, Histology, Biology, 65–88. Indiana University Press, Bloomington.
Chinsamy, A. 2023. Palaeoecological deductions from osteohistology. Biology Letters 19 (8): 20230245. Crossref
Chinsamy, A. and Raath, M.A. 1992. Preparation of fossil bone for histological examination. Palaeontologia Africana 29: 39–44.
Chinsamy, A. and Tumarkin-Deratzian, A. 2009. Pathologic bone tissues in a Turkey vulture and a nonavian dinosaur: implications for interpreting endosteal bone and radial fibrolamellar bone in fossil dinosaurs. The Anatomical Record 292: 1478–1484. Crossref
Chinzorig, T., Beguesse, K.A., Canoville, A., Phillips, G., and Zanno, L.E. 2022. Chronic fracture and osteomyelitis in a large-bodied ornithomimosaur with implications for the identification of unusual endosteal bone in the fossil record. The Anatomical Record 306: 1864–1879.
Cubo. J., Woodward, H., Wolff, E., and Horner, J.R. 2015. First reported cases of biomechanically adaptive bone modeling in non-avian dinosaurs. PLoS ONE 10 (7): e0131131. Crossref
Desojo, J.B., Baczko, M.B. von, Ezcurra, M.D., Fiorelli, L.E., Martinelli, A.G., Bona, P., Trotteyn, M.J., and Lacerda, M. 2024. Cranial osteology and paleoneurology of Tarjadia ruthae: An erpetosuchid pseudosuchian from the Triassic Chañares Formation (late Ladinian–?early Carnian) of Argentina. The Anatomical Record 307: 890–924. Crossref
Desojo, J.B., Fiorelli, L.E., Ezcurra, M.D., Martinelli, A.G., Ramezani, J., Da-Rosa, Á.A.S., Baczko, M.B. von, Trotteyn, M.J., Montefeltro, F.C., Ezpeleta, E., and Langer, M.C. 2020. The Late Triassic Ischigualasto Formation at Cerro Las Lajas (La Rioja, Argentina): fossil tetrapods, high-resolution chronostratigraphy, and faunal correlations. Nature Scientific Reports 10: 12782. Crossref
Desojo, J.B., Heckert, A.B., Martz, J.W., Parker, W.G., Schoch, R.R., Small, B.J., and Sulej, T. 2013. Aetosauria: a clade of armoured pseudosuchians from the Upper Triassic continental beds. In: S.J. Nesbitt, J.B. Desojo, and R.B. Irmis (eds.), Anatomy, Phylogeny and Palaeobiology of Early Archosaurs and their Kin. Geological Society of London Special Publication 379: 203–239. Crossref
Dutour, O. 2022. Paleopathology of infectious diseases. In: L.A. Grauer (ed.), The Routledge Handbook of Paleopathology, 324–337. Willey Blackwell, Chichester. Crossref
Ekhtiari, S., Chiba, K., Popovic, S., Crowther, R., Wohl, G., Kin On Wong, A., Tanke, D.H., Dufault, D.M., Geen, O.D., Parasu, N., Crowther, M.A., and Evans, D.C. 2020. First case of osteosarcoma in a dinosaur: a multimodal diagnosis. Lancet Oncology 21: 1021–1022. Crossref
Erickson, G.M. and Tumanova, T.A. 2000. Growth curve of Psittacosaurus mongoliensis Osborn (Ceratopsia: Psittacosauridae) inferred from long bone histology. Zoological Journal of the Linnean Society 130: 551–566. Crossref
Ezcurra, M.D., Bandyopadhyay, S., Sengupta, D.P., Sen, K., Sennikov, A.G., Sookias, R.B., Nesbitt, S.J., and Butler, R.J. 2023. A new archosauriform species from the Panchet Formation of India and the diversification of Proterosuchidae after the end-Permian mass extinction. Royal Society Open Science 10: 230387. Crossref
Ezcurra, M.D., Fiorelli, L.E., Martinelli, A.G., Rocher, S., Baczko, M.B. von, Ezpeleta, M., Taborda, J.R.A., Hechenleitner, E.M., Trotteyn, M.J., and Desojo, J.B. 2017. Deep faunistic turnovers preceded the rise of dinosaurs in southwestern Pangaea. Nature Ecology & Evolution 1: 1477–1483. Crossref
Foffa, D., Butler, R.J., Nesbitt, S.J., Walsh, S., Barrett, P.M., Brusatte, S.L., and Fraser, N.C. 2020. Revision of Erpetosuchus (Archosauria: Pseudosuchia) and new erpetosuchid material from the Late Triassic “Elgin Reptile” fauna based on μCT scanning techniques. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 111: 209–233. Crossref
Foth, C., Evers, S.W., Pabst, B., Mateus, O., Flisch, A., Patthey, M., and Rauhut, O.W. 2015. New insights into the lifestyle of Allosaurus (Dinosauria: Theropoda) based on another specimen with multiple pathologies. PeerJ 3: e940. Crossref
García, R.A., Cerda, I.A., Heller, M., Rothschild, B.M., and Zurriaguz, V. 2017. The first evidence of osteomyelitis in a sauropod dinosaur. Lethaia 50: 227–236. Crossref
Gerstenfeld, L.C., Edgar, C.M., Kakar, S., Jacobsen, K.A., and Einhorn, T.A. 2007. Osteogenic growth factors and cytokines and their role in bone repair. In: F. Bronner, M.C. Farach-Carson, and A.G. Mikos (eds.), Engineering of Functional Skeletal Tissues, Topics in Bone Biology, Vol. 3, 17–45. Springer, London. Crossref
Gonzalez, R., Gallina, P.A., and Cerda, I.A. 2017. Multiple paleopathologies in the dinosaur Bonitasaura salgadoi (Sauropoda: Titanosauria) from the Upper Cretaceous of Patagonia, Argentina. Cretaceous Research 79: 159–170. Crossref
Hao, B.-Q., Feng, H., Zhao, Z.-Q., Ye, Y., Wan, D.-X., and Peng, G.-Z. 2020. Different types of bone fractures in dinosaur fossils. Historical Biology 33: 1636–1641. Crossref
Hamm, C.A., Hampe, O., Schwarz, D., Witzmann, F., Makovicky, P.J., Brochu, C.A., Reiter, R., and Asbach, P. 2020. A comprehensive diagnostic approach combining phylogenetic disease bracketing and CT imaging reveals osteomyelitis in a Tyrannosaurus rex. Nature Scientific Reports 10: 18897. Crossref
Haridy, Y., Witzmann, F., Asbach, P., Schoch, R.R., Fröbisch, N., and Rothschild, B.M. 2019. Triassic cancer-osteosarcoma in a 240-million-year-old stem-turtle. Journal of the American Medical Association Oncology 5: 425–426. Crossref
Heckert, A.B., Viner, T.C., and Carrano, M.T. 2021. A large, pathological skeleton of Smilosuchus gregorii (Archosauriformes: Phytosauria) from the Upper Triassic of Arizona, USA, with discussion of the paleobiological implications of paleopathology in fossil archosauromorphs. Palaeontologia Electronica 24 (2): a21. Crossref
Hedrick, B.P., Gao, C., Tumarkin‐Deratzian, A.R., Shen, C., Holloway, J.L., Zhang, F., Hankenson, K.D., Liu, S., Anné, J., and Dodson, P. 2016. An injured Psittacosaurus (Dinosauria: Ceratopsia) from the Yixian formation (Liaoning, China): implications for Psittacosaurus biology. The Anatomical Record 299: 897–906. Crossref
Herbst, E.C., Doube, M., Smithson, T.R., Clack, J.A., and Hutchinson, J.R. 2019. Bony lesions in early tetrapods and the evolution of mineralized tissue repair. Paleobiology 45: 676–697. Crossref
Hurum, J.H., Bergan, M., Muller, R., Nystuen, J.P., and Klein, N. 2006. A Late Triassic dinosaur bone, offshore Norway. Norwegian Journal of Geology 86: 117–123.
Jentgen-Ceschino, B., Stein, K., and Fischer, V. 2020. Case study of radial fibrolamellar bone tissues in the outer cortex of basal sauropods. Philosophical Transactions of the Royal Society B 375: 20190143. Crossref
Kato, K.M., Rega, E.A., Sidor, C.A., and Huttenlocker, A.K. 2020. Investigation of a bone lesion in a gorgonopsian (Synapsida) from the Permian of Zambia and periosteal reactions in fossil non-mammalian tetrapods. Philosophical Transactions of the Royal Society B 375: 20190144. Crossref
Klein, N. and Sander, M. 2008. Ontogenetic stages in the long bone histology of sauropod dinosaurs. Paleobiology 34: 247–263. Crossref
Khurana, J.S. 2019. The surgical pathology of bone infections. In: J.S. Khurana (ed.), Bone Pathology, 179–186. Humana Press, Dordrecht. Crossref
Lovell, N.C. 1997. Trauma analysis in paleopathology. American Journal of Physical Anthropology 104 (25): 139–170. Crossref
Lucas, S.G. 2000. Pathological aetosaur armor from the Upper Triassic of Germany. Stuttgarter Beiträge zur Naturkunde B 281: 1–6.
McWhinney, L., Carpenter, K., and Rothschild, B.M. 2001. Dinosaurian humeral periostitis: a case of a juxtacortical lesion in the fossil record. In: D.H. Tanke, K. Carpenter, and M.W. Skrepnick (eds.), Mesozoic Vertebrate Life, 364–377. Indiana University Press, Bloomington.
Moodie, R.L. 1923. Paleopathology: An Introduction to the Study of Ancient Evidences of Disease. 567 pp. University of Illinois Press, Urbana.
Moskowitz, R.W., Howell, D.S., Goldberg, V.M., and Mankin, H.J. 1984. Osteoarthritis: Diagnosis and Management. 585 pp. W.B. Saunders Co., Philadelphia.
Nesbitt, S.J., Brusatte, S.L., Desojo, J.B., Liparini, A., França, M.A. de, Weinbaum, J.C., and Gower, D.J. 2013. Anatomy, phylogeny and palaeobiology of early archosaurs and their kin. In: S.J. Nesbitt, J.B. Desojo, and R.B. Irmis (eds.), Anatomy, Phylogeny and Palaeobiology of Early Archosaurs and their Kin. Geological Society of London Special Publication 379: 1–7. Crossref
Nesbitt, S.J., Stocker, M.R., Parker, W.G., Wood, T.A., Sidor, C.A., and Angielczyk, K.D. 2017. The braincase and endocast of Parringtonia gracilis, a Middle Triassic suchian (Archosauria: Pseudosuchia). In: C.A. Sidor and S.J. Nesbitt (eds.), Vertebrate and Climatic Evolution in the Triassic Rift Basins of Tanzania and Zambia. Journal of Vertebrate Paleontology 37 (Supplement 6): 122–141. Crossref
Paes-Neto, V.D., Desojo, J.B., Brust, A.C.B., Schultz, C.L., Da-Rosa, Á.A.S., and Soares, M.B. 2021. Intraspecific variation in the axial skeleton of Aetosauroides scagliai (Archosauria: Aetosauria) and its implications for the aetosaur diversity of the Late Triassic of Brazil. Anais da Academia Brasileira de Ciências 93 (Supplement 2): e20201239. Crossref
Parker, W.G., Reyes, W.A., and Marsh, A.D. 2023. Incongruent ontogenetic maturity indicators in a Late Triassic archosaur (Aetosauria: Typothorax coccinarum). The Anatomical Record 307: 1254–1270. Crossref
Ponce, D.A. 2024. Osteohistología de Archosauriformes triásicos sudamericanos (Reptilia: Eucrocopoda): implicancias paleobiológicas. 180 pp. Ph.D. Thesis. Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata.
Pradelli, L.A., Leardi, J.M., and Ezcurra, M.D. 2022. Body size disparity of the archosauromorph reptiles during the first 90 million years of their evolution. Ameghiniana 59: 47–77. Crossref
Redelstorff, R., Hayashi, S., Rothschild, B.M., and Chinsamy, A. 2014. Non-traumatic bone infection in stegosaurs from Como Bluff, Wyoming. Lethaia 48: 47–55. Crossref
Rega, E.A., Noriega, K., Sumida, S.S., Huttenlocker, A., Lee, A., and Kennedy, B. 2012. Healed fractures in the neural spines of an associated skeleton of Dimetrodon: implications for dorsal sail morphology and function. Fieldiana Life and Earth Sciences 2012 (5): 104–111. Crossref
Resnick, D. 2002. Disorders of Bones and Joints. 412 pp. Saunders, Philadelphia.
Reyes, W A., Martz, J.W., and Small, B.J. 2024. Garzapelta muelleri gen. et sp. nov., a new aetosaur (Archosauria: Pseudosuchia) from the Late Triassic (middle Norian) middle Cooper Canyon Formation, Dockum Group, Texas, USA, and its implications on our understanding of the morphological disparity of the aetosaurian dorsal carapace. The Anatomical Record: e25379. Crossref
Reyes, W.A., Parker, W.G., and Marsh, A.D. 2020. Cranial anatomy and dentition of the aetosaur Typothorax coccinarum (Archosauria: Pseudosuchia) from the Upper Triassic (Revueltian–mid Norian) Chinle formation of Arizona. Journal of Vertebrate Paleontology 40: e1876080. Crossref
Rothschild, B.M. 2009. Scientifically rigorous reptile and amphibian osseous pathology: lessons for forensic herpetology from comparative and paleopathology. Applied Herpetology 6: 47–79. Crossref
Rothschild, B.M. 2010. Macroscopic recognition of non-traumatic osseous pathology in the post-cranial skeletons of crocodilians and lizards. Journal of Herpetology 44: 13–20. Crossref
Rothschild, B.M. and Depalma, R. 2013. Skin pathology in the Cretaceous: Evidence for probable failed predation in a dinosaur. Cretaceous Research 42: 44–47. Crossref
Rothschild, B.M. and Martin, L.D. 1993. Paleopathology: Disease in the Fossil Record. 386 pp. CRC Press, Boca Raton.
Rothschild, B.M. and Martin, L.D. 2006. Skeletal impact of disease. New Mexico Museum of Natural History and Science Bulletin 33: 1–226.
Rothschild, B.M., Schultze, H.-P., and Pellegrini, R. 2013. Osseous and other hard tissue pathologies in turtles and abnormalities of mineral deposition. In: D. Brinkman, P. Holroyd, and J. Gardner (eds.), Morphology and Evolution of Turtles, Vertebrate Paleobiology and Paleoanthropology, 501–534. Springer, Dordrecht. Crossref
Samathi, A., Weluwanarak, J., Duanyai, P., Kaikaew, S., and Suteethorn, S. 2023. An unusual metatarsal of theropod dinosaur from the lower cretaceous of Thailand: the first detailed study of paleopathology in Megaraptora. Historical Biology 36: 467–472. Crossref
Shelton, C.D. and Sander, P.M. 2017. Long bone histology of Ophiacodon reveals the geologically earliest occurrence of fibrolamellar bone in the mammalian stem lineage. Comptes Rendus Palevol 16: 397–424. Crossref
Shelton, C.D., Chinsamy, A., and Rothschild, B.M. 2019. Osteomyelitis in a 265-million-year-old titanosuchid (Dinocephalia, Therapsida). Historical Biology 31: 1093–1096. Crossref
Surmik, D., Słowiak-Morkovina, J., Szczygielski, T., Kamaszewski, M., Kalita, S., Teschner, E.M., Dróżdż, D., Duda, P., Rothschild, B.M., and Konietzko-Meier, D. 2022. An insight into cancer palaeobiology: does the Mesozoic neoplasm support tissue organization field theory of tumorigenesis? BMC Ecology and Evolution 22 (1): art. 143. Crossref
Taborda, J.R.A., Desojo, J.B., and Dvorkin, E.N. 2021. Biomechanical skull study of the aetosaur Neoaetosauroides engaeus using finite element analysis. Ameghiniana 58: 401–415. Crossref
Tumarkin-Deratzian, A.R. 2007. Fibrolamellar bone in wild adult Alligator mississippiensis. Journal of Herpetology 41: 341–345. Crossref
van der Merwe, A.E., Maat, G.J.R., and Steyn, M. 2010. Ossified haematomas and infectious bone changes on the anterior tibia: histomorphological features as an aid for accurate diagnosis. International Journal of Osteoarchaeology 20: 227–239. Crossref
Waldron, T. 2009. Paleopathology. 279 pp. Cambridge University Press, Cambridge.
Weston, D.A. 2011. Nonspecific infection in paleopathology: Interpreting periosteal reactions. In: L.A. Grauer (ed.), A Companion to Paleopathology, 492–512. Willey Blackwell, Chichester. Crossref
Woodruff, D.C., Wolff, E.D.S., Wedel, M.J., Dennison, S., and Witmer, L.M. 2022. The first occurrence of an avian-style respiratory infection in a non-avian dinosaur. Nature Scientific Reports 12: 1954. Crossref
Acta Palaeontol. Pol. 69 (3): 395–402, 2024
https://doi.org/10.4202/app.01141.2024