

New parasitic organisms in a productid brachiopod Eomarginifera lobata from the lower Carboniferous of the Moscow Basin, Russia
OLEV VINN, ALEKSANDR A. MIRONENKO, and MARK A. WILSON
Vinn, O., Mironenko, A.A., and Wilson, M.A. 2024. New parasitic organisms in a productid brachiopod Eomarginifera lobata from the lower Carboniferous of the Moscow Basin, Russia. Acta Palaeontologica Polonica 69 (3): 403–410.
Bioclaustrations are among the best ways that parasitic associations are preserved. A new bioclaustration, Haplorygma productidophilia csp. nov., is here described from the ventral interior of the Carboniferous productid brachiopod Eomarginifera lobata. The location and morphology of the structures in the ventral valve differ from the structure in the dorsal valve, suggesting that two different organisms infested the shells of Eomarginifera lobata. It is possible that whether it was ventral or dorsal valve was an important selection criterion for the infesting organisms, which were likely parasites. The proportion of infested productids was low in the population (5.8%). This low infestation rate could indicate that productids had effective methods for resisting parasites, or that the parasites were ineffective in spreading through the brachiopod population.
Key words: Brachiopoda, symbiosis, bioclaustrations, Mississippian, lower Carboniferous, Eastern Europe.
Olev Vinn [olev.vinn@ut.ee; ORCID: https://orcid.org/0000-0002-2889-2544 ], Department of Geology, Institute of Ecology and Earth Sciences, University of Tartu, 50411 Tartu, Estonia.
Aleksandr A. Mironenko [paleometro@gmail.com ; ORCID: https://orcid.org/0000-0003-1913-8395 ], Geological Institute of the Russian Academy of Sciences, Pyzhevski Lane 7, 119017 Moscow, Russia.
Mark A. Wilson [mwilson@wooster.edu; ORCID: https://orcid.org/0000-0002-4651-0589 ], Department of Earth Sciences, The College of Wooster, Wooster, OH, 44691 USA.
Received 21 March 2024, accepted 16 May 2024, published online 17 September 2024.
Copyright © 2024 O. Vinn et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Parasitic associations have a long evolutionary history (Conway-Morris 1981; De Baets and Littlewood 2015; Leung 2017; De Baets et al. 2021a, b). Fossils of parasites occur throughout the post-Cambrian Phanerozoic (Baumiller and Gahn 2002; De Baets et al. 2021a, b). The earliest brachiopod parasites have been described from the lower Cambrian (Bassett et al. 2004). Bioclaustrations are among the best ways that parasitic associations are preserved when a skeletonized host organism embeds a symbiont within its mineral tissues (Palmer and Wilson 1988; Słowiński et al. 2020). There are also other ways by which parasitic associations can be preserved. Vinn et al. (2023) described pits in the valve margin of the brachiopod Porambonites from the Upper Ordovician of Estonia. These symbiont cavities in host organisms have only rarely been reported from the Carboniferous, and then only from brachiopods (Baliński and Sun 2010). The earliest known brachiopod parasite, Eodiorygma acrotretophilia, dating from the early Cambrian, inhabited a lingulate brachiopod with a phosphatic shell (Bassett et al. 2004). It is believed to have been a solitary suspension-feeding organism similar to an entoproct. Tubes attributed to Diorygma atrypophilia, found in the Devonian atrypid brachiopod Desquamatia subzonata from Poland, are thought to have belonged to suspension-feeding phoronids (Biernat 1961). Open-ended tubes identified as Burrinjuckia spiriferidophilia, extending inward from the inner surfaces of brachial valves in three Emsian spiriferid brachiopods, have uncertain biological affinities but were likely suspension feeders; possible culprits including Polychaeta, Tunicata, Cnidaria, Arthropoda, or Phoronida (Chatterton 1975). Haplorygma, a tube-dwelling organism with potential entoproct or annelid affinities, has been found within the shells of Tylothyris laminosa and Spiriferellina cf. insculpta in the Mississippian of South China (Baliński and Sun 2010). Additionally, parasitic polychaetes have been documented in the Early Cretaceous cold seep brachiopod Peregrinella multicarinata (Kiel 2008).
The evolution of parasitic associations has recently been an object of thorough analysis in multiple studies (Zapalski 2007; De Baets and Littlewood 2015; Huntley and De Baets 2015; De Baets et al. 2011, 2021a, b; Huntley et al. 2021; Van Dijk and De Baets 2021). Data on fossil parasites suggest that biodiversity controls parasite prevalence, and that parasitism was likely scale dependent and has increased through the Phanerozoic (De Baets et al. 2021a, b). The parasitic relationships were likely important drivers of brachiopod evolution, but the available data are scarce and additional studies are needed.
This paper: (i) describes two new fossil brachiopod parasites from the lower Carboniferous of Russia; (ii) discusses the palaeoecology of these parasitic associations; (iii) compares modern brachiopod symbiosis with the Carboniferous of Russia.
Institutional abbreviations.—PIN, Borisiak Palaeontological Institute of the Russian Academy of Science, Moscow, Russia.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: urn:lsid:zoobank.org:pub:F529C048-2116-4979-837F-53401E73302A
Geological setting
The productid brachiopods studied in the present article were collected from the lower part of the Dashkovka Member of the Gurovo Formation in the Borshchevsky Quarry (54° 25′ 25.0” N, 36° 48′ 37.3” E), which is located approximately 150 km southwest of Moscow and about midway between Tula and Kaluga (Vinn and Mironenko 2021: fig.1; Van Iten et al. 2022: fig. 1). In the Borshchevsky Quarry, the Gurovo Formation differs substantially in lithology from other Serpukhovian formations of Central Russia. It mainly consists of light-colored dolostone and limestone. The Dashkovka Member consists of several thick (2–5 m) beds of dark (gray-green and red-burgundy) clay and palygorskite shale alternating with thin (ca. 0.3 m) interbeds of dolomitic siltstone. The stratigraphy of this undescribed site is very similar to that of the well-studied Zaborie locality (Kabanov 2003).
In general, dark clay and shale typically form under dysoxic or hypoxic conditions (Potter et al. 1980). The dark gray shale in the Dashkovka Member contains abundant remains of pelagic fauna, including nautiloid cephalopods, fish scales, teeth, and coprolites, conodonts, and graptolites. Benthic fauna such as corals, gastropods, bivalves and crinoids, which are characteristic of the Carboniferous dolostones and limestones of adjacent formations, either is absent or represented by rare and mostly small juvenile specimens. These facts support the idea that there was a relative lack of oxygen in the lower depths of water. Nevertheless, levels of dissolved oxygen in the waters on the sea floor likely improved at times, as indicated by rare occurrences of mobile benthos such as sea urchins and trilobites. Some benthic organisms, such as conulariids and Sphenothallus, are also quite numerous in several layers of black clay and siltstone interbeds (Vinn and Mironenko 2021; Van Iten et al. 2022).
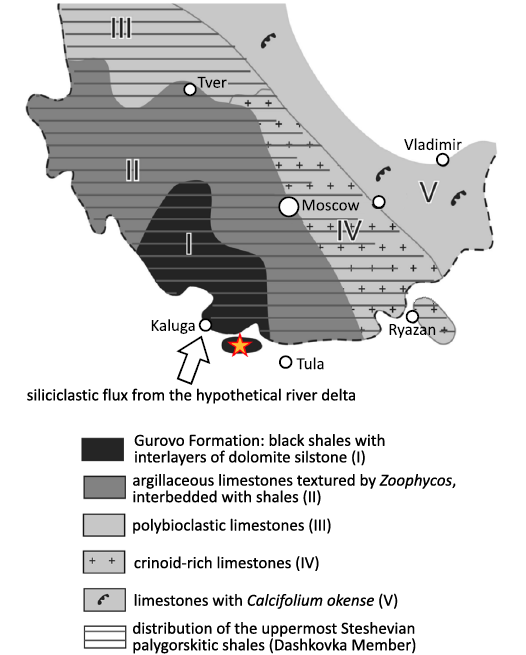
Fig. 1. Geological map with location of Borshchevsky Quarry indicated (modified after Vinn and Mironenko 2021: fig. 1).
The depositional models of the Gurovo Formation have been a topic of debate for a long time, with various researchers presenting different arguments in support of two mutually conflicting hypotheses regarding the origin of the dark gray shale in the Dashkovka Member in the middle part of the formation. Specifically, Shvetsov (1932) proposed that the shale was deposited in deep water during the early Serpukhovian transgression. In contrast, Kabanov et al. (2012) argued that the shale was deposited in a shallow lagoon receiving a continuous influx of terrigenous sediment from a low-energy river delta.
The presence within the dark gray-green shale of a large number of pyritized cephalopod shells falsifies the second hypothesis, since modern cephalopods are stenohaline animals that cannot tolerate desalination, which occurs frequently in the vicinity of large river deltas (Vinn and Mironenko 2021). On the other hand, three specimens of horseshoe crabs of the genus Belinurus were found in dark red-burgundy clays, otherwise practically devoid of fossils (AAM unpublished observation). The family Belinuridae is thought to have inhabited freshwaters (Bicknell and Pates 2020), so this discovery suggests that the red-burgundy clay could have deposited if not in freshwater, then in brackish conditions. Therefore, it is quite possible that both hypotheses do not exclude, but complement each other and that different layers of dark clays were formed under different conditions. The black-green clays, containing pyritized cephalopods, could have formed under deeper open marine conditions with normal salinity, whereas the red-burgundy clays, containing horseshoe crabs, could have formed under strong influence of the river delta.
Although in general benthic fauna is rare in the clays of the Dashkovka Member of the Gurovo Formation, there are several layers with a nearly monotypic complex of very abundant Eomarginifera lobata brachiopods (Kabanov 2003). These complexes also include rare specimens of the brachiopod species Schuchertella radialis and Cleiothyridina pectinata which do not show any bioclaustrations. These brachiopods occur in approximately 1 to 100 ratio with Eomarginifera lobata. All these complexes are confined to the top of black-green clays and to the base of dark burgundy clays. Therefore, the massive flourishing of brachiopods occurred during transitional conditions between clearly marine and presumably brackish environments.
Material and methods
Brachiopods from the Borshchevsky Quarry (Fig. 1) were examined and photographed using low magnification reflected light and low and high magnification scanning electron microscopy (SEM). Low vacuum SEM imaging was conducted in both secondary electron (SE) and backscattered electron (BSE) modes using the TESCAN VEGA II and III electron microscopes in the Palaeontological Institute of the Russian Academy of Science in Moscow. The Z Score Calculator for 2 Population Proportions using two-tailed hypothesis was used to test the differences in infestation of dorsal and ventral valves in productid Eomarginifera lobata (https://www.socscistatistics.com/tests/ztest/default2.aspx). All brachiopod specimens are housed in the Borisiak Palaeontological Institute of the Russian Academy of Science in Moscow (PIN RAS), under collection numbers PIN 5918.
Results
Location of bioclaustrations.—Eight ventral valves (5.8%) of 138 isolated ventral valves contain possible parasite traces. A single dorsal valve of 34 isolated dorsal valves contains a possible trace of a parasite. The dorsal and ventral valves do not differ significantly in their infestation rates (z = 0.6698, p = 0.50286.
Bioclaustrations are located in various places within the ventral valve interiors. In the dorsal valve of specimen 1 (PIN 5918/1), there is swollen spine that is turned backwards towards the valve’s umbo (Fig. 2A). The swollen spine has an opening in the top with broken edges. In specimen 2 (PIN 5918/2) there are three short tubular structures in the ventral interior; two structures are located within the adductor muscle field, one in each scar. The one tubular structure in the left adductor scar is sealed off. The third structure is located in the middle of the left diductor muscle scar. In the ventral valve of specimen 3 (PIN 5918/3), a short basally widened tubular structure is located laterally within the right adductor scar. In the ventral valve of specimen 4 (PIN 5918/4), a long tubular structure is located in the front of the right diductor scar and is oriented towards the umbo. In the ventral valve of specimen 5 (PIN 5918/5), a short tubular structure is located within the edge of the right adductor scar. In the ventral valve of specimen 6 (PIN 5918/6), a short tubular structure with an angular cross section is located within the right adductor scar and is oriented towards the sulcus on the anterior commissure. In the ventral valve of specimen 7 (PIN 5918/7), a basally strongly widened, very short, tubular structure is located within the frontal part of the right diductor muscle scar. In the ventral valve of specimen 8 (PIN 5918/8), a short tubular structure is located in the mantle cavity near the left diductor scar and oriented towards the anterior commissure. In the ventral valve of specimen 9 (PIN 5918/9), there is a possible short tubular structure within the right adductor scar.
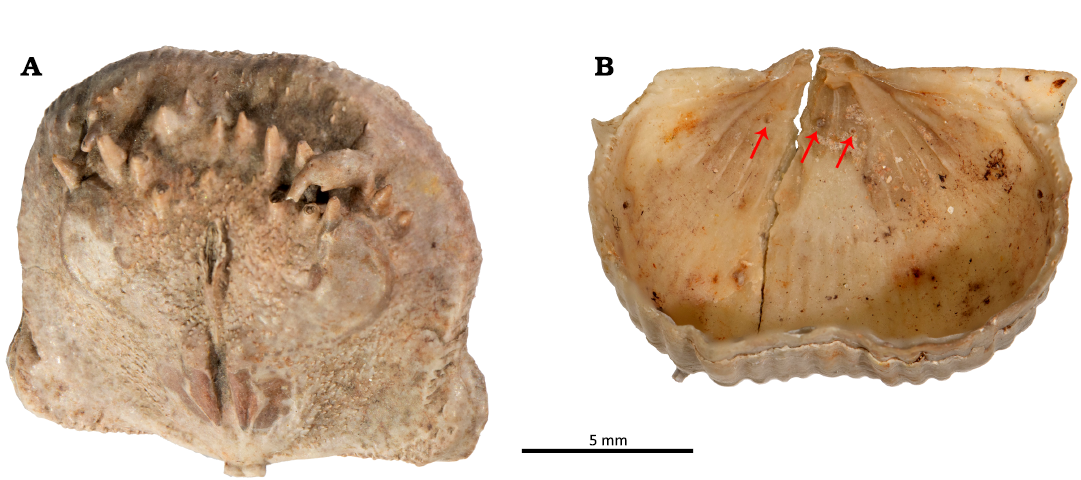
Fig. 2. Haplorygma productidophilia csp. nov. in productid brachiopods Eomarginifera lobata (Sowerby, 1821) from Gurovo Formation, Missisipian, Serpukhovian, Borshchevsky Quarry, central Russia. A. PIN 5918/1, dorsal valve interior showing swollen and backwards turned infested spine. B. PIN 5918/2, ventral valve interior with three tubes (arrows).
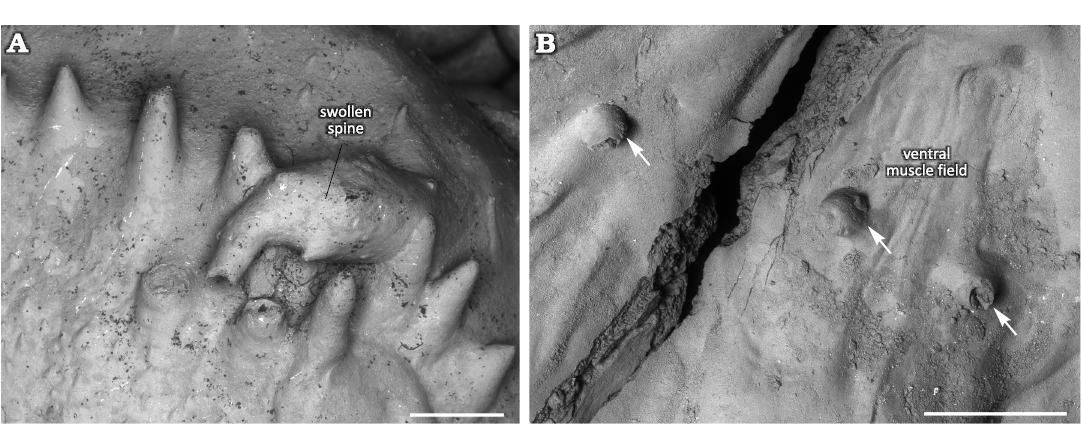
Fig. 3. Haplorygma productidophilia csp. nov. in productid brachiopods Eomarginifera lobata (Sowerby, 1821) from Gurovo Formation, Missisipian, Serpukhovian, Borshchevsky Quarry, central Russia. A. PIN 5918/1, dorsal valve interior showing swollen and backwards turned infested spine. B. PIN 5918/2, ventral valve interior with three tubes (arrows), associated with the ventral muscle field. Scale bars 1 mm.
Swollen spine.—Normal spines are oriented towards the anterior commissure in the dorsal valve interior. They are about 1 mm in diameter and up to 3 mm long. A marginal spine is turned backwards towards the valve’s umbo and is markedly swollen (Fig. 2A). The swollen spine is three times longer than normal spines and also about three times wider. The swelling is ellipsoid and occupies the middle 2/3 of the spine. It surface is generally smooth, though with some tubercles and short outgrowths. The initial non-swollen part of the spine has similar orientation to the normal spines. The swollen part is turned about 100° backwards whereas its non-swollen top is again turned about 75° backwards. The swollen spine has an aperture on its top with broken edges.
Systematic palaeontology
In their revision of ichnotaxonomy, Bertling et al. (2022) recommended that bioclaustrations be named as cecidotaxa rather than as traditional ichnotaxa. Since cecidotaxa are also governed by the International Code of Zoological Nomenclature, we agree and have therefore designated this new bioclaustration as a cecidospecies (csp.).
Genus Haplorygma Baliński & Sun, 2010
Type species: Haplorygma dorsalis Baliński & Sun, 2010; middle Tournaisian, Mississippian, Carboniferous, South China.
Haplorygma productidophilia csp. nov.
Figs. 2B, 3B, 4A, D, 5B.
ZooBank LSID: urn:lsid:zoobank.org:act:4A7CCE0C-4176-4E91-A96 D-9125DC9F617D
Etymology: After occurrence in a productid brachiopod.
Holotype: PIN 5918/4, complete tube.
Type locality: Borshchevsky Quarry, Russia (54°25′25.0” N, 36°48′37.3” E).
Type horizon: Gurovo Formation (Serpukhovian), Mississippian, Carboniferous.
Material.—Ten variably preserved tubes in Eomarginifera lobata (Sowerby, 1821) ventral valves (PIN 5918/2–9).
Diagnosis.—Simple, variably oriented with distal part upturned dorsally, microscopic tubular outgrowth of the inner surface of the secondary shell layer in the ventral valve, with circular aperture and widened base, often within muscle scars.
Description.—Simple, variably oriented with distal part upturned dorsally, microscopic tubular outgrowth of the inner surface of the secondary shell layer of the convex ventral valve of productid brachiopods. The tubes often have a widened base in the form of a small bump. The apertures of the tubes vary from perfectly circular to oval, and in a single case angular. There can be a single keel-like structure in the tube interior. The longer tubes have constant diameter. The structure of the tube wall is microlaminar. The tube wall thickness is often variable around the aperture. The tubular structures mostly occur within the adductor or diductor muscle scars. The diameter of the aperture is greatly variable from 100 to 350 µm. The tubes can grow up to 2 mm long but usually are 250 to 500 µm long. The tube wall is up to 70 µm thick.
Remarks.—This new species resembles most closely Haplorygma dorsalis Baliński & Sun, 2010, from southern China (Baliński and Sun 2010: 693, fig. 1A, B) in being a simple tubular outgrowth of the inner surface of the secondary shell layer of a brachiopod. It differs, however, by the larger size and location on the ventral interior. Unlike this new species, H. dorsalis never has a circular aperture, it is never located within muscle scars, and it does not have a widened base.
Stratigraphic and geographic range.—Type locality and horizon only.
Discussion
Interpretation of bioclaustrations.—The location and morphology of these bioclaustration structures in the ventral valve differ from the structure in the dorsal valve, suggesting that two different organisms infested the shells of Eomarginifera lobata. There is no significant difference in infestation rate of the dorsal and ventral valves, but considering the possibility that different organisms infested the ventral and dorsal valves, it is likely that the brachiopod valve was an important selection criterion for the infesting organisms. The organisms infesting the ventral valves created tubular structures associated with muscle scars. It is reasonable to assume that the location of these structures within muscle scars was not accidental and represents the site selection of the symbiont. In the case where the symbiont targeted muscle scars where protein-rich muscles were located, it is possible that it may have consumed brachiopod tissues. Many tubular structures in Paleozoic brachiopod mantle cavities have been interpreted as domiciles of filter-feeding organisms and therefore as potential kleptoparasites (Biernat 1961, 1964; Chatterton 1975; Bassett et al. 2004; Baliński and Sun 2010; Vinn et al. 2014, 2022). The latter life mode does not fit at all with the location of the tubular structures within or between muscle bundles of the host brachiopod; it would have been an unfortunate site selection for a filter-feeding organism. The muscles would have prevented the organism from successfully filtering food particles. Most of these tubular structures are also too short to allow the symbiont to reach to the mantle cavity were the food-rich waters were located. Thus most likely the organisms responsible for these tubular bioclaustrations were not filter-feeding. The only accessible food resource for the symbionts would have been the brachiopod muscles or other soft tissues. Thus, most likely the organisms that infested ventral muscle fields were parasites. The tubular shape suggests a worm-like parasite. Moreover, one tube of the possible parasite was sealed off by the brachiopod (Fig. 5B1), which could either indicate that the brachiopod actively killed the parasite or alternatively that the parasite had a shorter life span than the host brachiopod. However, the lack of signs of overgrowth attempts in all other tubes suggests that the parasite was very efficient in keeping its structure’s aperture opened.
In the dorsal valve an unknown organism lived within the spine and made the spine swell. This swelling of the spine could have resulted from an immune reaction of the host brachiopod but it could also have been chemically initiated by the symbiont in order to create a larger domicile. It is likely that this organism specially targeted dorsal valves as there are no spines in the interior of ventral valves that would be suited for the domicile. The swollen spine has an opening in its top that directed towards the brachiopod lophophore in the mantle cavity (Fig. 4A). If the opening is not the result of breakage of the spine, then the symbiont had access to the waters of mantle cavity and may have been a suspension feeder. In the latter case it would have benefitted from the food particles collected by the host brachiopod. Thus, the organism that inhabited the swollen spine could have been a kleptoparasite (Zhang et al. 2020). Both organisms presumably were not useful to the host brachiopod, but the worm-like organisms that infested the ventral muscle field likely caused harm to the host brachiopod.
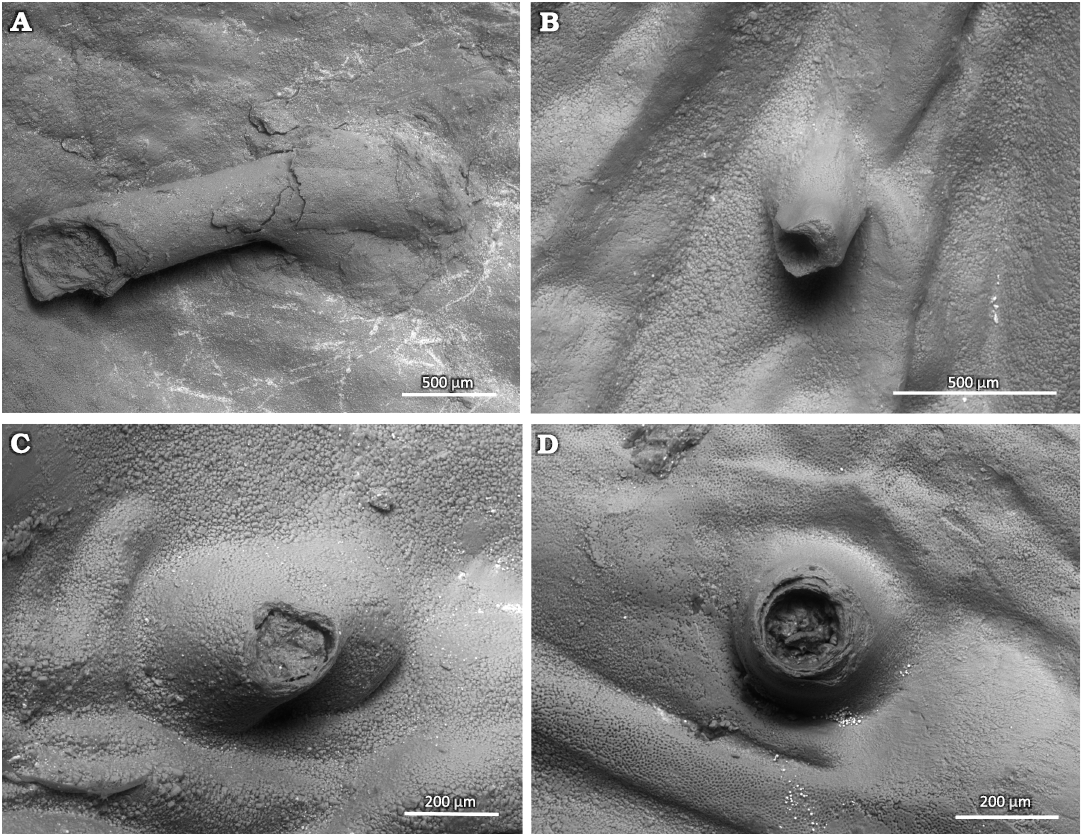
Fig. 4. Tubes of parasitic Haplorygma productidophilia csp. nov. from Gurovo Formation, Missisipian, Serpukhovian, Borshchevsky Quarry, central Russia. A. Holotype PIN 5918/4, long tube with widened base. B. PIN 5918/6, short tube without widened tube base. C. PIN 5918/3, broken specimen with widened tube base. D. PIN 5918/5, short tube with only slightly widened base.
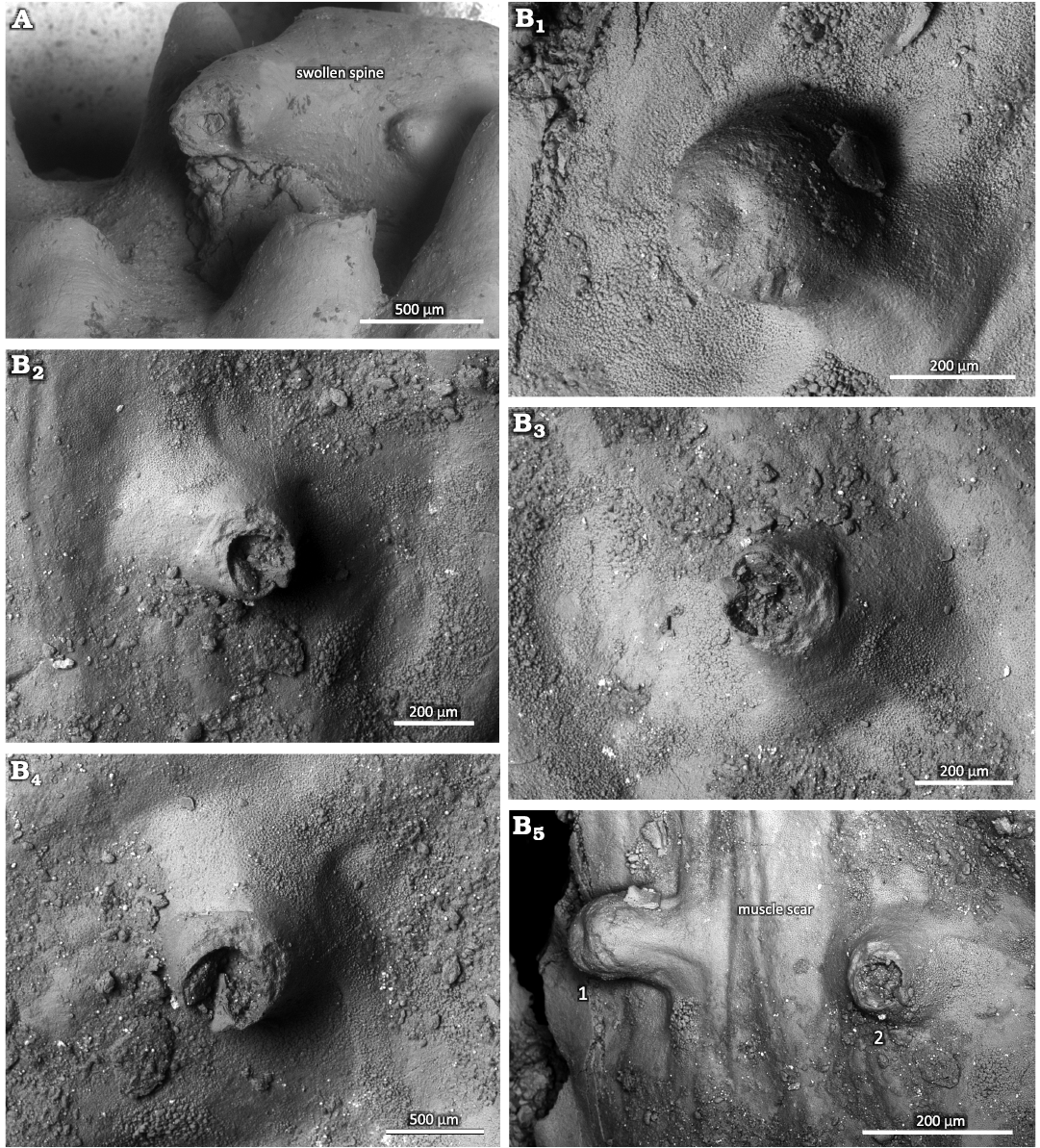
Fig. 5. Haplorygma productidophilia csp. nov. in productid brachiopods Eomarginifera lobata (Sowerby, 1821) from Gurovo Formation, Missisipian, Serpukhovian, Borshchevsky Quarry, central Russia. A. PIN 5918/1, top of swollen spine. B. PIN 5918/2, tube with sealed off aperture (B1); short tube with only slightly widened base, tube interior with possible keel-like structure (B2); short tube with widened base (B3); short tube with only slightly widened base, tube interior with possible keel-like structure (B4); two tubes (1 and 2) within the muscle scar (B5).
Infestation rate.—The infestation rates of fossil brachiopods by organisms inhabiting their shell interiors are not well known despite being important for understanding the evolutionary paleoecology of parasitic associations (De Baets et al. 2021a). Nevertheless, there are some data for the Ordovician brachiopods (Vinn et al. 2023). Most previously described cases are based on few infested specimens and have no information about the frequency of such interactions in the brachiopod population. Biotic interactions between living brachiopods and spionid polychaete worms, collected around the San Juan Islands (USA), show that proportions of infested brachiopods range 3.4–29.1% (Rodrigues 2007). Thus, the infestation rate of the productids here (5.8%) is not high. This low infestation rate could indicate that productids had effective resistance against parasites, or that the parasites were ineffective in spreading within the brachiopod population. In the case of ectoparasites, the infestation rates can be much higher as shown in the early Cambrian lingulids where as much as 47.8% of the specimens can be infested by kleptoparasitic tubeworms (Zhang et al. 2020). On the other hand, infestation rates can be quite low in the case of Paleozoic brachiopod endoparasites (De Baets et al. 2021b). The number of parasite specimens per host brachiopod, usually one and only rarely three, is also very low. This could indicate that the parasites were not breeding inside the brachiopod or that their earliest growth stages lived outside of brachiopods. The low number of parasites could also indicate that they had high virulence so that the larger number of parasites could have been fatal to the host and no bioclaustrations could form.
Parasitic associations in the Carboniferous.—Previously, only two parasitic associations involving brachiopod endobionts were known from the Carboniferous. In the Carboniferous of China, one specimen of Haplorygma dorsalis has been found in dorsal valve of Tylothyris laminosa, and another specimen has been reported from dorsal valve of Spiriferellina cf. insculpta (Baliński and Sun 2010). The affinity of the tube-dwelling Haplorygma dorsalis is enigmatic, but its annelid relationship and kleptoparasitic nature is highly probable (Baliński and Sun 2010). In addition, there are numerous parasitic associations that occur between other invertebrates such as crinoid/blastoid-platyceratid associations (Baumiller and Gahn 2018; Thomka and Brett 2021). The crinoid-Cladochonus symbiotic associations occurred during the Mississippian (Donovan and Lewis 1999). Cladochonus corallites grew in a ring around an erect column of a living crinoid and often caused malformations such as swellings of the crinoid stem, and it was partially embedded by the crinoid skeleton (Donovan and Lewis 1999). These interactions have often been classified as parasitism (Vinn 2017). The symbiotic rugosans occur in the calices of crinoids in the Carboniferous. The rugosan location on the crinoid suggests that the coral actively harvested food with its tentacles from the adoral groove of the crinoid’s arm and thus could have been a kleptoparasite (Donovan et al. 2005).
Conclusions
Two types of bioclaustrations occur in the productid brachiopod Eomarginifera lobata from the Mississippian of central Russia. Simple, variably oriented with distal part upturned dorsally, microscopic tubular outgrowth of the inner surface of the secondary shell layer of the convex ventral valve of Eomarginifera lobata. The parasitic organisms infesting the ventral valves created tubular structures associated with muscle scars. We assume that the location of these structures within muscle scars was not accidental and represents the site selection of the parasite. The organisms responsible for these tubular bioclaustrations were not filter-feeding. The only accessible food resource for the symbionts would have been the brachiopod muscles or other soft tissues. In addition to the latter parasites, an unknown organism lived within the spine of the dorsal valve and made the spine swell. The organism that inhabited the swollen spine could have been a kleptoparasite.
Acknowledgements
We are grateful to Kenneth De Baets (University of Warsaw, Poland) and an anonymous reviewer for the constructive comments on the manuscript. Financial support to OV was provided by a Sepkoski Grant from the Paleontological Society.
References
Baliński, A. and Sun, Y. 2010. Tubular shell infestations in some Mississippian spirilophous brachiopods. Acta Palaeontologica Polonica 55: 689–694. Crossref
Bassett, M.G., Popov, L.E., and Holmer, L.E. 2004. The oldest-known metazoan parasite? Journal of Paleontology 78: 1214–1216. Crossref
Baumiller, T.K. and Gahn, F.J. 2002. Fossil record of parasitism on marine invertebrates with special emphasis on the platyceratid–crinoid interaction. Paleontological Society Papers 8: 195–210. Crossref
Baumiller, T.K. and Gahn, F.J. 2018. The nature of the platyceratid–crinoid association as revealed by cross-sectional data from the Carboniferous of Alabama (USA). Swiss Journal of Palaeontology 137: 177–187. Crossref
Bertling, M., Buatois, L.A., Knaust, D., Laing, B., Mángano, M.G., Meyer, N., Mikuláš, R., Minter, N.J., Neumann, C., Rindsberg, A.K., and Uchman, A. 2022. Names for trace fossils 2.0: theory and practice in ichnotaxonomy. Lethaia 55: 1–19. Crossref
Biernat, G. 1961. Diorygma atrypophilia n. gen., n. sp.—a parasitic organism of Atrypa zonata Schnur. Acta Palaeontologica Polonica 6: 17–28.
Biernat, G. 1964. Middle Devonian Atrypacea (Brachiopoda) from the Holy Cross Mountains, Poland. Acta Palaeontologica Polonica 9: 277–356.
Chatterton, B.D.E. 1975. A commensal relationship between a small filter feeding organism and Australian Devonian spiriferid brachiopods. Paleobiology 1: 371–378. Crossref
Conway-Morris, S. 1981. Parasites and the fossil record. Parasitology 82: 489–509. Crossref
De Baets, K. and Littlewood, D.T.J. 2015. The importance of fossils in understanding the evolution of parasites and their vectors. Advances in Parasitology 90: 1–51. Crossref
De Baets, K., Huntley, J.W., Klompmaker, A.A., Schiffbauer, J.D., and Muscente, A.D. 2021a. The fossil record of parasitism: its extent and taphonomic constraints. In: K. De Baets and J.W. Huntley (eds.), The Evolution and Fossil Record of Parasitism. Topics in Geobiology 50: 1–50. Crossref
De Baets, K., Huntley, J.W., Scarponi, D., Klompmaker, A.A., and Skawina, A. 2021b. Phanerozoic parasitism and marine metazoan diversity: dilution versus amplification. Philosophical Transactions of the Royal Society B: Biological Sciences 376 (1837): 20200366. Crossref
De Baets, K., Klug, C., and Korn, D. 2011. Devonian pearls and ammonoid–endoparasite co-evolution. Acta Palaeontologica Polonica 56: 159–180. Crossref
Donovan, S.K. and Lewis, D.N. 1999. An epibiont and the functional morphology of the column of a platycrinitid crinoid. Proceedings of the Yorkshire Geological Society 52: 321–323. Crossref
Donovan, S.K., Lewis, D.N., and Kabrna, P. 2005. An unusual crinoid coral association from the Lower Carboniferous of Clitheroe, Lancashire. Proceedings of the Yorkshire Geological Society 55: 301–304. Crossref
Huntley, J.W. and De Baets, K. 2015. Trace fossil evidence of trematode–bivalve parasite–host interactions in deep time. Advances in Parasitology 90: 201–231. Crossref
Huntley, J., De Baets, K., Scarponi, D., Linehan, L., Epa, Y., Jacobs, G., and Todd, J. 2021. Bivalve mollusks as hosts in the fossil record. In: K. De Baets and J.W. Huntley (eds.), The Evolution and Fossil Record of Parasitism: Coevolution and Paleoparasitological Techniques. Topics in Geobiology 50: 251–287. Crossref
Kabanov, P.B. 2003. Stratotype of the Serpukhovian Stage in Zaborie Quarry. Part I. Lithofacial characteristics. Stratigraphy and Geological Correlation 11: 18–35.
Kabanov, P.B., Alekseeva, T.V., and Alekseev, A.O. 2012. Serpukhovian stage (Carboniferous) in type area: sedimentology, mineralogy, geochemistry and section correlation. Stratigraphy and Geological Correlation 20: 15–41. Crossref
Leung, T.L. 2017. Fossils of parasites: what can the fossil record tell us about the evolution of parasitism?. Biological Reviews 92: 410–430. Crossref
Kiel, S. 2008. Parasitic polychaetes in the Early Cretaceous hydrocarbon seep-restricted brachiopod Peregrinella multicarinata. Journal of Paleontology 82: 1215–1217. Crossref
Palmer, T.J. and Wilson, M.A. 1988. Parasitism of Ordovician bryozoans and the origin of pseudoborings. Palaeontology 31: 939–949.
Potter, P.E., Maynard, J.B., and Pryor, W.A. 1980. Sedimentology of Shale. 310 pp. Springer, New York. Crossref
Rodrigues, S.C. 2007. Biotic interactions recorded in shells of recent rhynchonelliform brachiopods from San Juan Island, USA. Journal of Shellfish Research 26: 241–252. Crossref
Shvetsov, M.S. 1932. Obŝaâ geologičeskaâ karta Evropejskoj časti SSSR: List. 58. Severo-zapadnaâ četvert lista. 184 pp. Gosnauchtekizdat, Moskva.
Słowiński, J., Surmik, D., Duda, P., and Zatoń, M. 2020. Assessment of serpulid–hydroid association through the Jurassic: A case study from the Polish Basin. PLoS ONE 15: e0242924. Crossref
Sowerby, J. 1821. The Mineral Conchology of Great Britain, Vol. 3. 184 pp. Published by the author, London.
Thomka, J.R. and Brett, C.E. 2021. Parasitism of Paleozoic crinoids and related stalked echinoderms: paleopathology, ichnology, coevolution, and evolutionary paleoecology. In: K. De Baets and J.W. Huntley (eds.), The Evolution and Fossil Record of Parasitism. Topics in Geobiology 50: 289–316. Crossref
Van Dijk, J. and De Baets, K. 2021. Biodiversity and host-parasite (co)extinction. In: K. De Baets and J.W. Huntley (eds.), The Evolution and Fossil Record of Parasitism: Coevolution and Paleoparasitological Techniques. Topics in Geobiology 50: 75–97. Crossref
Van Iten, H., Mironenko, A.A., and Vinn, O. 2023. A new conulariid from the Upper Mississippian (early Serpukhovian) of Central Russia (Moscow Basin): systematics, microstructure, and growth abnormalities. PalZ 97: 311–322. Crossref
Vinn, O. 2017. Symbiosis in Late Devonian–Mississippian corals: a review. Palaeobiodiversity and Palaeoenvironments 97: 723–729. Crossref
Vinn, O. and Mironenko, A.A. 2021. Discovery of plywood structure in Sphenothallus from Gurovo Formation (Mississipian), Central Russia. Annales Societatis Geologorum Poloniae 91: 67–74. Crossref
Vinn, O., De Baets, K., Isakar, M., and Toom, U. 2023. Parasite induced shell damage in brachiopod Porambonites (Porambonites) laticaudata from Late Ordovician (Sandbian) of Estonia. Estonian Journal of Earth Sciences 72: 110–113. Crossref
Vinn, O., Wilson, M.A., and Toom, U. 2014. Earliest rhynchonelliform brachiopod parasite from the Late Ordovician of northern Estonia (Baltica). Palaeogeography, Palaeoclimatology, Palaeoecology 411: 42–45. Crossref
Vinn, O., Wilson, M.A. Isakar, M., and Toom, U. 2022. New bioclaustration of a symbiont in the mantle cavity of Clitambonites schmidti (Brachiopoda) from the Sandbian (Upper Ordovician) of Estonia. Palaios 37: 520–524. Crossref
Zapalski, M.K. 2007. Parasitism versus commensalism: the case of tabulate endobionts. Palaeontology 50: 375–380. Crossref
Zhang, Z., Strotz, L.C., Topper, T.P., Chen, F., Chen, Y., Liang, Y., Zhang, Z., Skovsted, C.B., and Brock, G.A. 2020. An encrusting kleptoparasite-host interaction from the early Cambrian. Nature Communications 11 (1): 2625. Crossref
Acta Palaeontol. Pol. 69 (3): 403–410, 2024
https://doi.org/10.4202/app.01156.2024