

Comparison of Recent and sub-fossil sponge communities of West Antarctica
MAGDALENA ŁUKOWIAK, BASLAVI CÓNDOR-LUJÁN, VICTOR CORRÊA SEIXAS, ALVARO ARTEAGA, LUIS CERPA, KATARZYNA ZAREMBA, and JONATAN AUDYCKI
Łukowiak, M., Cóndor-Luján, B., Seixas, V.C., Arteaga, A., Cerpa, L., Zaremba, K., and Audycki, J. 2025. Comparison of Recent and sub-fossil sponge communities of West Antarctica. Acta Palaeontologica Polonica 70 (1): 43–56.
Sponges comprise a diverse and important benthic invertebrate group in many ecoregions, being a dominating component in the Antarctic communities. Based on two piston cores from the Admiralty Bay, West Antarctica, we isolated the sponge spicules from the sediments and compared the sub-fossil sublittoral sponge assemblage with the Recent sponge fauna of this area. In addition, we performed statistical analysis to compare the species composition from and within each core. While no significant shifts in the composition of sponge assemblages along the cores were noticed, our study revealed a noteworthy distinction between the documented sponge species in the area and those identified based on loose spicules within the sediment. The sedimentological record appeared to capture more sponge species than identified in contemporary faunistic studies. Out of the 27 sponge taxa recognized in the sediment, merely seven were confirmed as presently inhabiting the area. Of the remaining 20 taxa, several were documented in nearby regions such as Maxwell Bay or South Shetland Islands in general, but 12 species had not been previously recorded in this locality or its adjacent areas. Conversely, the sediments did not indicate the presence of nine modern species previously reported in this area. We attribute these discrepancies to various factors, including the potential oversights in recognizing sponges characterized by small size, fragility, or encrusting habits during faunistic studies. In cases where the certain taxa lack a fossil record, we suggest that the simplicity of their spicule types made the recognition in sediments challenging. In summary, our findings underscore that Admiralty Bay remains substantially understudied in terms of sponge species inhabiting this area, highlighting the need for further comprehensive studies in this region.
Key words: Porifera, sediment spicules, sponge taxonomy, Admiralty Bay, Antarctica, Holocene.
Magdalena Łukowiak [mlukowiak@twarda.pan.pl; ORCID: https://orcid.org/0000-0002-5282-5341 ], Institute of Paleobiology, Polish Academy of Sciences, ul. Twarda 51/55, 00-818 Warszawa, Poland.
Baslavi Cóndor-Luján [bcondorl@unmsm.edu.pe; ORCID: https://orcid.org/0000-0001-7832-7319 ], Carrera de Biología Marina, Facultad de Ciencias Veterinarias y Biológicas, Universidad Científica del Sur, Antigua Panamericana Sur Km. 19, Villa El Salvador, Lima, Peru; Departamento de Zoología, Facultad de Ciencias Biológicas, Universidad Nacional Mayor de San Marcos, Lima, Peru; Carrera de Biología Marina, Facultad de Ciencias Veterinarias y Biológicas, Universidad Científica del Sur, Antigua Panamericana Sur Km. 19, Villa El Salvador, Lima, Peru.
Victor Corrêa Seixas [vcseixas@id.uff.br; ORCID: https://orcid.org/0000-0002-2067-9490 ] Instituto de Biologia, Departamento de Biologia Marinha Universidade Federal Fluminense, Niterói, Rio de Janeiro, Brazil.
Alvaro Arteaga [alvaromarteagab@gmail.com; ORCID: https://orcid.org/0000-0001-6001-8358 ] Carrera de Biología Marina, Facultad de Ciencias Veterinarias y Biológicas, Universidad Científica del Sur, Antigua Panamericana Sur Km. 19, Villa El Salvador, Lima, Peru.
Luis Cerpa [lcerpa@gmail.com; ORCID: https://orcid.org/0000-0001-8717-5142 ], Instituto Geológico, Minero y Metalúrgico, INGEMMET, Av. Canadá 1470, San Borja, Lima, Peru.
Katarzyna Zaremba [kg.zaremba@student.uw.edu.pl], Department of Hydrobiology, Faculty of Biology, University of Warsaw, Żwirki i Wigury 101, 02-089 Warsaw, Poland.
Jonatan Audycki [j.audycki@uw.edu.pl; ORCID: https://orcid.org/0009-0004-9507-3217 ], Institute of Evolutionary Biology, Faculty of Biology, University of Warsaw, Żwirki i Wigury 101, 02-089 Warsaw, Poland.
Received 24 April 2024, accepted 15 January 2025, published online 11 March 2025.
Copyright © 2025 M. Łukowiak et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Sponges comprise a diverse and abundant benthic invertebrate community in the Antarctic ecosystems being the dominating biota next to bryozoans and echinoderms (Sarà et al. 1992) and playing important roles in marine benthic community structure, dynamics and nutrition cycling (McClintock et al. 2005; Siciński et al. 2011; Downey et al. 2012; Angulo-Preckler et al. 2018).
In the Southern Ocean, approximately 397 sponge species have been documented, encompassing 296 demosponges and 50 hexactinellids (Downey et al. 2012). However, these estimations are approximated, as earlier studies reported 352 species of demosponges in the Antarctic (McClintock et al. 2005). Despite affinities with the Magellanic region (southern South America) and the Falkland Islands, the sponge community in the Antarctic is remarkably heterogeneous (Sarà et al. 1992). Although many Antarctic species seemed to have a circumpolar distribution (Koltun 1964; Sarà et al. 1992; McClintock et al. 2005), a systematic review on literature has cast doubt on the consistency of this pattern (Downey et al. 2012). There is also a high level of endemism in the Antarctic sponges observed. Of the 346 Antarctic demosponge and hexactinellid species recognized, 42% (146 species) were determined to be endemic (Downey et. al. 2012). Moreover, the distribution of sponges in this area is patchy and uneven (Gutt and Koltun 1995; Downey et al. 2012).
While Southern Ocean sponges had been widely studied (e.g., Carter 1877; Topsent 1901a, 1907, 1908, 1913, 1915, 1916; Kirkpatrick 1907a, b, 1908; Burton 1929, 1930, 1934; Tanita 1959; Koltun 1964, 1969, 1976; Barthel et al. 1990; Calcinai and Pansini 2000; Ríos 2007; Ríos et al. 2004; Schejter et al. 2006, 2019, 2024; Bertolino et al. 2007, 2009, 2020; Ríos and Cristobo 2007a, b, 2014; Plotkin and Janussen 2008; Janussen and Reiswig 2009; Goodwin et al. 2011, 2012, 2014, 2019; Downey et al. 2012; Göcke and Janussen 2013a, b; Kersken et al. 2016; Fernández et al. 2020), certain areas are unevenly sampled and inadequately understood. Even in well-studied regions, sampling may be too incomplete to confidently claim that most sponge species present have been detected (Downey et al. 2012).
Nevertheless, the Antarctic Peninsula seems to be one of the comparatively well studied areas (e.g., Ríos and Cristobo 2007a, b, 2014; Downey et al. 2012; Cárdenas et al. 2016) and the sponge fauna from the South Shetland Islands has been described in several taxonomic studies (Topsent 1917; Hamilton et al. 1997; Pisera 1997; Campos et al. 2007a, b; Vieira et al. 2010a, b; Siciński et al. 2011; Hajdu et al. 2016; Fernández et al. 2021).
Exploration of sponge fauna within a specific region typically relies on examination of live specimens. However, alternative methods for faunal assessment have demonstrated efficacy in capturing a more extensive range of sponge taxa compared to traditional faunistic studies (Łukowiak 2016). Those include analysis of the dissociated sponge skeletal elements (spicules and skeleton fragments) that can accumulate in the surface sediment (Gutt et al. 2015). Notably, the dissociated sponge spicules offer versatility for various applications (for more details see Łukowiak 2020). For instance, some studies including spicule-based analyses have been performed in order to determine silica content present in the Scotia Sea (Hendry et al. 2010) and in the Bransfield Strait (Maldonado et al. 2019). Furthermore, sponge spicules retrieved from the cores have been employed to reconstruct the dynamics of both marine (e.g., Bertolino et al. 2014, 2017; Łukowiak et al. 2018) and freshwater sponge assemblages globally (e.g., Paduano and Fell 1997; Candido et al. 2000; Volkmer-Ribeiro et al. 2007; Rasbold et al. 2019). These investigations have provided insights into temporal changes in sponge richness and abundance, contributing to a more comprehensive understanding of sponge ecology worldwide. In this study we have examined dissociated sponge spicules retrieved from two sedimentary cores from Admiralty Bay to reconstruct and describe the past sponge communities from the loose spicule record in order to evaluate temporal changes in the soft bottom, sublittoral sponge community in decennial time spans and compare the local sub-fossil sponge community with the Recent. To our knowledge, this is the first attempt to contrast the modern and subfossil Antarctic sponge diversity using the sponge spicules from the cores.
Institutional abbreviations.—ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
Material and methods
Study area.—Admiralty Bay is located at the center of King George Island being its largest embayment (Central South Shetland Islands, north of the Antarctic Peninsula, West Antarctica) with an area of 122 km2 and maximum depth of 535 m, harboring three inlets: Ezcurra, Martel, and Mackellar. The local hydrodynamics are driven by the heterogeneity of the seafloor in Admiralty Bay and the circulation in the Bransfield Strait (Szafrański and Lipski 1982). Waters entering the bay from Bransfield Strait are derived from the neighboring Weddell Sea or the Bellingshausen Sea, depending on regional water circulation, winds, and seasonal patterns (Gordon and Nowlin 1978). Regarding the bottom sediments, these contain diverse portions of randomly dispersed clastic materials coming from the glaciers and subglacial streams or drifting ice to the Admiralty Bay. Furthermore, the bed that contains accumulated sediment can reach a thickness of 150 m, as a Recent sediment cover presumably initiated during the deglaciation of the last Holocene glaciation (Siciński et al. 2011).
Sampling.—Procedures were executed under the framework of the ORCA Project during the Peruvian Antarctic Expeditions ANTAR XXVI (2019) and XXVII (2020). Samples were collected using an OSIL Piston core and a Van Veen grab (30×50 cm2) deployed at different depths onboard the Peruvian oceanographic research vessel BAP Carrasco, in two different sites along the Admiralty Bay, both off Ezcurra Inlet: GANT-20-07 (a and b) and GANT-19-08 (Fig. 1).
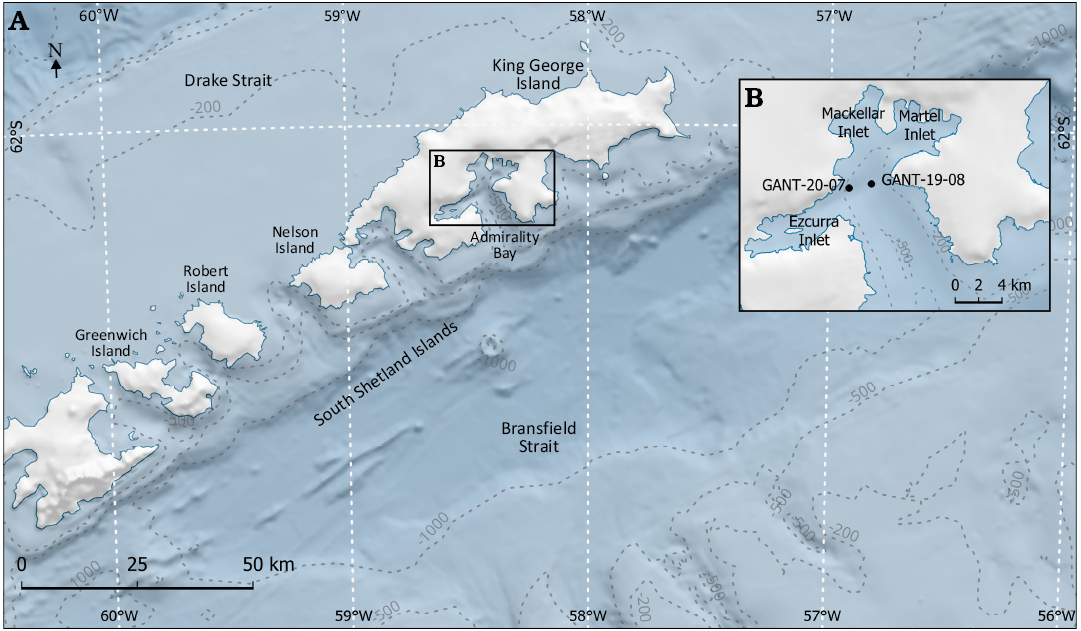
Fig. 1. A. Schematic map of South Shetland Islands, featuring geographic and depth characteristics. B. Admiralty Bay with sampling sites. Database source: SCAR Antarctic Digital Database (ADD) Version 7.0 and derived product from the General Bathymetric Chart of the Oceans (GEBCO) 2024 Grid.
Two piston cores of different length were retrieved. Piston core N°4 (C4, 338 cm long) was deployed at 282 m depth in GANT-20-07a (62°08’45.09’’S, 58°28’51.54’’W) in 2020. In this core the sediments were light green clay-silt transitioning to silt-sand towards the core base. Structures identified included organic matter, flaser lamination, polychaete burrows, plant roots, rock fragments, nodules, and sand lenses in different segments of the core. This structural combination suggests a dynamic and variable environment over time, indicating a complex and diverse sedimentary background e.g., changes in environmental conditions, alternating from marine to intertidal habitats. The piston core N°8 (C8, 140 cm long) was deployed at 423 m depth in GANT-19-08 (62°07’48.00’’S, 58°25’48.00’’W) in 2019, but no sedimentological data for this core is available. Both cores were divided into two halves and one of the halves was subsampled (2–3 cm3) every 10 cm (when possible). The core sample designation combines the core number (C4 or C8) with the depth from which the sediment was retrieved (e.g., -0, -210, etc.). Surface sediment sample N°4 was obtained from the grab deployed at 280 m depth at GANT-20-07b (62°08’45.31’’S, 58°28’51.52’’W) and surface sediment sample N°8 was taken from the piston core N°8. Surface sediment samples (6×5×1 cm3) were obtained from the most superficial part of the collected sediment (either grab or piston core). All samples were packed in plastic bags, conserved at room temperature (14°C), delivered to the Henryk Arctowski Polish Antarctic Station and shipped to Poland by the end of February 2020.
Sample extraction and processing.—The core sediment (mainly fine-grained silts, silty clay sands and sandy clay silts) was sampled every 10 cm to extract disassociated sponge spicules. The sediment was first treated with 30% solution of hydrogen peroxide (H2O2) to remove organic matter. If the sediment was too muddy, causing clumping of sediment particles, additional treatment with softener Extran MA 02 was performed to remove the finest sediment particles. The residue was then washed and dried. From the dry sediment, the representative sponge spicules were handpicked under a binocular microscope NIKON ZM7 under magnification ×40. The picked spicules were then hand-mounted on Scanning Electron Microscope stubs, covered with platinum and photographed under the SEM Philips XL-20 at the Institute of Paleobiology, Polish Academy of Sciences.
Taxonomic assignment of dissociated spicules.—The obtained spicules had been assigned to sponge taxa noted from the South Shetland Islands with a special emphasis on the Admiralty Bay species based on the literature. When no match was found among sponges noted in the vicinity, more distant Antarctic and sub-Antarctic sponge communities were taken under consideration. The sponge spicules had been assigned to a single taxon whenever their morphology was diagnostic enough to assign them to a definite taxon with a high confidence. This was done when the analyzed spicules were identical in terms of morphology and size to spicules of living sponge taxa. However, there were cases when the morphology of the analyzed spicules corresponded with more than one sponge taxon. In one case the spicules were assigned to a genus with two possible species match (Hymedesmia [Hymedesmia] croftsae or H. [H.] gaussiana), but for the purposes of analysis we consider this taxon as Hymedesmia (Hymedesmia) croftsae. In other cases, when the morphology of the analyzed spicules was too general or corresponded with more than two sponge taxa, bulk categories were established. Additionally, some spicules of triaxial symmetry difficult to assign were broadly identified as hexactinellid spicules (see SOM: table S1, Supplementary Online Material available at http://app.pan.pl/SOM/app70-Lukowiak_etal_SOM.pdf). However, these categories were excluded from the analysis due to their ambiguity and difficulty in the interpretation of the results. The studied material is deposited in the Institute of Paleobiology, Polish Academy of Sciences under the number ZPAL Pf. 33.
Sediment dating.—The dating of the examined core samples was not possible, due to the disturbance of the samples. For this reason the approximate age of the samples was ascertained based on the previous studies that dealt with the dating of the Admiralty Bay sediments considering 137Cs and 210Pb radionuclides (Nascimento et al. 2009). The sedimentation rate was estimated as ranging from 0.11±0.01 cm y-1 to 0.46±0.05 cm y-1 (0.35 cm y-1 on average; for more details see Nascimento et al. 2009). Based on this data, our cores were estimated as to cover at least the last 400 years for the shorter core (C8) and 966 years for the longer core (C4). For more details see SOM: table S2.
Statistical analysis.—Differences in species composition between the cores were tested using a permutational multivariate analysis of variance (PERMANOVA) based on a presence-absence data and using Jaccard similarity matrix considering different data sets: (i) grouping different depths from each core considering all subsamples (i.e., core N°4 from 0 to 388 cm and core N°8 from 0 to 140 cm); (ii) grouping different range depths (cores N°4 and N°8) down to 140 cm; (iii) and grouping different subsamples within each core by 2, 3, 4, 5, 6, and 7 subsamples. Differences in species composition among cores were assessed in a Non-metric Multidimensional Scaling (n-MDS) analyses. The Jaccard similarity matrix was also used for cluster analysis to determine similarities among within each core independently. Analyses were performed in R (R Core Team 2018) using vegan package (Oksanen et al. 2018) and all graphs were plotted with ggplot2 package (Wickham 2016) or using basic R commands (see SOM for R code).
Results
Spicule assignments.—Basing on the dissociated spicules we have recorded in total the presence of 27 sponge taxa in the sediment samples, from which twenty-four have been assigned to species level (Figs. 2, 3, and Table 1). In core 4 (C4) which was richer in sponge taxa recognized, 26 taxa were recorded, and in core 8 (C8), only 15. In turn, from 15 taxa recorded in C8, only one was absent in C4 (Table 1).
Table 1. Sponge taxa list recognized from area of Admiralty Bay or other nearest area. * species that do not produce mineral skeleton; ** Alvaro Arteaga, Philippe Willenz, Eduardo Hajdu, Bernabé Moreno, Natalia Venturini, Luis Cerpa, Luis Quipuzcoa, and Báslavi Cóndor-Luján; symbols: + present, – absent.
|
Species/species association |
Admiralty Bay records |
Other nearest area of
occurrence (if absent |
Literature |
||
|
Sediment (C4) |
Sediment (C8) |
Living (literature data) |
|||
|
Anoxycalyx (Scolymastra) joubini |
+ |
– |
+ |
|
|
|
Iophon unicorne |
+ |
+ |
+ |
|
|
|
Isodictya sp. |
+ |
+ |
+ |
|
|
|
Sphaerotylus antarcticus |
+ |
+ |
+ |
|
|
|
Tethyopsis longispina |
+ |
+ |
+ |
|
|
|
Tedania charcoti |
+ |
– |
+ |
|
|
|
Rossella (Tedaniopsis) sp. |
+ |
+ |
+ |
|
|
|
Acanthorhabdus fragilis |
+ |
+ |
– |
South Shetland Islands (Clarence Island) |
|
|
Antarctotetilla leptoderma |
+ |
– |
– |
South Shetland Islands |
|
|
Cinachyra antarctica |
+ |
+ |
– |
South Shetland Islands |
|
|
Cinachyra barbata |
+ |
+ |
– |
Maxwell Bay |
|
|
Chondrocladia (Chondrocladia) antarctica |
+ |
+ |
– |
Antarctica East Abyssal Province |
|
|
Guitarra dendyi |
+ |
+ |
– |
South Shetland Islands |
|
|
Iophon terranovae |
+ |
+ |
– |
Antarctic Peninsula |
|
|
Mycale (Aegogropila) denticulata |
– |
+ |
– |
Terra Nova Bay |
|
|
Myxodoryx hanitschi |
+ |
– |
– |
Antarctic Peninsula |
|
|
Nullarbora penniformis |
+ |
+ |
– |
Eastern Weddell Sea |
|
|
Polymastia invaginata |
+ |
+ |
– |
Maxwell Bay |
|
|
Rhizaxinella strongylata |
+ |
+ |
– |
Chile |
|
|
Clathria (Axosuberites) parva |
+ |
– |
– |
SW Africa |
|
|
Hymedesmia (H.) croftsae / gaussiana |
+ |
– |
– |
Falklands, Antarctic Peninsula |
|
|
Hymerhabdia imperfecta |
+ |
– |
– |
Chile |
|
|
Iophon hesperidesi |
+ |
– |
– |
South Shetland Islands |
|
|
Siphonochalina fortis |
+ |
– |
– |
Falklands |
|
|
Sphaerotylus capitatus |
+ |
– |
– |
Kapp Norvegia (Weddell Sea) |
Arntz et al. 1990 (as Sphaerotylus schoenus) |
|
Tedania (Tedaniopsis) sarai |
+ |
– |
– |
Argentine Sea |
|
|
Tedania sp. |
+ |
– |
– |
South Shetland Islands |
|
|
Clathria (Axosuberites) flabellata |
– |
– |
+ |
|
|
|
Haliclona (Soestella) chilensis |
– |
– |
+ |
|
|
|
Haliclona sp. |
– |
– |
+ |
|
|
|
Homaxinella balfourensis |
– |
– |
+ |
|
|
|
Hymeniacidon sp. |
– |
– |
+ |
|
|
|
Iophon cf. unicorne |
– |
– |
+ |
|
|
|
Iophon sp. |
– |
– |
+ |
|
|
|
Latrunculia (L.) biformis |
– |
– |
+ |
|
|
|
Mycale (Oxymycale) acerata |
– |
– |
+ |
|
Arteaga et al.** (unpublished data) |
|
Myxilla (Burtonanchora) lissostyla |
– |
– |
+ |
|
|
|
Mycale sp. |
– |
– |
+ |
|
|
|
Phorbas domini |
– |
– |
+ |
|
|
|
Phorbas cf. domini |
– |
– |
+ |
|
|
|
Rossella podagrosa |
– |
– |
+ |
|
|
|
Rossella cf. racovitzae |
– |
– |
+ |
|
|
|
Tedania (Tedaniopsis) tenuicapitata |
– |
– |
+ |
|
|
|
Dendrilla antarctica* |
– |
– |
+ |
South Shetland Islands |
|
|
In total |
26 taxa |
15 taxa |
24 taxa |
|
|
|
in total 27 different taxa |
|||||
The most common species observed in both cores and along all the depths were Iophon unicorne (24 and 9 for C4 and C8, respectively), Cinachyra antarctica (18 and 7 records, respectively), and C. barbata (16 and 8 records, respectively). Less common were Isodictya sp. (14 and 4 records, respectively), Iophon hesperidesi (12 in C4 only), and Acanthorhabdus fragilis (8 and 4 records, respectively). Only single appearances of Tedania (Tedaniopsis) sarai, Tedania (Tedaniopsis) charcoti, Hymedesmia (Hymedesmia) croftsae, Clathria (Axosuberites) parva, and Siphonochalina fortis were recorded. For more details see SOM: table S1.
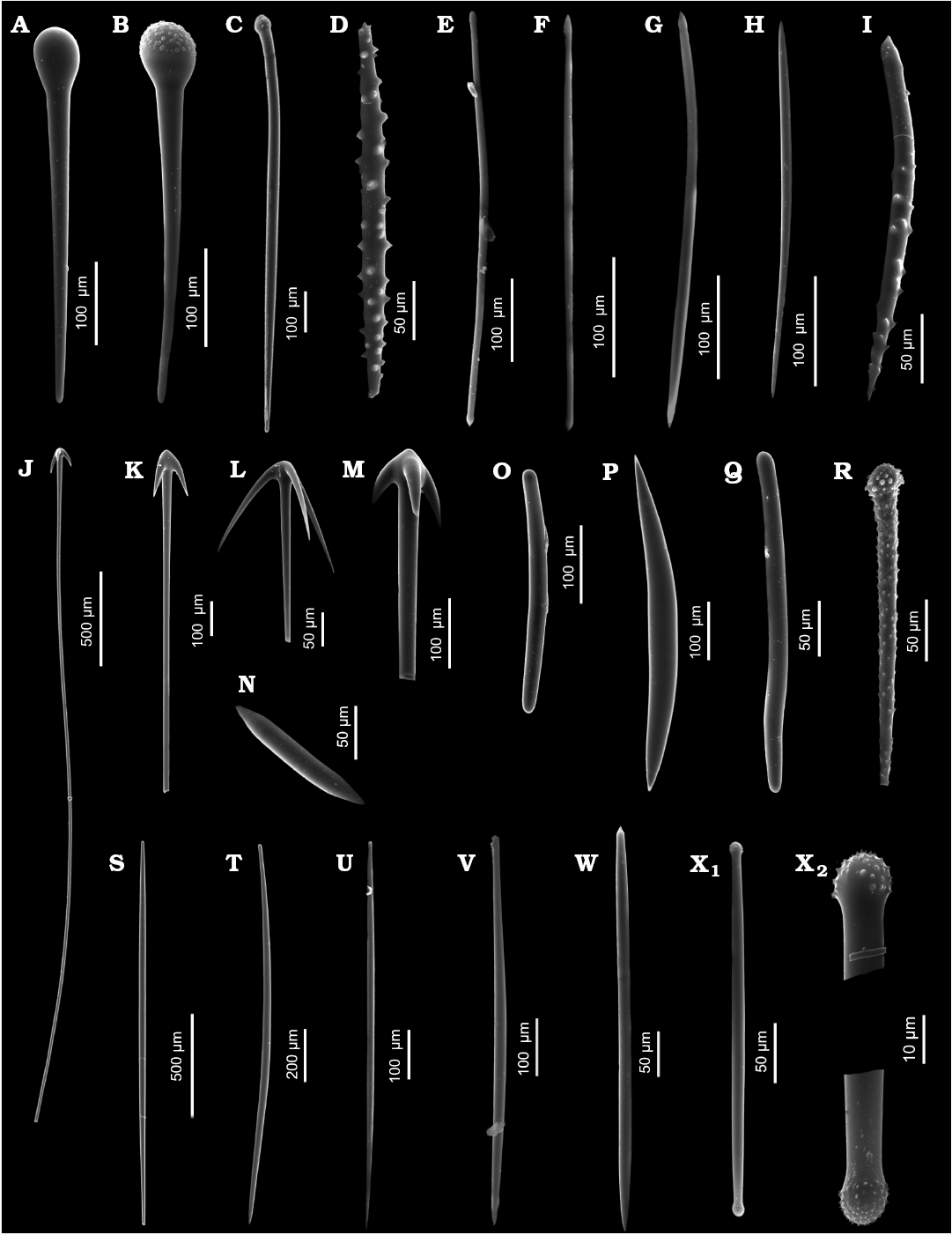
Fig. 2. Sediment sponge spicules belonging to sponges that have never been noted from area of Admiralty Bay: A, B. Exotyles of Guitarra dendyi (Kirkpatrick, 1907). A. ZPAL Pf. 33/4060-27. B. ZPAL Pf. 33/410-17. C. Spherostyle of Sphaerotylus capitatus (Vosmaer, 1885), ZPAL Pf. 33/4280S-66. D. Acanthorhabd of Acanthorhabdus fragilis Burton, 1929, ZPAL Pf. 33/4080-37. E. Tornote of Tedania sp., ZPAL Pf. 33/4080-33. F. Tornote of Tedania (Tedaniopsis) sarai Bertolino et al., 2007, ZPAL Pf. 33/4280S-46. G. Tornote of Tedania (Tedaniopsis) charcoti Topsent, 1907, ZPAL Pf. 33/4060-18. H. Anisoxea of Clathria (Axosuberites) parva Lévi, 1963, ZPAL Pf. 33/C4310-14. I. Acanthostyle of Myxodoryx hanitschi (Kirkpatrick, 1907b), ZPAL Pf. 33/4060-35. J, K. Anatriaenes of Cinachyra antarctica (Carter, 1872). J. ZPAL Pf. 33/4150-23. K. ZPAL Pf. 33/C4200-05. L. Anatriaene of Antarctotetilla leptoderma (Sollas, 1886), ZPAL Pf. 33/4160-03. M. Anatriaene of Cinachyra barbata Sollas, 1886, ZPAL Pf. 33/4030-27. N. Short oxea of Siphonochalina fortis Ridley, 1881, ZPAL Pf. 33/4080-20. O. Strongyle of Rhizaxinella strongylata Bertolino, Costa, & Pansini in Bertolino et al., 2020, ZPAL Pf. 33/4190-46. P. Anisoxea of Cinachyrella barbata Sollas, 1886, ZPAL Pf. 33/410-16. Q. Strongyloxea of Hymerhabdia imperfecta Bertolino, Costa, & Pansini in Bertolino et al., 2020, ZPAL Pf. 33/4080-13. R. Acanthostyle of Hymedesmia (Hymedesmia) croftsae Goodwin et al., 2016 / gausiana Hentschel, 1914, ZPAL Pf. 33/4280S-10. S. Style of Chondrocladia (Chondrocladia) antarctica Hentschel, 1914, ZPAL Pf. 33/4210-07. T. Style of Polymastia invaginata Kirkpatrick, 1907b, ZPAL Pf. 33/4050-22. U. Style of Nullarbora penniformis (Göcke & Janussen, 2013a), ZPAL Pf. 33/4280S-23. V. Mycalostyle of Mycale (Aegogropila) denticulata Bertolino et al., 2009, ZPAL Pf. 33/4080-34. W. Style of Iophon terranovae Calcinai & Pansini, 2000, ZPAL Pf. 33/4050-14. X. Tylote (X1) and details (X2) of Iophon hesperidesi Rios et al., 2004, ZPAL Pf. 33/4150-42.
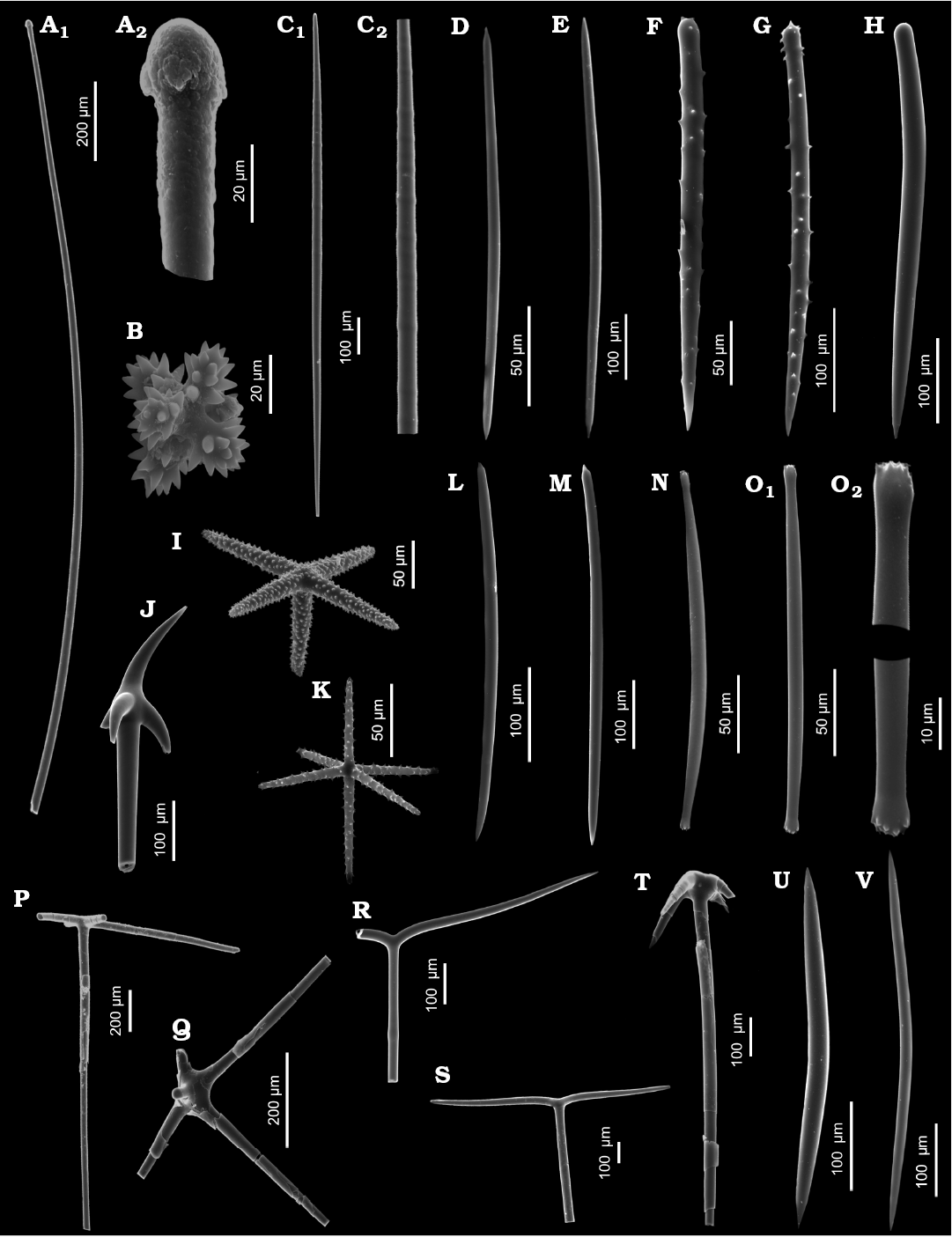
Fig. 3. Sediment sponge spicules that belong to species that were already noted from the area of Admiralty Bay. A. Exotyle (A1) and details (A2) of Sphaerotylus antarcticus Kirkpatrick, 1907b, ZPAL Pf. 33/4170-17. B. Dermal hexactine of Anoxycalyx (Scolymastra) joubini (Topsent, 1916), ZPAL Pf. 33/2503-03. C. Polytylote style (C1) and details (C2) of Sphaerotylus antarcticus Kirkpatrick, 1907b, ZPAL Pf. 33/4130-38. D. Oxea of Haliclona (Gellius) tylotoxa (Hentschel, 1914)–Pyloderma latrunculioides (Ridley & Dendy, 1886)–Mycale (Oxymycale) acerata Kirkpatrick, 1907b–Pseudosuberites montiniger (Carter, 1880) bulk group, ZPAL Pf. 33/4210-18. E. Anisoxea of Pyloderma latrunculioides (Ridley & Dendy, 1886)–Haliclona (Gellius) tylotoxa (Hentschel, 1914)–Mycale (Oxymycale) acerata Kirkpatrick, 1907b–Pseudosuberites montiniger (Carter, 1880) bulk group, ZPAL Pf. 33/4310-07. F, G. Acanthostyles of unknown origin. F. ZPAL Pf. 33/4280S-45. G. ZPAL Pf. 33/4280S-02. H. Style of Clathria (Axosuberites) flabellata (Topsent, 1916)–Myxilla (Burtonanchora) lissostyla Burton, 1938–Myxilla (Burtonanchora) asigmata (Topsent, 1901b)–Megaciella annecten bulk group (Ridley & Dendy, 1886), ZPAL Pf. 33/4050-26. I. Micropentactine of Rossella sp., ZPAL Pf. 33/4150-33. J. Hypodermal pentactine of Rossella sp., ZPAL Pf. 33/4020-08. K. Hexactine of Rossella sp., ZPAL Pf. 33/4150-34. L–O. Spicules of Iophon unicorne Topsent, 1907. L. ZPAL Pf. 33/4030-04. M. ZPAL Pf. 33/2503-06. N. ZPAL Pf. 33/4060-31. O. ZPAL Pf. 33/4150-46 (O1) and details (O2). P. Dermal pentactines of Anoxycalyx (Scolymastra) joubini (Topsent, 1916) or Rossella sp., ZPAL Pf. 33/4180-18. Q. Hypodermal pentactine of Rossella sp., ZPAL Pf. 33/450-11. R, S. Orthodiaenes of Tethyopsis longispina (Lendenfeld, 1907). R. ZPAL Pf. 33/104-02. S. ZPAL Pf. 33/4120-12). T. Pentactine of Rossella sp., ZPAL Pf. 33/4050-13. U. Isoxea of Isodictya sp., ZPAL Pf. 33/160-03. V. Isoxea of Isodictya sp., ZPAL Pf. 33/4280S-42.
Sponge community stability over time.—Core N°4 (C4): The sediment core, extending to a depth of approximately 340 cm below the sea floor and spanning about 966 years up to around 1054 CE, encompasses a total of 34 samples, with an additional sample collected from the surface sediment dredge (Fig. 4). The most diverse sample, boasting the recognition of 12 sponge taxa, is the surface sample (C4-0). Following closely are the samples from ~229 and 171 years ago (C4-80 and C4-60, respectively) with respectively 10 and 9 identified species. The samples dating back to around 86 (C4-30) and 600 years ago (C4-210), showed a slightly lower diversity with 7 recognized species. Four samples, from before ~343, 429, 486, and 886 years ago (C4-120, -150, -170, and -310, respectively) contained six recognized taxa. Meanwhile, the samples from ~114, 314, 457, 514, 686 and 966 years ago (C4-40, -110, -160, -180, -240, and -338, respectively) captured five taxa. Four taxa were noted in 7 samples, originating from ~29, 57, 200, 371, 571, 600, and 771 years ago (C4-10, -20, -70, -130, -200, -220, and -270, respectively), while three taxa were identified in five samples dating back to ~257, 286, 714, 800, and 857 years ago (C4-90, -100, -250, -280, and -300, respectively). The remaining samples exhibited a diversity of only two taxa (C4-140, -190, -230, -290, -300, -320, respectively), and two others consisted of a single taxon (C4-260 and -330).
In this core the intervals from 966 to 629 years ago (338 to 220 cm of depth), and from 571 to 257 years ago (200 to 90 cm of depth), are quite uniform with 3.2 and 4.25 taxa respectively recognized on average with the highest record of 7 recognized taxa (in the sample from the depth of 210 cm) in between these two intervals. Starting from 229 years ago (C4-80), with the second highest record of ten taxa recognized, the average number of recognized taxa reaches 6.88 up to the end of the core. The highest record of 12 taxa is observed in the surface sample. For more details see Fig. 4 (orange).
Core N°8 (C8): The sediment core, extending approximately 140 cm and encompassing the last 400 years up to around 1620 CE, yielded a total of 14 samples, complemented by an additional sample extracted from the surface sediment dredge (Fig. 4). Among these samples, the most diverse (with 7 identified sponge taxa) was C8-80, dating back around 229 years ago. Preceding this, the samples from ~343 (C8-120) and 286 (C8-100) years ago revealed the presence of six recognized species, while those dated as ~314 and 400 years old (C8-110 and -140) exhibited five taxa. Notably, samples dating to ~200, 257, and 371 years ago (C8-70, -90, and -130, respectively) were characterized by four identified species. In contrast, the remaining samples demonstrated lower richness in recognized sponge taxa, with three taxa identified in the surface sample and the one from ~114, 143, and 171 years ago (C8-40, -50, and -60, respectively), and a solitary taxon recognized in the samples from ~29 (C8-10) and 86 (C8-30) years ago. Interestingly, the sample from around 57 years ago (C8-20) did not contain any identifiable sponge spicules.
The general trend shows a quite uniform number of taxa recognized from the oldest sample (earlier than ~400 years ago) to the sample from 257 years ago with 5 taxa recognized on average. Then, 229 years ago, a maximum of recognized sponge taxa (7) is observed in this core and drops to a single taxon recognized in the sample from ~86 years ago. After the sample with no spicules recognized (~57 years ago), there is an increase of the number of recognized taxa to three in the surface sample. For more details see Fig. 4 (blue).
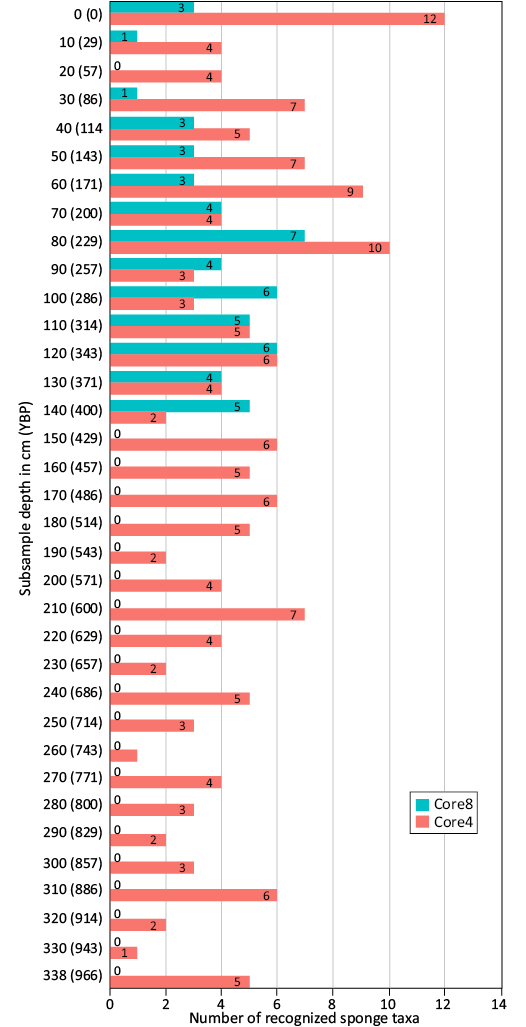
Fig 4. Sponge species richness (richness expressed in number of species) by depth [expressed in cm and estimated years before present (YBP)].
Core similarities and differences.—Cores samples displayed some differences. The PERMANOVA showed no significant differences between the cores when considering all the subsamples of both cores (p = 0.0744) but it was significant when only including the subsamples down to 140 cm (the same depth for both cores, p = 0.0207). A subtle differentiation was also observed in the nMDS analyses, considering both data sets (Fig. 5A, B). However, no significant difference was detected in the PERMANOVA when testing groups composed of different number of subsamples (2, 3, 4, 5, 6, and 7) within each core (SOM: table S3). Moreover, no visible pattern was recovered in the cluster analyses (Fig. 6).
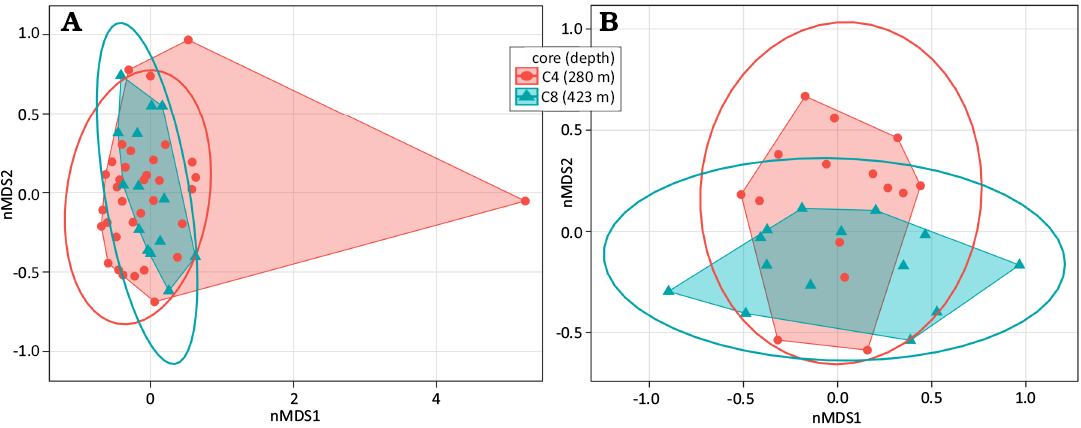
Fig. 5. nMDS considering subsamples from both cores (A) and both cores until 140 cm (B). Stress for A = 0.1775289, B = 0.2059217.
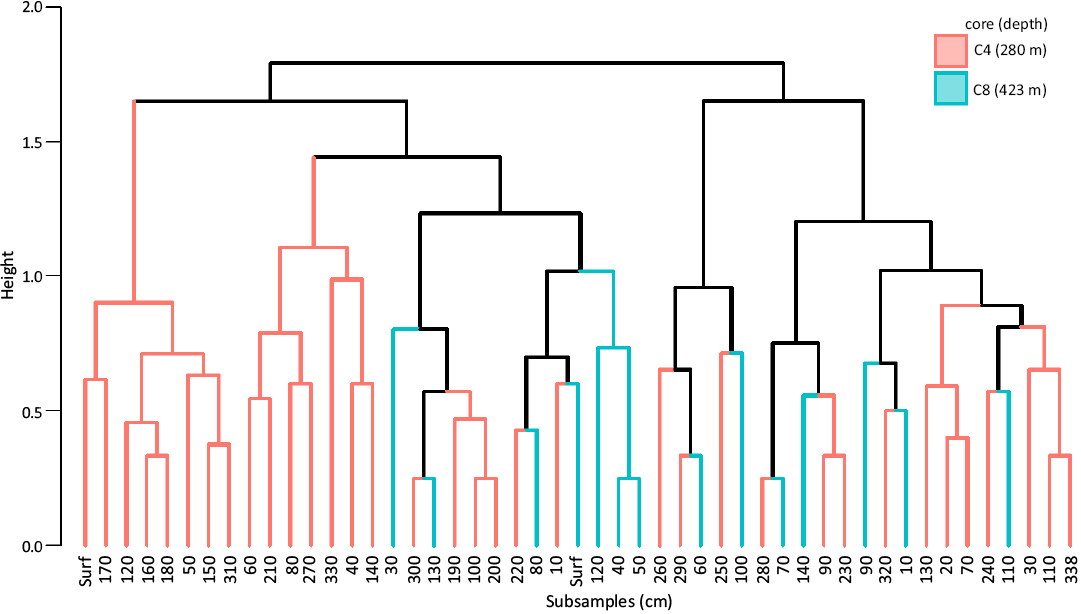
Fig. 6. Dendrogram from cluster analysis based on the Ward method (ward.d2) using Jaccard distance matrix. Subsamples from cores C4 (orange) and C8 (green) were considered. Surf, sediment surface.
Modern vs. sub-fossil sponge community.—The core data was merged to get a fuller picture of sponge communities of the Admiralty Bay area. Both cores differ not only in depth of sampling, but also in sub-fossil record history, and that is why they were treated not as replicates but one merged dataset.
Out of the 27 taxa identified in the sediment cores, only seven overlap with the currently recognized taxa in the Admiralty Bay area (Table 1). Of the remaining 20 taxa, 8 are currently documented in nearby regions like the South Shetland Islands (6 taxa) and Maxwell Bay (2 taxa). Conversely, 12 taxa are presently found outside the South Shetland Islands, spanning regions such as the Antarctic Peninsula (1), Antarctica East Abyssal Province (1), Terra Nova Bay (2), Weddell Sea (2), Argentine Sea (1), SW Africa (1), Falkland Islands (2), and Chile (2).
In the Admiralty Bay surveys of Recent sponges, 24 sponge taxa were recorded; intriguingly, 17 of these were not identified in the sediment samples (Table 1). However, when taxa which do not produce mineral skeleton (Dendrilla antarctica) or which are assigned in the surveys as “confer” (Iophon cf. unicorne, Phorbas cf. domini, Rossella cf. racovitzae), or identified only to generic level (Haliclona sp., Hymeniacidon sp., Iophon sp., Mycale sp., Tedania sp.) are excluded from the analysis, only nine taxa living on this area today [Clathria (Axosuberites) flabellata, Homaxinella balfourensis, Haliclona (Soestella) chilensis, Latrunculia (L.) biformis, Mycale (Oxymycale) acerata, Myxilla (Burtonanchora) lissostyla, Phorbas domini, Rossella podagrosa, and Tedania (Tedaniopsis) tenuicapitata], are not recognized in the fossil record (Table 1).
Sponge fauna fluctuations.—While there isn’t a clear pattern in the distribution of taxa along the cores, both cores exhibit quite a consistent trend in the changes of recognized taxa over time intervals in the intervals where data for both cores exists, e.g., from 10 cm of depth to 140 cm. However, in these intervals the average number of taxa in core 8 is approximately 3.71 while in core 4 it is 4.38. This might be due to different water depths the cores were retrieved from (423 m for core 8 vs. 282 m for core 4). In the strata where both cores data overlap, the number of recognized species in the core 4 vary from two to 10, while in the core 8 from zero (57 years ago) to seven (229 years ago). This decline in the number of recognized sponge taxa in the core 8 and a gradual recovery with 3 sponge taxa recorded in the surface sediments, could be attributed to various factors influencing coastal benthic communities. This could have resulted from a brief, one-time event or disturbance that either disrupted the living community or removed the spicular record from the sediment. Potential causes for disturbances in Antarctic benthic fauna include iceberg grounding during the summer, and iceberg ripping the seabed benthos in winter, as well as factors like high wind and wave action, hypoxia/anoxia, volcanism, temperature stress, freshwater inundation, localized pollution, and ultraviolet (UV) radiation (Barnes and Conlan 2007). Given the absence of similar changes in the spicular record in the second core, it is presumed that this disturbance had a highly localized rather than a global character, unrelated to general climatic events like temperature stress, pollution, freshwater inundation, or volcanism. Trawling was ruled out due to the absence of data indicating its occurrence in this area. Wave action was also excluded from consideration, as its influence is limited to shallow depths, extending only to approximately 20 meters (Barnes and Conlan 2007). However, the direct impact of ice, particularly iceberg activity, can reshape sediment in waters as deep as 550 meters (Barnes and Lien 1988; Dowdeswell et al. 1993). The biological consequences of such ice-related disturbances include the loss of benthic biomass, alterations in abundance and diversity patterns, changes in community structure and function (Gutt 2001; Gutt et al. 2015) and shifts in population structure (Peck and Bullough 1993; Brown et al. 2004). Hypoxia (or anoxia), e.g., by brine or macroalgae accumulation, is also considered a potential factor, especially in ice-covered bays and fjords where water circulation is confined by a narrow neck or sill (Kvitek et al. 1998; Bromberg et al. 2000).
Differences between the taxonomic composition of the living and the fossil sponge communities.—There can be several potential reasons for the fact that among 27 sponge taxa recognized in the sediments, 8 taxa are not recorded in the Admiralty Bay nor in the vicinity (South Shetland Islands and Maxwell Bay) today. The first reason could be insufficient sampling during the faunistic studies of this area. Many of these sponges could have been overlooked and/or are difficult to sample due to their fragileness (like in case of e.g., Acanthorhabdus fragilis, Nullarbora penniformis), or encrusting nature [like in case of e.g., Hymerhabdia imperfecta and Hymedesmia (Hymedesmia) croftsae/gaussiana]. The other specimens might have been overlooked because they are characterized by a small habitus (like e.g., Rhizaxinella strongylata, Chondrocladia [Chondrocladia] antarctica, and Sphaerotylus capitatus). The oversight problem of small, cryptic and excavating sponge taxa in faunistic studies has already been discussed by Łukowiak (2016) who described four sponge species unnoted so far from a shallow water reef of Bocas del Toro, Panama, by means of sediment dissociated spicules. Moreover, on the list of sponges recognized in the sediments, there are some species that were described relatively lately as new, e.g., Hymerhabdia imperfecta, Rhizaxinella strongylata (recognized from Chilean coastal waters by Bertolino et al. 2020). The comparatively recent recognition of these species might be the reason why they have not been identified from the areas other than the type locality yet.
The next reason taken under consideration when comparing discrepancies in sponge taxa recorded in sediments and in surveys is the transport of disassociated spicules. The archipelago of the South Shetland Islands extends for approximately 500 km in a southwest/northeast direction and lies on the southern edge of the Drake Passage (Stein and Rakusa-Suszczewski 1983). Admiralty Bay is situated at inward shores of King George Island, which lies near the center of the South Shetland Islands and is characterized by a two-phase flow system typical for fiords (Pruszak 1980). Within the Bay, the surface currents outflow the water from Admiralty Bay into Bransfield Strait and only the winds blowing from SW-W can cause an inflow of surface waters into Admiralty Bay (Pruszak 1980). The waters are carried into the Bay by the tide through the lower part of the profile from the Bransfield Strait. The direction of the movements of water masses in the Bransfield Strait is probably north-eastwards (Toporkov 1973). These waters are a part of the East Wind Drift turning southwards before reaching Drake Passage and moving along the South Shetland Islands (Pruszak 1980). The spicules of sponge species which have not been noted living in this area could have been transported by the currents from the surroundings, however a long-distance transport, namely form Chilean waters or Falkland Islands, is very unlikely. On the other hand, according to biogeographical analyses performed by Downey et al. (2012), there is a strong link of the Southern American sponge fauna with the Antarctic one. There is a minimal chance that the spicules found in the most superficial strata were carried by the scientific vessels coming from Punta Arenas (Chile) or Ushuaia (Argentina) which are the mandatory stops for Antarctic research vessels from (at least) Brazil and Peru, while heading to the Admiralty Bay. This could be the case of Hymerhabdia spicules which had been found in the surface sediment. The other taxa e.g., Rhizaxinella strongylata, Clathria parva, and Siphonochalina fortis were noted in the deeper intervals (from 80 to 310 cm), what makes the transport of spicules by vessels impossible. Also, the current changes in the sponge community composition over time need to be taken under consideration. This might be the case of Iophon terranovae, Myxodoryx hanitschi, Mycale (Aegogropila) denticulata, Tedania (Tedaniopsis) sarai, and Siphonochalina fortis which tend to have rather massive or big habitus so their overlooking during the faunistic studies is rather unlikely.
The other factor that might play a role in the sampling bias can be the patchiness of the sponge fauna (Gutt and Koltun 1995) that may inhabit regions depending on many ecological parameters such as, among others, turbidity (Zea 1994), water flow (Bell and Barnes 2003), nutrient concentrations (Wilkinson and Cheshire 1989; Campos et al. 2013), substratum type (Bell and Barnes 2000; Powell et al. 2010) and predation (Waddell and Pawlik 2000). These factors might have changed over time causing the dispersal of sponge faunas to other, more suitable areas; the selection of wrong or too few sampling areas for the faunistic studies could have also played a role in not capturing the full variety of sponge fauna of this region.
The lack of recognition of some sponge taxa in the sediments may be caused by various reasons, but the main seems to be the difficulty in the correct identification of morphologically simple spicule types. From among five species that seem not to have fossil record in the sediments, four [Haliclona (Soestella) chilensis, Homaxinella balfourensis, Latrunculia (L.) biformis and Tedania (Tedaniopsis) tenuicapitata] produce morphologically simple spicules, namely oxeas or styles which are difficult to identify and assign. Admittedly, in the last two cases, styles are accompanied by other, more characteristic, spicule types i.e., the microscleres discasters in L. (L.) biformis, and tornotes in T. (T.) tenuicapitata. However, due to their size, microscleres are generally overlooked or tend not to preserve in the fossil record (MŁ unpublished data). Moreover, the tips of the tornotes can be easily altered during the diagenetic processes and thus, their recognition can be obscured.
Conclusions
For the first time, the core samples from the Admiralty Bay were employed to reveal the historical changes of sponge communities spanning one thousand years, offering a valuable comparison with the present-day living sponge fauna in the area. Surprisingly, despite the absence of specific trends or patterns in the sponge spicule record over the past millennium, both cores, especially in overlapping depths, displayed a consistent trend in the changes of recognized taxa across various time intervals.
A single event of the decline of spicular record that has been documented in one of the cores (C8) that was retrieved from the greater depth could possibly be related to local hypoxia event or iceberg activity, but more replicates from nearby areas are needed to test this hypothesis.
Intriguingly, upon comparing the sediment spicular record with the contemporary sponge fauna surveyed in the area, the sedimentological record proved to be richer, showcasing three more sponge taxa retrieved from the sediments compared to the observed Recent fauna. Conversely, among species documented in the surveys, at least nine were not recognized in the fossil state. The disparities between the fossil record and the list of sponge taxa currently present in the studied area could be potentially explained by various factors influencing the sedimentary record, including misassignment of simple morphologically spicule types, possible post-mortem transport of spicules, faunal changes, and the patchiness of the modern sponge fauna. On the other hand, factors influencing the living sponge fauna include insufficient recognition of the diverse Recent sponge species in this region and the oversight of small, cryptic, and fragile sponge species during faunistic studies.
Acknowledgements
We acknowledge Wai Long Ng-Cutipa (Geological Survey of Spain, Madrid, Spain and Instituto Geológico, Minero y Metalúrgico, Lima, Peru) for helping us with the subsampling of the cores. We would like to thank Viviana Frisone (Museo di Archeologia e Scienze Naturali “G. Zannato”, Vicenza, Italy), Michelle Kelly (NIWA, Wellington, New Zealand), and a two anonymous reviewer whose suggestions helped to considerably improve this manuscript. This study was developed under the framework of the ORCA Project “Evolución del Volcanismo Submarino en el Estrecho de Bransfield: Relación de las Emanaciones Hidrotermales con la Biodiversidad y el Cambio Climático”, lead by Instituto Geológico Minero y Metalúrgico, in collaboration with Universidad Científica del Sur (Lima, Perú) (Project N°050-2019-PRO99). We are indebted to the Peruvian Antarctic National Program from the Ministry of Foreign Affairs of Peru as well as the Peruvian Navy for the logistical support during the Peruvian Expedition ANTAR XXVI and XXVII. We thank the Norwegian Polar Institute for providing the free-to-use Quantarctica 3.2v package, and the SCAR Antarctic Digital Database (ADD) (http://www.scar.org/add).
Editor: Andrzej Kaim.
References
Angulo-Preckler, C., Figuerola, B., Núñez-Pons, L., Moles, J., Martín-Martín, R., Rull-Lluch, J., Gómez-Garreta, de A., and Avila, C. 2018. Macrobenthic patterns at the shallow marine waters in the caldera of the active volcano of Deception Island. Antarctica. Continental Shelf Research 157: 20–31 Crossref
Arntz, W., Ernst, W., and Hempel I. 1990. The Expedition Antarktis V1114 (Epos leg 3) and Vlll.5 of RV ‘Polarstern’ in 1989. Berichte zur Polarforschung 68: 214.
Barnes, D.K.A. and Conlan, K.E. 2007. Disturbance, colonization and development of Antarctic benthic communities. Philosophical Transactions of the Royal Society B 362: 11–38. Crossref
Barnes, P.W. and Lien, R. 1988. Icebergs rework shelf sediments to 500 m off Antarctica. Geology 16: 1130–1133. Crossref
Barthel, D., Tendal, O., and Panzer, K. 1990. Ecology and taxonomy of sponges in the eastern Weddell Sea shelf and slope communities. In: W. Arntz, W. Ernst, and I. Hempel (eds.), The Expedition Antarktis VII/4 (EPOS Leg 3) and VII/5 of RV “Polarstern” in 1989. Reports on Polar Research 90: 120–130.
Bell, J.J. and Barnes, D.K.A. 2000. The distribution and prevalence of sponges in relation to environmental gradients within a temperate sea lough: inclined cliff surfaces. Diversity and Distribution 6: 305–323. Crossref
Bell, J.J. and Barnes, D.K.A. 2003. Effect of disturbance on assemblages: an example using Porifera. The Biological Bulletin 205: 144–159. Crossref
Bertolino, M., Calcinai, B., and Pansini, M. 2009. Two new species of Poecilosclerida (Porifera, Demospongiae) from Terra Nova Bay (Antarctic Sea). Journal of the Marine Biological Association of the United Kingdom 89: 1671–1679. Crossref
Bertolino, M., Calcinai, B., Cattaneo-Vietti, R., Cerrano, C., Lafratta, A., Pansini, M., Pica, D., and Bavestrello, G. 2014. Stability of the sponge assemblage of Mediterranean coralligenous concretions along a millennial time span. Marine Ecology 35: 149–158. Crossref
Bertolino, M., Costa, G., Bavestrello, G., Pansini, M., and Daneri, G. 2020. New sponge species from Seno Magdalena, Puyuhuapi Fjord and Jacaf Canal (Chile). European Journal of Taxonomy 715: 1–49. Crossref
Bertolino, M., Costa, G., Bavestrello, G., Pansini, M., and Daneri, G. 2020. New sponge species from Seno Magdalena, Puyuhuapi Fjord and Jacaf Canal (Chile). European Journal of Taxonomy 715: 1–49. Crossref
Bertolino, M., Costa, G., Carella, M., Cattaneo-Vietti, R., Cerrano, C., Pansini, M., Quarta, G, Calcagnil, L., and Bavestrello, G. 2017. The dynamics of a Mediterranean coralligenous sponge assemblage at decennial and millennial temporal scales. Plos One 12 (5): e0177945. Crossref
Bertolino, M., Schejter, L., Calcinai, B., Cerrano, C., and Bremec, C. 2007. Sponges from a submarine canyon of the Argentine Sea. In: M.R. Custódio, G. Lôbo-Hajdu, E. Hajdu, and G. Muricy (eds.), Porifera Research. Biodiversity, Innovation and Sustainability, 189–201. Livros de Museu Nacional 28, Rio de Janeiro.
Bromberg, S., Nonato, E.F., Corbisier, T.N., and Petti, M.A.V. 2000. Polychaete distribution in the near-shore zone of Martel Inlet, Admiralty Bay (King George Island, Antarctica). Bulletin of Marine Science 67: 175–188.
Brown, K.M., Fraser, KP., Barnes, D.K.A., and Peck, L.S. 2004. Links between the structure of an Antarctic shallow water community and ice-scour frequency. Oecologia 141: 121–129. Crossref
Burton, M. 1929. Porifera. Part II. Antarctic sponges. British Antarctic (“Terra-Nova”) Expedition, 1910. British Museum Natural History Reports (Zoology) 6: 393–458.
Burton, M. 1930. Report on a collection of sponges from South Georgia and from Campbell Island, South Pacific, obtained by Dr. Kohl-Larsen. Senckenbergiana 12: 331–335.
Burton, M. 1932. Sponges. Discovery Reports 6: 237–392. Crossref
Burton, M. 1934. Sponges. Further Zoological Results of the Swedish Antarctic Expedition 1901–1903 3: 1–58.
Burton, M. 1938. Non-calcareous sponges. Scientific Reports of the Australasian Antarctic Expedition, 1911–1914 (C. Zoology and Botany). 9 (3): 5–22.
Calcinai, B. and Pansini, M. 2000. Four new demosponge species from Terra Nova Bay (Ross Sea, Antarctica). Zoosystema 22: 369–381.
Campos, L.S., Barboza, C.A.M., Bassoi, M., Bernardes, M., Bromberg, S., Corbisier, T., Fontes, R.C., Gheller, P.F., Hajdu, E., Kawall, H.G., Lange, P.K., Lanna, A.M., Lavrado, H.P., Monteiro, G.C.S., Montone, R., Morales, T., Moura, R.B., Nakayama, C.R., Oackes, T., Paranhos, R., Passos, F.D., Petti, M.A.V., Pellizari, V.H., Rezende, C.E., Rodrigues, M., Rosa, L.H., Secchi, E., Tenenbaum, D.R., and Yoneshigue-Valentin, Y. 2013. Environmental processes, biodiversity and changes in Admiralty Bay, King George Island, Antarctica. In: C. Verde and G. Di Prisco (eds.), Adaptation and Evolution in Marine Environments— The Impact of Global Change on Biodiversity, Vol. 2, 127–156. Series “From Pole to Pole”, Springer-Verlag, Berlin. Crossref
Campos, M., Mothes, B., and Veitenheimer Mendes, I.L. 2007a. Antarctic sponges (Porifera, Demospongiae) of the South Shetland Islands and vicinity. Part II. Poecilosclerida. Revista Brasileira De Zoologia 24: 742–770. Crossref
Campos, M., Mothes, B., and Veitenheimer Mendes, I.L. 2007b. Esponjas Antárticas (Porifera, Demospongiae) das Ilhas Shetland do Sul e áreas próximas. Parte I. Spirophorida, Astrophorida, Hadromerida, Halichondrida e Haplosclerida. Revista Brasileira De Zoologia 24: 687–708. Crossref
Candido, J.L., Volkmer-Ribeiro, C., Filho, F.L.S., Turcq, B.J., Desjardins, T., and Chauvel, A. 2000. Microsclere variations of Dosilia pydanieli (Porifera, Spongillidae) in Caracaranã Lake (Roraima-Brazil): Palaeoenvironmental implications. Biociências 2: 77–92.
Carter, H.J. 1872. On two new sponges from the Antarctic Sea, and on a new species of Tethya from Shetland; together with observations on the reproduction of sponges commencing from zygosis of the sponge animal. Annals and Magazine of Natural History, Ser 4 9: 409–435. Crossref
Carter, H.J. 1880. Description of two new sponges. In: W.S.M. d’Urban (ed.), The Zoology of Barents Sea. Annals and Magazine of Natural History, Serie 5 6: 253–277. Crossref
Carter, H.J. 1877. Antarctic and subantarctic sponges. The Annals and Magazine of Natural History, Ser 4 20: 38–42. Crossref
Cattaneo-Vietti, R., Bavestrello, G., Cerrano, C., Gaino, E., Mazzella, L., Pansini, M., and Sarà, M. 2000. The role of sponges in the Terra Nova Bay ecosystem. In: F.M. Faranda, L. Guglielmo, and A. Ianora (eds.), Ross Sea Ecology, 539–549. Springer Verlag, Berlin. Crossref
Cárdenas, C.A., Newcombe, E.M., Hajdu, E., Gonzalez-Aravena, M., Geange, S.W., and Bell, J.J. 2016. Sponge richness on algae-dominated rocky reefs in the western Antarctic Peninsula and the Magellan Strait. Polar Research 25: 30532. Crossref
Desqueyroux, R. 1975. Esponjas (Porifera) de la region antartica chilena. Cahiers de Biologie Marine 16: 47–82.
Dowdeswell, J.A., Villinger, H., Whittington, R.J., and Marienfeld, P. 1993. Iceberg scouring in Scoresby Sund and on the East Greenland continental shelf. Marine Geology 111: 37–53. Crossref
Downey, R.V., Griffiths, H.J., Linse, K., and Janussen, D. 2012. Diversity and distribution patterns in high southern latitude sponges. PLoS ONE 7 (7): e41672. Crossref
Fernández, A.A., Lemiña, N.A., and Schejter, L. 2021. Sponges (Porifera: Demospongiae) recorded at the South Shetland Islands and near the Antarctic Peninsula during the Argentinian Summer Antarctic Expedition in 2012. Biology and Life Sciences Forum 2: 38. Crossref
Goodwin, C., Berman, J., and Hendry, K. 2019. Demosponges from the sublittoral and shallow-circalittoral (<24 m depth) Antarctic Peninsula with a description of four new species and notes on in situ identification characteristics. Zootaxa 4658: 461–508. Crossref
Goodwin, C., Brewin, P., and Brickle, P. 2012. Sponge biodiversity of South Georgia island with descriptions of fifteen new species. Zootaxa 3542: 1–48. Crossref
Goodwin, C., Jones, J., Neely, K., and Brickle, P. 2011. Sponge biodiversity of the Jason Islands and Stanley, Falkland Islands with descriptions of twelve new species. Journal of the Marine Biological Association of the UK 91: 275–301. Crossref
Goodwin, C., Jones, J., Neely, K., and Brickle, P. 2014. Sponge biodiversity of Beauchêne and the Sea Lion Islands and south-east East Falkland, Falkland Islands, with a description of nine new species. Journal of the Marine Biological Association of the United Kingdom 96: 263–290. Crossref
Goodwin, C., Jones, J., Neely, K., and Brickle, P. 2016. Sponge biodiversity of Beauchêne and the Sea Lion Islands and south-east East Falkland, Falkland Islands, with a description of nine new species. In: C.H.L. Schönberg, J. Fromont, N.A. Hooper, S. Sorokin, W. Zhang, and N. de Voogd (eds.), New Frontiers in Sponge Science. Journal of the Marine Biological Association of the United Kingdom 96: 263–290. Crossref
Gordon, A.L. and Nowlin, W.D.J. 1978. The basin waters of the Bransfield Strait. Journal of Physical Oceanography 8: 258–264. Crossref
Göcke, C. and Janussen, D. 2013a. Demospongiae of ANT XXIV/2 (SYSTCO I) Expedition—Antarctic Eastern Weddell Sea. Zootaxa 3692: 28–101. Crossref
Göcke, C. and Janussen, D. 2013b. Hexactinellida of the genus Rossella, of ANT XXIV/2 (SYSTCO I) Expedition—Antarctic Eastern Weddell Sea. Zootaxa 3692: 102–122. Crossref
Gutt, J. 2001. On the direct impact of ice on marine benthic communities, a review. Polar Biology 24: 553–564. Crossref
Gutt, J. and Koltun, V. 1995. Sponges of the Lazarev and the Weddell Sea, Antarctica: explanations for their patchy occurrence. Antarctic Science 7: 227–234. Crossref
Gutt, J., Cummings, V., Dayton, P., Isla, E., Jentsch, A., and Schiaparelli, S. 2015. Antarctic Marine Animal Forests: Three-Dimensional Communities in Southern Ocean Ecosystems. In: S. Rossi, L. Bramanti, A. Gori, and C. Orejas Saco del Valle (eds.), Marine Animal Forests, 1–30. Springer, Cham. Crossref
Hajdu, E., Fonseca, C., Schories, D., and Kohlberg, G. 2016. Sponges, Porifera. In: D. Schories and G. Kohlberg (eds.), Marine Wildlife, King George Island, Antarctica: Identification Guide, 56–78. Dirk Schories publications, Rostock.
Hamilton, P.B., Poulin, M., and Yang, J.-R. 1997. A new diatom genus Porannulus (Bacillariophyta), associated with marine sponges around King George Island, South Shetland Islands, Antarctica. Diatom Research 12: 229–242. Crossref
Hendry, K.R., Leng, M.J., Robinson, L.F., Sloane, H.J., Blusztjan, J., Rickaby, R.E.M., Georg R.B., and Halliday, A.N. 2010. Silicon isotopes in Antarctic sponges: an interlaboratory comparison. Antarctic Science 23: 34–42. Crossref
Hentschel, E. 1914. Monaxone Kieselschwämme und Hornschwämme der deutschen Südpolar Expedition 1901–1903. Deutschen Südpolar Expedition Zoology 7: 37–141.
Janussen, D. and Reiswig, H.M. 2009. Hexactinellida (Porifera) from the ANDEEP III Expedition to the Weddell Sea, Antarctica. Zootaxa 2136: 1–20. Crossref
Kersken, D., Feldmeyer, B., and Janussen, D. 2016. Sponge communities of the Antarctic Peninsula: influence of environmental variables on species composition and richness. Polar Biology 39: 851. Crossref
Kirkpatrick, R. 1907a. Porifera, Hexactinellida. National Antarctic Expedition (S.S. ‘Discovery’) 1901–1904. Natural History 3: 1–25. Crossref
Kirkpatrick, R. 1907b. Preliminary report on the Monaxonellida of the National Antarctic Expedition. Annals and Magazine of Natural History Series 7 20: 271–291.
Kirkpatrick, R. 1908. Porifera (Sponges). II. Tetraxonida, Dendy. National Antarctic Expedition, 1901–1904. Natural History 4: 1–56.
Koltun, V.M. 1964. Sponges of the Antarctic I. Tetraxonida and Cornacuspongida [in Russian]. In: E.P. Pavilovskii, A.P. Andriyashev, and P.V. Ushakov (eds.), Biologičeskie otčety Sovetskoj Antarktičeskoj ekspedicii (1955–1958), Vol. 2, 6–133. Akademâ Nauk SSSR, Moskva.
Koltun, V.M. 1969. Porifera. Antarctic Map Folio Series 11: 13–14.
Koltun, V.M. 1976. Porifera. Part 1: Antarctic Sponges. Report B.A.N.Z. Antarctic Research Expedition 1929–1931, B (Zoology and Botany) 5: 163–198.
Kvitek, R.G., Conlan, K.E., and Iamietro, P.J. 1998. Black pools of death: hypoxic, brine-filled ice gouge depressions become lethal traps for benthic organisms in a shallow Arctic embayment. Marine Ecology Progress Series 162: 1–10. Crossref
Lendenfeld, R. von. 1907. Tetraxonia der Deutschen Südpolar-Expedition 1901–1903. In: E. Von Drygalski (ed.), Deutsche Südpolar-Expedition 1901–1903 9. Zoologie 1: 303–342.
Lévi, C. 1963. Spongiaires d’Afrique du Sud. (1) Poecilosclérides. Transactions of the Royal Society of South Africa 37 (1): 1–72. Crossref
Łukowiak, M. 2016. Spicular analysis of surficial sediments as a supplementary tool for studies of modern sponge communities. Helgoland Marine Research 70: 5. Crossref
Łukowiak, M. 2020. Utilizing sponge spicules in taxonomic, ecological and environmental reconstructions: a review. PeerJ 8: e10601. Crossref
Łukowiak, M., Cramer, K.L., Madzia, D., Hynes, M.G., Norris, R.D., and O’Dea, A. 2018. Historical change in a Caribbean reef sponge community and long-term loss of sponge predators. Marine Ecology Progress Series 601: 127–137. Crossref
Maldonado, M., López-Acosta, M., Sitjà, C., García-Puig, M., Galobart, C., Ercilla, G., and Leynaert, A. 2019. Sponge skeletons as an important sink of silicon in the global oceans. Nature Geoscience 12: 815–822. Crossref
McClintock, J.B., Amsler, C.D., Baker, B.J., and van Soest, R.W.M. 2005. Ecology of Antarctic marine sponges: an overview. Integrative and Comparative Biology 45: 359–668. Crossref
Nascimento, M.G. do, de Castro, M.C., Figueira, R.C.L., Montone, R.C., Mahiques, M.M. de, and Tessler, M.G. 2009. Application of 137Cs and 210Pb radionuclides to determine sedimentation rates of recent sediments from Admiralty Bay, Antarctica Peninsula. International Nuclear Atlantic Conference—INAC 2009 Rio de Janeiro, RJ, Brazil, September 27 to October 2, 2009. Associação Brasileira De Energia Nuclear, Rio de Janeiro.
Nascimento, M.G. do, Figueira, R.C.L., Vendrame, A.C, Montone, R.C., Mahiques, M.M. de, Tessler, M.G., and Martins, C. 2009. Application of 137Cs and 210Pb radionuclides to determine sedimentation rates of recent sediments from admiraty Bay, Antarctica Peninsula. Associacao Brasileira de Odontologia Rio de Janeiro (ABORJ).
Oksanen, J., Blanchet, F., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P., O’Hara, R.B., Simpson, G., Solymos, P., Stevens, M., Szoecs, E., and Wagner, H. 2018. Vegan: Community Ecology Package. R package version 2.5-3 [available online, https://CRAN.R-project.org/package=vegan].
Paduano, G.M. and Fell, P.E. 1997. Spatial and temporal distribution of freshwater sponges in Connecticut lakes based upon analysis of siliceous spicules in dated sediment cores. Hydrobiologia 350: 105–121. Crossref
Peck, L.S. and Bullough, L.W. 1993 Growth and population structure in the infaunal bivalve Yoldia eightsi in relation to iceberg activity at Signy Island. Antarctic Marine Biology 117: 235–241. Crossref
Pisera, A. 1997. Hexactinellid sponges of the Admiralty Bay, King George Island, Antarctica. In: P. Głowacki (ed.), Proceedings of the 24th Polar Symposium, Polish Polar Studies, 203–205. Warsaw.
Plotkin, A.S. and Janussen, D. 2008. Polymastiidae and Suberitidae (Porifera: Demospongiae: Hadromerida) of the deep Weddell Sea, Antarctic. Zootaxa 1866: 95–135. Crossref
Powell, A.L., Hepburn, L.J., Smith, D.J., and Bell, J.J. 2010. Patterns of sponge abundance across a gradient of habitat quality in the Wakatobi Marine National Park, Indonesia. The Open Marine Biology Journal 4: 31–38. Crossref
Pruszak, Z. 1980. Currents circulation in the waters of Admiralty Bay (region of Arctowski. Station on King George Island). Polar Research 1: 55–74.
Rasbold, G.G., Stevaux, J.C., Parolin, M., Leli, I.T., Luz, L.D., Guerreiro, R.L., and Brito, H.D. 2019. Sponge spicules as indicators of paleoenvironmental changes in island deposits—Upper Paraná River, Brazil. Palaeogeography, Palaeoclimatology, Palaeoecology 536: 109391. Crossref
Ridley, S.O. 1881. Spongida collected during the Expedition of H.M.S Alert in the Straits of Magellan and on the coast of Patagonia. Proceedings of the Zoological Society of London 1881: 107–139.
Ridley, S.O. and Dendy, A. 1886. Preliminary report on the Monaxonida collected by H.M.S. Challenger. Part I. Annals and Magazine of Natural History 18: 325–351, 470–493. Crossref
Ríos, P. 2007. Esponjas del Orden Poecilosclerida de las campañas españolas de bentos antártico. 527 pp. Ph.D. Thesis, Universidade De Santiago De Compostela, Santiago De Compostela.
Ríos, P. and Cristobo, F.J. 2007a. A new species of Phorbas (Porifera: Poecilosclerida) from the Bellingshausen Sea, Antarctica. Journal of the Marine Biological Association of the United Kingdom 87: 1485–1490. Crossref
Ríos, P. and Cristobo, F.J. 2007b. Sponges of genus Myxilla Schmidt, 1862, collected in Antarctic waters by Spanish Antarctic expeditions. In: M. Custódio, G. Lôbo-Hajdu, E. Hajdu, and G. Muricy (eds.), Porifera Research: Biodiversity, Innovation and Sustainability, 525–546. Série Livros 28. Universidad Nacional de Rio de Janeiro, Rio de Janeiro.
Ríos, P. and Cristobo, J. 2014. Antarctic Porifera database from the Spanish benthic expeditions. ZooKeys 401: 1–10. Crossref
Ríos, P., Cristobo, F., and Urgorri, V. 2004. Poecilosclerida (Porifera, Demospongiae) collected by the Spanish Antarctic expedition BENTART-94. Cahiers de Biologie Marine 45: 97–119.
Sarà, M., Balduzzi, A., Barbieri, M., Bvestrello, G., and Burlando, B. 1992. Biogeographic traits and checklist of Antarctic demosponges. Polar Biology 12: 559–585. Crossref
Schejter, L., Calcinai, B., Cerrano, C., Bertolino, M., Pansini M., Giberto, D., and Bremec, C. 2006. Porifera from the Argentina Sea: diversity in Patagonian scallop beds. Italian Journal of Zoology 73: 373–385. Crossref
Schejter, L., Cristobo, J., and Ríos, P. 2019. Coelosphaera (Coelosphaera) koltuni sp. nov. (Porifera: Demospongiae): a new species from South Orkney Islands, Antarctica. Marine Biodiversity 49: 1987–1996. Crossref
Schejter L., Cristobo, J., and Ríos, P. 2024. New records of demosponges (Porifera) from the South Orkney Islands (Antarctica) with a checklist for the region. Zootaxa 5403: 401–430. Crossref
Siciński, J., Jażdżewski, K. De Broyer, C., Presler, P., Ligowski, R., Nonato, E.F., Corbisier, T.N., Petti, M.A.V., Brito, T.A.S., Lavrado, H.P., Błażewicz-Paszkowycz, M., Pabis, K., Jażdżewska, A., and Campos, L.S. 2011. Admiralty Bay benthos diversity—a census of a complex polar ecosystem. Deep Sea Research Part II 58: 30–48. Crossref
Sollas, W.J. 1886. Preliminary account of the Tetractinellid sponges Dredged by H.M.S. ‘Challenger’ 1872-76. Part I. The Choristida. Scientific Proceedings of the Royal Dublin Society (new series) 5: 177–199.
Sollas, W.J. 1888. Report on the Tetractinellida collected by H.M.S. Challenger, during the years 1873–1876. Report on the scientific results of the voyage of H.M.S. Challenger during the years 1873–76. Zoology 25 (part 63): 1–458.
Stein, M. and Rakusa-Suszczewski, S. 1983. Geostrophic currents in the South Shetlands Islands area during FIBEX. Memoirs of National Institute of Polar Research Tokyo 27: 24–34.
Szafrański, Z. and Lipski, M. 1982. Characteristics of water temperature and salinity at Admiralty Bay (King George Island, South Shetland Islands, Antarctic) during the austral summer. Polish Polar Research 3: 7–24.
Tanita, S. 1959. Sponges. Biological Results of the Japanese Antarctic Research Expedition 1: 1–8. Crossref
Toporkov, L.G. 1973.Hydrochemical studies in the Bransfield Strait [in Russian]. Trudy Sovetskoj Antarktičeskoj Ekspedicji 56: 96–102.
Topsent, E. 1901a. Notice préliminaire sur les éponges recueuillies par l’Expédition Antarctique Belge. Archives de Zoologie expérimentale et générale 3: 5–16.
Topsent, E. 1901b. Spongiaires. Résultats du voyage du S.Y. ‘Belgica’en 1897-99 sous le commandement de A. de Gerlache de Gomery. Expédition antarctique belge. Zoologie 4: 1–54.
Topsent, E. 1907. Poecilosclérides nouvelles recueillies par le Français dans l’Antarctique. Bulletin du Muséum national d’histoire naturelle, Paris 13: 69–76. Crossref
Topsent, E. 1908. Spongiaires. In: L. Joubin, (ed.), Expédition antarctique française (1903–1905) commandée par le Dr. Jean Charcot, 1–37. Masson & Cie, Paris.
Topsent, E. 1913. Spongiaires de l’Expédition Antarctique Nationale Ecossaise. Transactions of the Royal Society of Edinburgh 49: 579–643. Crossref
Topsent, E. 1915. Spongiaires recueillis par la ‘Scotia’ dans l’Antarctique (1903–1904). Transactions of the Royal Society of Edinburgh 51: 35–43. Crossref
Topsent, E. 1916. Diagnoses d’éponges recueillies dans l’Antarctique par le “Pourquoi-pas?”. Bulletin du Muséum d’Histoire Naturelle Paris 3: 163–172.
Topsent, E. 1917. Spongiaires. In: L. Joubin, (ed.), Deuxième Expédition antarctique française (1908–1910) commandée par le Dr. Jean Charcot, Sciences Physiques: Documents Scientifiques, 1–88. Masson & Cie, Paris.
Uriz, M.J. 1988. Deep-water sponges from the continental shelf and slope off Namibia (Southwest Africa): Classses Hexactinellida and Demospongia. Monografías de Zoología Marina 3: 9–157.
Vieira, W.F., Hajdu, E., Lopes, D.A., Cárdenas, P., Newcombe, E., and Campos, L. 2010a. Porifera of Project MABIREH: new species and records of Acarnidae from King George Island, Antarctica. VIII World Sponge Conference, 356. Girona.
Vieira W.F., Hajdu, E., Lopes, D.A., Cárdenas, P., Newcombe, E., and Campos, L. 2010b. Taxonomy of sponges of IPY Project MABIREH (King George Island). World sponge conference, Girona, conference paper. XXXI SCAR Open Science Conference. Buenos Aires [available online, https://www.researchgate.net/publication/260789625].
Volkmer-Ribeiro, C., De Drago, E., and Parolin, M. 2007. Spicules of the freshwater sponge Ephydatia facunda indicate lagoonal paleoenvironment at the Pampas of Buenos Aires Province, Argentina. (Proceedings of the 9th International Coastal Symposium). Journal of Coastal Research SI 50: 449–452. Crossref
Vosmaer, G.C.J. 1885. The Sponges of the ‘Willem Barents’ Expedition 1880 and 1881. Bijdragen tot de Dierkunde 12 (3): 1–47. Crossref
Wickham, H. 2016. Ggplot2: Elegant Graphics for Data Analysis. 213 pp. Springer, New York. Crossref
Wilkinson, C.R. and Cheshire, A.C. 1989. Patterns in the distribution of sponge populations across the Central Great Barrier Reef. Coral Reefs 8: 127–134. Crossref
Zea, S. 1994. Patterns of coral and sponge abundance in stressed coral reefs at Santa Marta, Colombian Caribbean. In: R.W.M. Van Soest, T.M.G. Van Kempen, and J.C. Braekman (eds.), Sponges in Time and Space, 257–264. AA Balkema, Rotterdam.
Acta Palaeontol. Pol. 70 (1): 43–56, 2025
https://doi.org/10.4202/app.01166.2024