

First Iberian aspidothoracid megasecopteran insect and associated plants evidencing herbivory in a tropical Carboniferous forest from León, Spain
ARTAI A. SANTOS, ANTONIO HERNÁNDEZ-ORÚE, ENRIQUE PEÑALVER, STEPHEN MCLOUGHLIN, JOSÉ B. DIEZ, and ANDRÉ NEL
Santos, A.A. Hernández-Orúe, A., Peñalver, E., McLoughlin, S., Diez, J.B., and Nel, A. 2025. First Iberian aspidothoracid megasecopteran insect and associated plants evidencing herbivory in a tropical Carboniferous forest from León, Spain. Acta Palaeontologica Polonica 70 (1): 115–124.
We describe Aspidothorax hispanicus sp. nov. from Gzhelian, Pennsylvanian strata of León, Spain, representing the first occurrence of Aspidothoracidae in the Iberian Peninsula. This discovery expands the paleogeographical range of the family, previously known only from the Russian Federation and France. The new insect is preserved in close association with foliar remains of medullosan (Alethopteris zeilleri) and callistophytalean (Pseudomariopteris cordato-ovata) seed-ferns whose environmental preferences suggest that the new insect species inhabited humid tropical forests. The fossil leaves bear six types of damage, probably produced by insects, belonging to three functional feeding groups: margin feeding, hole feeding, and piercing and sucking. This diversity of interactions highlights varied feeding strategies, including chewing, piercing and sucking behaviors, evidencing a more complex range of herbivory in the area than previously known. The stylet mouthparts of Megasecoptera make these insects strong candidates for producing the piercing and sucking damage on the associated plants. The presence of dark patches and spots on the fossil wing, probably represents a camouflage strategy against predators, such as Palaeodictyoptera and other active hunters. The dark wing apex might also reflect the presence of sexual dimorphism or courtship behavior. This new assemblage of insects, in addition to plants and plant-insect interactions, contributes to a broader paleoecological understanding of the Carboniferous forests of the La Magdalena Coalfield.
Key words: Insecta, Megasecoptera, Paleoptera, plant-insect interactions, tropical paleoecosystem, Gzhelian, Carboniferous, León Province, Spain.
Artai A. Santos [artaisantos@gmail.com; ORCID: https://orcid.org/0000-0002-2399-8825 ] and Stephen McLoughlin [steve.mcloughlin@nrm.se; ORCID: https://orcid.org/0000-0001-6723-239X ], Department of Paleobiology, Swedish Museum of Natural History, Box 50007, S-104 05 Stockholm, Sweden.
Antonio Hernández-Orúe [anthernn@gmail.com; ORCID: https://orcid.org/0000-0002-6428-8889 ], Nanclares de la Oca, Álava, 01230, Spain.
Enrique Peñalver [e.penalver@igme.es; ORCID: https://orcid.org/0000-0001-8312-6087 ], Instituto Geológico y Minero de España (IGME), CSIC, C/Cirilo Amorós 42, 46004, Valencia, Spain.
José B. Diez [jbdiez@uvigo.es; ORCID: https://orcid.org/0000-0001-5739-7270 ], Departamento de Xeociencias Mariñas e Ordenación do Territorio, Facultade de Ciencias do Mar, Centro de Investigación Mariña, Universidade de Vigo (CIM-UVIGO), 36310 Vigo, Spain.
André Nel [andre.nel@mnhn.fr; ORCID: https://orcid.org/0000-0002-4241-7651 ], Institut Systématique Evolution Biodiversité (ISYEB), Museum National d’Histoire Naturelle, CNRS, Sorbonne Université, Université des Antilles, EPHE, 57 rue Cuvier, CP 50, Paris, 75005, France.
Received 26 August 2024, accepted 24 January 2025, published online 19 March 2025.
Copyright © 2025 A.A. Santos et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Megasecoptera, an order of insects within the superorder Palaeodictyopterida, is common in Carboniferous and Permian entomofaunas worldwide (Prokop et al. 2016; Pecharová et al. 2020). This superorder is distinguished by its piercing and sucking mouthparts, likely used for feeding on the inner tissues of various vascular plants (Kukalová-Peck 1991b; Labandeira and Phillips 1996; Engel et al. 2013; Pecharová et al. 2020). However, most research on Megasecoptera has focused on the taxonomy of fossils, leaving their paleoecology, paleohabitats, and feeding strategies largely unexplored. Therefore, clarifying both the taxonomy and lifestyles of these insects and their host plants (Santos et al. 2022, 2023, 2024; Garrouste et al. 2024) is crucial for resolving the paleoenvironments and paleoecological interactions in Carboniferous equatorial forests.
The rich Pennsylvanian (late Carboniferous) entomofaunas of the United Kingdom, Germany, and France are well known, in contrast to those of the Iberian Peninsula. However, recent investigations in Portugal and Spain have helped to fill this gap, with the descriptions of new specimens of Palaeodictyopterida (Palaeodictyoptera, Megasecoptera), Odonatoptera (Meganisoptera), Archaeorthoptera (Orthoptera, Caloneurodea), Dictyoptera and its sister order Paoliida (see Santos et al. 2023: table 1, and references therein). Recently described Iberian megasecopterans are the anchineurid Anchineura hispanica Carpenter, 1963, the corydaloidid Corydaloides leonensis Santos et al., 2023, and the mischopterid Mischoptera bergidensis Santos et al., 2023 (see Table 1 for details on previous Megasecoptera occurrences in the Iberian Peninsula).
Table 1. List of Carboniferous Megasecoptera recorded from the Iberian Peninsula; modified from Santos et al. (2023).
|
Family |
Species |
Age |
Locality |
References |
|
Anchineuridae |
Anchineura hispanica Carpenter, 1963 |
“Saberian”, Gzhelian, Late Pennsylvanian |
Garaño, La Magdalena Coalfield,León Province, Spain |
|
|
Corydaloididae |
Corydaloides leonensis Santos et al., 2023 |
Stephanian B, Gzhelian, Late Pennsylvanian |
Tremor de Arriba, El Bierzo Coalfield, León Province, Spain |
|
|
Mischopteridae |
Mischoptera bergidensis Santos et al., 2023 |
Stephanian B, Gzhelian, Late Pennsylvanian |
Santa Marina de Torre, El Bierzo Coalfield, León Province, Spain |
|
|
Aspidothoracidae |
Aspidothorax hispanicus sp. nov. |
“Saberian”, Gzhelian, Late Pennsylvanian |
Carmen mine, La Magdalena Coalfield, León Province, Spain |
this study |
Here, we describe the fourth Iberian representative of this order and the first record of Aspidothoracidae Handlirsch, 1919, from Spain, this family was previously known only from the Pennsylvanian of France and the Permian of the Russian Federation. We also describe and identify various plant fossils closely associated with this new megasecopteran. The plant fossils were also analyzed for plant-insect interactions which are identified and discussed in this work.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: urn:lsid:zoobank.org:pub:D50E4135-5AC0-4A32-8499-EF0085141FC3.
Institutional abbreviations.—MUPE, Museo Paleontológico de Elche, Elche, Alicante, Spain.
Other abbreviations.—C, costa; CuA: cubitus anterior; CuP, cubitus posterior; DTs, Damage Types; FFGs, Functional Feeding Groups; MA: median anterior; MP, median posterior; m-cua, basalmost crossvein between M and CuA; PCu, postcubital vein; RA, radius anterior; RP, radius posterior; rp-ma: basalmost crossvein between RP and MA.
Material and methods
The insect wing described herein, co-occurring with various plant fossils, was collected by José Luis Garrido (Elche, Alicante, Spain) close to the Carmen mine (Fig. 1), within the upper part of La Magdalena Coalfield succession (Castro 2005a). The stratigraphic position and paleoflora favor a “Saberian” (Gzhelian) age (Santos et al. 2023).
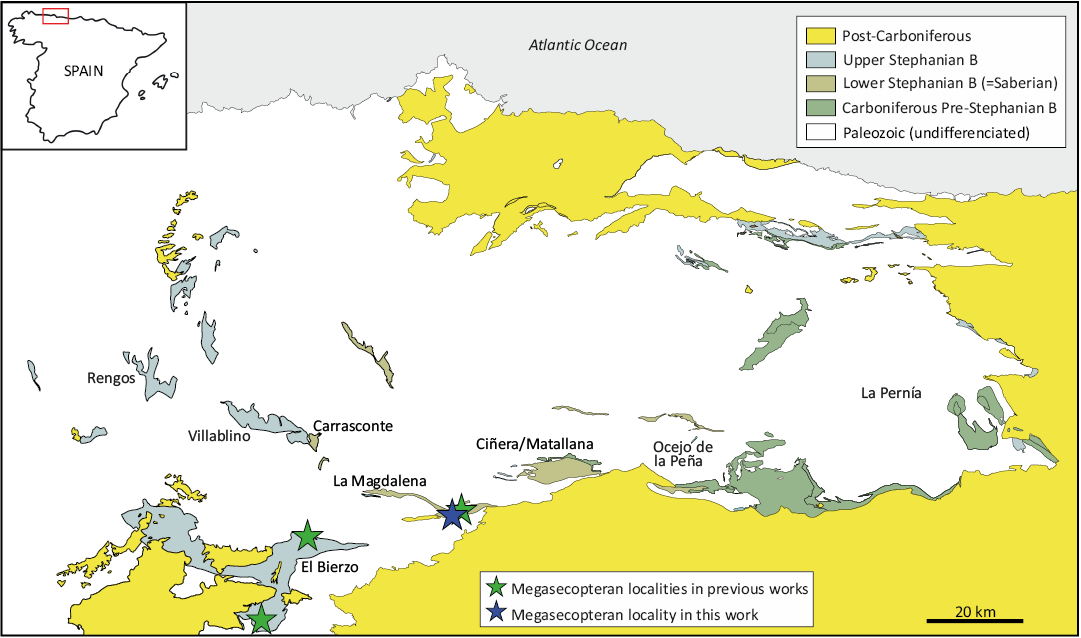
Fig. 1. Geological sketch map showing the La Magdalena Coalfield (León Province, NW Spain) and other coalfields previously yielding Carboniferous Megasecoptera in the northwestern Iberian Peninsula (see Table 1 for previous records).
Vein terminology for the insect wing (Fig. 2) follows that of Schubnel et al. (2020).
The fossil plants (Fig. 3) were identified using the extensive literature on Carboniferous floras from the region (Wagner 1968; Krings and Kerp 2000; Castro 2005b). We also analyzed the associated plant fossils for plant-arthropod interactions using the classification scheme developed by Labandeira et al. (2007) to categorize damage features. This framework classifies interactions on fossil leaves into various stereotypical morphotypes, referred to as Damage Types (DTs), each assigned an identifying number. Most DTs are associated with specific Functional Feeding Groups (FFGs). Furthermore, each DT is linked to a specific level of specialization, determined by its occurrence on various host plants (Labandeira et al. 2007).
The specimen is housed at the Museo Paleontológico de Elche (MUPE) provisionally labeled as MUPE-IWC-01.
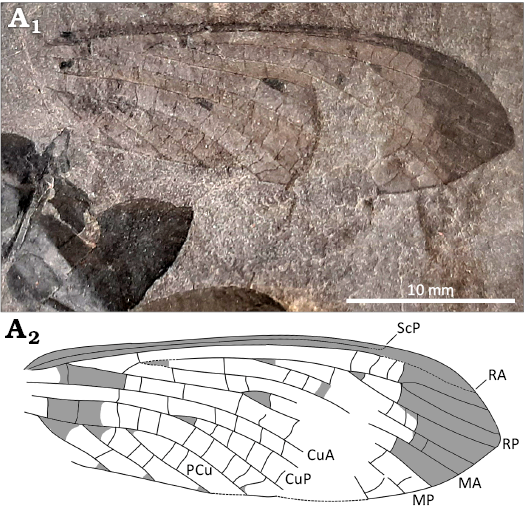
Fig. 2. Aspidothoracid megasecopteran insect Aspidothorax hispanicus sp. nov. holotype (MUPE-IWC-01) from mine heap near Carmen mine, La Magdalena Coalfield, León Province, Spain, “Saberian”: Gzhelian; A1, photography of the specimen, note fossils of the gymnosperm Pseudomariopteris cordato-ovata (Weiss, 1869) Gillespie et al., 1978 ex Krings et Kerp, 2000, with evidence of herbivory; A2, line drawing of wing venation.
Geological setting
La Magdalena Coalfield is located in northwestern Spain, in the north of León Province. This is one of the most studied post-Asturian coalfields in the Cantabrian region of the Iberian Peninsula and its paleoflora is one of the richest and best preserved in the district (Castro 2005a).
Based on plant fossils identified in previous studies, strata in La Magdalena Coalfield have been assigned to the Alethopteris zeilleri Megafloral Zone dated as “Saberian”, which in the northwestern Iberian Peninsula corresponds to the earliest part of the Gzhelian (Knight et al. 2023).
During the Gzhelian, the Cantabrian continental basins were located less than 5° south of the equator (Scotese and Golonka 1992). They were adjacent to the Paleotethys Sea and formed in the foreland of the Hercynian mountain range (Wagner and Castro 2011) during the peak of Southern Hemisphere glaciation at a time of stregthened latitudinal climatic and floristic gradients (McLoughlin 2001; Gastaldo et al. 2020). This region experienced a tropical, humid climate, which gradually became more arid as it transitioned into the Permian (Tabor and Poulsen 2008).
Strata within La Magdalena Coalfield have been subjected to facies analyses resulting in two broad interpretations of the depositional setting. Heward (1978) considered that mass flow deposits typical of alluvial fans were the most important facies in the succession. Heward (1978) differentiated conglomerates of proximal areas, sandy and conglomeratic lobes of middle fan regions, alluvial fan channels, associations of interlobes and interchannels, distal fans and lacustrine facies. Alternatively, Colmenero et al. (1996) argued that alluvial fan processes were restricted to areas flanking the mountains and breaches of some river levees, and that deposition occurred primarily in braided and meandering rivers. Bashforth et al. (2010) also interpreted most of the deposits as fluvial, but indicated that deposits in proximal areas derived from debris flows.
The studied sample was recovered from near the Carmen mine, from strata unassigned to a formal lithostratigraphic unit but corresponding to the uppermost parts of the La Magdalena succession (Castro 2005a). These beds were laid down during the most stable fluvial phases, with development of mire environments favoring the accumulation of coal.
Systematic palaeontology
Phylum Arthropoda Gravenhorst, 1843
Class Insecta Linnaeus, 1758
Superorder Palaeodictyopterida Bechly, 1996
Order Megasecoptera Brongniart, 1885
Family Aspidothoracidae Handlirsch, 1919
Genus Aspidothorax Brongniart, 1894
Type species: Aspidothorax triangularis Brongniart, 1894 (original designation) from Gzhelian (Pennsylvanian, Carboniferous) strata of Commentry, France.
Included species: Aspidothorax permianus Sinitshenkova & Aristov in Sinitshenkova et al., 2020, from Kazanian, Permian; Perm Region, Russian Federation, and Aspidothorax hispanicus sp. nov., from Gzhelian, Pennsylvanian, Carboniferous of Spain.
Aspidothorax hispanicus sp. nov.
Fig. 2.
Zoobank LSID: urn:lsid:zoobank.org:act:158B10CA-E6B4-40C1-8376 -6C724E66DB68.
Holotype: MUPE-IWC-01, well-preserved distal two-thirds of a wing co-preserved with Alethopteris zeilleri and Pseudomariopteris cordato-ovata plant fragments.
Type locality: Outcrop near Carmen mine, La Magdalena Coalfield, León Province, Spain (Fig. 1).
Type horizon: Un-named “Saberian”, Gzhelian, upper Carboniferous beds.
Diagnosis.—Wing characters only. RP with four branches; CuP and MP veins each with long fork; area between PCu and posterior wing margin broad, with five–six rows of cells in-between.
Description.—Wing hyaline except for darkened costal area, apex, and small spots at forks of RP, MP, and CuP; wing apex pointed; preserved part 29.6 mm long, wing 10.2 mm wide; costal area very narrow, 0.2 mm wide; ScP close to R/RA and terminating at C 8.5 mm from wing apex; R/RA weakly curved, with apical part of area between it and C slightly widened, with few weak and short crossveins; base of RP 22.5 mm from wing apex; RP posteriorly pectinate, with four main branches, all simple; base of M 26.9 mm basal to that of RP; base of MA 3.6 mm distally; RP and MA touching at one point near base of RP; MA simple, MP with two long branches; a strong crossvein m-cua between base of M and CuA; CuA simple; bases of CuA and CuP not preserved; CuP with two long branches; PCu posteriorly pectinate, with four branches preserved; five–six rows of cells between PCu and posterior margin of wing.
Remarks.—The new insect fossil is similar to the wings of megasecopterans belonging to Aspidothoracidae Handlirsch, 1919, and Bardohymenidae Zalessky, 1937. Owing to the lack of phylogenetic analyses of Megasecoptera, we compare the new fossil with all megasecopteran families.
Representatives of Protohymenidae Tillyard, 1924, have a very narrow area between C and RA and a very distinctive posterior branch of RA near its apex (Huang et al. 2021), unlike the new fossil. Mischopteridae Handlirsch, 1906, Foririidae Handlirsch, 1919, and Aspidohymenidae Martynov, 1930, have distinctive wing shapes, and the distal crossveins are arranged in several lines as in extant Chrysopidae (order Neuroptera). Representatives of Moravohymenidae Kukalová-Peck, 1972, have a broader area between RA and RP than the new fossil, and MA, MP and branches of RP slightly curve anteriorly in their distal portion. Anchineuridae Carpenter, 1963, Hanidae Kukalová-Peck, 1975, Caulopteridae Kukalová-Peck, 1975, Arcioneuridae Kukalová-Peck, 1975, Engisopteridae Kukalová-Peck, 1975, and Ancopteridae Kukalová-Peck, 1975, have many more veins and crossveins than the new fossil (Kukalová-Peck 1975, 1991a). Members of Alectoneuridae Kukalová-Peck 1975, have MA touching RP at only one point (Carpenter 1963; Kukalová-Peck 1975). Scytohymenidae Martynov, 1937, Aykhalidae Sinitshenkova, 1993, Sphecopteridae Carpenter, 1951, Brodiopteridae Carpenter, 1963, and Brodiidae Handlirsch, 1906, have a relatively simplified venation with very few crossveins (Carpenter 1992; Pecharová et al. 2015a; Prokop et al. 2017). Representatives of Xenopteraidae Ross et al. 2013 (replacement name for Xenopteridae Pinto, 1986) have a MA connected to RP (Pinto 1986; Pecharová et al. 2015b). Vorkutiidae (Vorkutia Rohdendorf, 1947, Siberiohymen Rohdendorf, 1961, and Fragmohymen Novokshonov, 1995) seem to constitute a composite group with taxa having varied venation styles, based on incomplete wings. Nevertheless, they all differ from the new fossil by the ScP ending in RA at the midwing (Rohdendorf 1947, 1961; Novokshonov 1995). Corydaloididae Handlirsch, 1906 (Corydaloides Brongniart, 1885), Sphecorydaloididae Pinto, 1994 (Sphecorydaloides Pinto, 1994) and Ischnoptilidae Carpenter, 1951 (Ischnoptilus Brongniart, 1894) have a MA that anastomoses with RP, and a CuA anastomosing with M for some distances, unlike in the new fossil.
The new fossil shares with Aspidothoracidae and Bardohymenidae the characters of vein MA not touching RP and CuA not touching M. Pecharová et al. (2020: 10) proposed the following emended diagnosis for Aspidothoracidae: “The fore and hindwings homonomous, venation nearly the same, wings narrowing gradually basally, not petiolate. The apex distinctly pointed. ScP terminates near RA. ScP and RA very close together, connected to costal margin; stem of M very close to, but separate from base of RA + RP; MA free from RP and not diverging towards it, connected by strong cross vein rp-ma; stem of Cu basally very close to M, but not connected to it; CuA not diverging towards MP, connected by a strong cross vein (m-cua); one anal vein A1 [PCu]. Cross veins numerous and nearly evenly distributed over the wing”. All the preserved characters of the new fossil conform well to this diagnosis, except for the presence of a strong crossvein rp-ma.
Pecharová et al. (2020: 2) proposed the following emended diagnosis for the Bardohymenidae: “Fore and hind wings subequal in length and shape (nearly homonomous), petiolate, similar in venation; CA+CP flattened and wide, running close to ScA+ScP (costal space narrow); ScP distinguishable as a separate vein only in proximal part of wing; distally continues along RA, RA connected with CA+CP and ScA+ScP except in the distal part of wing; RA diverging from CA+CP + ScA+ScP and forming an apical cell; RP originating at about midwing, gives rise to 2–5 branches, M lies close to RA+RP basally, but separate from it, diverging away from RA+RP in the second quarter of wing; M dividing into simple MA and MP near to the point at which RP separates from RA; MA connected to RP by a strong cross vein; Cu at the base connected with the stem of M; CuA connected to M by a strong cross vein, one long anal vein with posterior branches; cross veins usually arranged in 2 staggered rows”.
The new fossil shares the presence of more crossveins not arranged in two rows with Aspidothoracidae, in contrast to all taxa of Bardohymenidae. However, it shares the character of area between C and RA broadened apically and having some very short intervening crossveins with Bardynohymenidae. This broadening is similar to that of Aspidothorax triangularis Brongniart 1894, but is much weaker in the new fossil than in all Bardohymenidae (Pecharová et al. 2020: fig. 7). Thus, we assign the new fossil to Aspidothoracidae. Aspidothorax permianus differs from the new fossil in its simple CuP and MP vs with a long fork (Sinitshenkova et al. 2020). Aspidothorax triangularis differs from the new fossil in having a narrower area between PCu and the posterior wing margin, with one–two rows of intervening cells vs five–six (Carpenter 1992).
Stratigraphic and geographic range.—Known only from type locality and horizon.
Associated plants and plant-insect interactions
Preserved on the same bedding plane and almost overlapping the insect wing are several plant fossils (Fig. 3). The medullosan gymnosperm Alethopteris zeilleri Ragot, 1955 ex. Wagner, 1965 (Fig. 3A1, B) is widely distributed in Stephanian and lower Permian strata of tropical Laurasia (Wagner 1968; Zodrow 2007). The new specimens of A. zeilleri consist of typical alethopteroid pinnae with well-separated pinnules 7–22 mm long and of fairly constant (4–5 mm) width in the middle part of the pinnules. Pinnules are ovate to lorate, clearly confluent at the base and broadly attached to the rachis with subsidiary veins on both sides of the midvein (Fig. 3B). Pinnules are alternate with variable angles of insertion (40–80°) on the rachis. These specimens have a strong and distinctive midvein that extends about 4/5 of the pinnule length. Secondary veins are relatively dense (35–40 veins/cm measured), perpendicular to the pinnule margins.
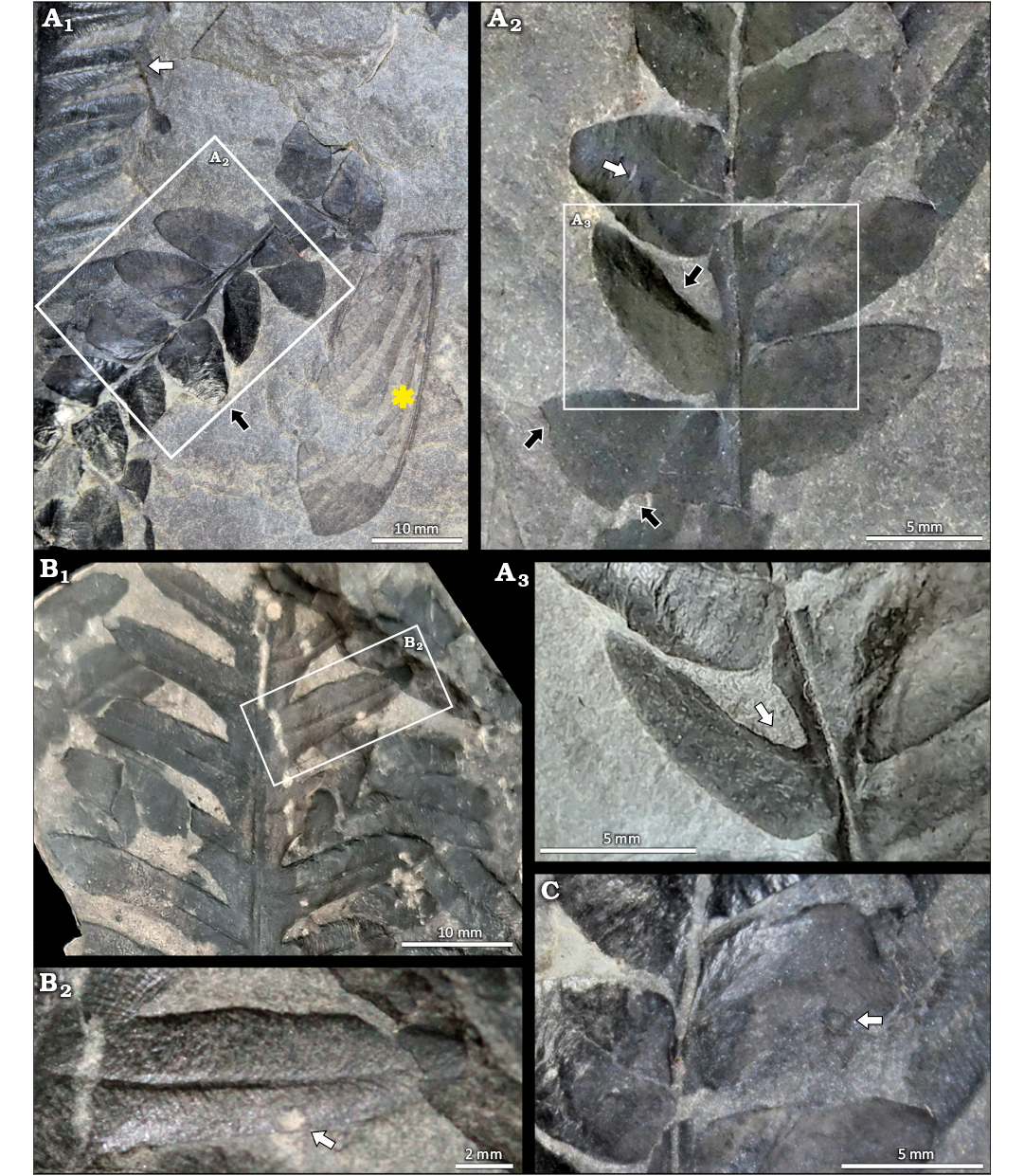
Fig. 3. Plant fossils associated with Aspidothorax hispanicus sp. nov. (MUPE-IWC-01, marked as yellow asterisk) from La Magdalena Coalfield, Spain; Gzhelian, upper Carboniferous. A. MUPE-IWC-01b; A1, evidence of medullosan Alethopteris zeilleri Ragot, 1955 ex. Wagner, 1965 (white arrow), and the callistophytalean Pseudomariopteris cordato-ovata (Weiss, 1869) Gillespie et al., 1978 emend. Krings and Kerp 2000 (black arrow), note that the white arrow also denotes insect damage (margin feeding DT013) in the apical part of a pinnule; A2, detail of P. cordato-ovata with evidence of plant-insect interactions, including hole feeding (DT007, white arrow) and margin feeding (DT012, black arrows); A3, detail showing margin feeding (DT012) with reaction rim on a P. cordato-ovata pinnule (white arrow). B. MUPE-IWC-01c; B1, specimen of A. zeilleri hosting hole feeding (DT001); B2, detail of the hole feeding (DT001) on a pinnule of A. zeilleri. C. MUPE-IWC-01d, detail of a pinnule of P. cordato-ovata, the white arrow shows piercing and sucking damage (DT046).
Some compressed pinnae fragments in this assemblage can be assigned confidently to the callistophytalean gymnosperm Pseudomariopteris cordato-ovata (Weiss, 1869) Gillespie et al. 1978 emend. Krings and Kerp 2000 (Figs. 2, 3A, C). Pseudomariopteris is common in late Carboniferous and early Permian floras of Europe and North America (Krings and Kerp 2000). Representatives of this genus have been interpreted to possess liana-like growth habits in forested areas (Krings and Kerp 2000). The new specimens have typical slightly asymmetrical oblong mariopterid pinnules that are 5–9 mm long, 4–5 mm wide, with entire margins, slightly pointed apices and a vaulted limb. Pinnules are inserted at 30–50° to the rachis, broadly attached, but slightly constricted at the base with the basiscopic side decurrent under the constriction and extending as a narrow flange down the rachis. The midvein is decurrent, extending about two-thirds of the pinnule length. The secondary veins are indistinct owing to poor preservation, but are bifurcated. Cuticle details were not recoverable.
Several herbivory features were identified on these plant remains and are referable to six DTs belonging to three FFGs. These FFGs are margin feeding (DT012, DT013), hole feeding (DT001, DT003, DT007) and piercing and sucking damage (DT046). Alethopteris zeilleri hosts evidence of margin feeding and hole feeding (DT012, DT001, and DT013; Fig. 3A1, B). Pseudomariopteris cordato-ovata has a greater number and diversity of interactions, including margin feeding (DT012; Fig, 3A2, A3), and elliptical hole feeding with a weakly developed reaction rim (DT003; Fig. 3A2); the reaction rims observed in these interactions reflect a well-developed plant response to the damage, indicating that the plant was alive when the damage occurred. Finally, P. cordato-ovata also hosts a more specialized damage type (DT046; Fig. 3C) consisting of an isolated, slightly concave, styletal puncture into the leaf tissue, 0.6–0.7 mm in diameter and characterized by dark carbonized material with a central depression. These piercing and sucking marks were probably caused by insects with stylet-like mouthparts.
Discussion
Previous studies have indicated that the diversity of Carboniferous insects in the Iberian Peninsula was relatively low compared to other parts of Europe (Carpenter 1992; Santos et al. 2023). Nevertheless, renewed interest in the study of Paleozoic insects and plant-arthropod interactions from the Iberian Peninsula in the past few years has revealed numerous new fossils, suggesting that insect occurrence and diversity in these deposits is much greater than previously recognized (Correia et al. 2020, 2021; Nel et al. 2022a, b; Santos et al. 2022, 2023, 2024). This renewed focus has opened new lines of research in the relationships between Paleozoic tropical plants and insects. The identification of Aspidothorax hispanicus sp. nov. in close association with herbivore-damaged gymnosperms (see reconstruction in Fig. 4) expands the complexity of Iberian Gzhelian terrestrial ecosystems.

Fig. 4. Reconstruction of megasecopteran insect Aspidothorax hispanicus sp. nov. on leaves of Pseudomariopteris cordato-ovata and Alethopteris zeilleri with evidence of herbivory in a tropical late Carboniferous forest of Iberia. Reconstruction by Pollyanna von Knorring, Swedish Museum of Natural History, Stockholm.
Both plant species found associated with the new insect wing, Alethopteris zeilleri and Pseudomariopteris cordato-ovata, have features that are informative of the climate and local vegetation structure. Based on the pinnules of A. zeilleri being coriaceous, with inrolled margins, thick cuticles with upper surface hairs, and deeply sunken stomata restricted to the lower surfaces, Šimůnek (1989), Kerp and Barthel (1993), and Zodrow (2007), interpreted the parent plant to be mesophytic, growing on the margin of peat-forming areas. DiMichele et al. (2006) pointed out that, in the Upper Pennsylvanian of Texas, A. zeilleri locally occurs in shales associated with organic accumulations, but tends to be more common in less organic-rich deposits. Bashforth and Zodrow (2007) reported this species in Middle Pennsylvanian deposits of Nova Scotia in non-peat-forming habitats but in proximity to swamps, where they may have been exposed to intense sunlight or wind. Further, Bashforth et al. (2010), in their work on the vegetation of La Magdalena, cited Alethopteris and Pseudomariopteris as typical of non-peat-forming communities located in marginal wetlands and interfluve wetlands. These authors noted that both genera had a clear preference for disturbed sites, closer to rivers, in interfluve wetlands over other “seed-ferns”. The co-occurrence of A. zeilleri and P. cordato-ovata indicates that Aspidothorax hispanicus likely lived in humid environments in alluvial settings, perhaps close to rivers in communities of arborescent Medullosales (A. zeilleri) and climbing/scrambling (liana-like) Callistophytales (P. cordato-ovata). Despite the Megasecoptera wing being disarticulated, it lacks abrasion or other evidence of transport, as do the plant remains. Thus, this assemblage seems to reflect minimal transport.
Several of the damage types observed on the studied plant remains have been found in other Carboniferous assemblages of northwestern Spain (Santos et al. 2022). However, we report damage types DT046, DT013, DT003, and DT007 for the first time in the Carboniferous of Spain. This diversity of interactions is indicative of a diverse and well-established entomofauna in the area, and is consistent with previous interpretations that the Carboniferous insect diversity from this region was much higher than recorded so far (Santos et al. 2022, 2023). Linking the damage to specific families or genera of insects is challenging, especially when the damage is very generalized, as is the case for DT012, DT001 or DT003, which could have been produced by several types of insects or other arthropods with chewing mouthparts. The more specialized piercing and sucking scars were probably made by insects with stylet mouthparts. Megasecoptera are strong candidates for this kind of damage, as insects of this group possessed piercing and sucking mouthparts (Prokop et al. 2016), probably to penetrate and feed on the inner tissues of various vascular plants (Kukalová-Peck 1991b; Labandeira and Phillips 1996; Engel et al. 2013; Prokop et al. 2016; Pecharová et al. 2020). Diverse genera of Megasecoptera were present in the region based on several records from Carboniferous deposits, including species of Anchineuridae (Carpenter 1963; Brauckmann 1993), Corydaloididae (Santos et al. 2023), Mischopteridae (Santos et al. 2023), and Aspidothoracidae; (Table 1). Future studies on the entomofauna and plant-insect interactions in the region may help to link insects with some interactions.
The new herbivorous species Aspidothorax hispanicus likely fed on the vegetation of the Carboniferous tropical forests of the area, which also provided shelter and protection from predators. Notably, A. hispanicus possessed patterning traits that may have helped avoid or deter predators. Dark patches and spots similar to those on the new species have been observed on other members of the genus, such as A. triangularis, for which this disruptive coloration was interpreted as a camouflage mechanism (Pecharová et al. 2020). The dark patch on the wings of A. hispanicus likely also served as effective camouflage, allowing the insect to blend into a dappled-light background. This camouflage would have offered protection from predators, such as griffinflies (Meganisoptera) (Nel et al. 2009; Pecharová et al. 2020), large damselfly-like species in the “Protozygopteran” group of Odonatoptera (Nel et al. 2012, 2018), and potentially even vertebrate predators. Disruptive color patterns in flight are commonly present in both modern and fossil odonates (Jouault et al. 2022), typically as transverse bands and spots on the wings, much like those in A. hispanicus. However, the distinct dark area at the wing apex might also have played a role in sexual display or courtship, similar to the color variations in the pterostigmata of male and female Sympetrum species. Although we agree with Pecharová et al. (2020) that the spots’ most likely function was camouflage, the dark wing apex may have played an additional role in sexual dimorphism and courtship behavior.
Conclusions
The discovery of Aspidothorax hispanicus sp. nov. in Gzhelian, Pennsylvanian, deposits of León marks the first record of Aspidothoracidae in the Iberian Peninsula. This newly described species, found in close association with several plant remains, expands the distributions of this family and genus, which were previously identified only in France and the Russian Federation. The fossil represents the fourth megasecopteran species described from the Iberian Peninsula, contributing significantly to our understanding of the regional Carboniferous insect diversity.
The association of Aspidothorax hispanicus with gymnosperms, namely Alethopteris zeilleri and Pseudomariopteris cordato-ovata, suggests that these insects inhabited forested communities that included climbing plants/ground covers of humid alluvial environments. The presence of six damage types belonging to three functional feeding groups including margin feeding (DT012, DT013), hole feeding (DT001, DT003, DT007), and piercing and sucking (DT046), indicates diverse insect feeding strategies in the area during the Carboniferous. It also suggests the presence of chewing and piercing and sucking insect herbivores. The stylet mouthparts of Megasecoptera make insects of this order good candidates for the production of piercing and sucking damage found in the associated plants. Aspidothorax hispanicus sp. nov. probably used the vegetation for both nourishment and protection. Its wing markings, having coloration that likely served as camouflage, helped it blend into its environment and avoiding predators, such as griffinflies and damselfly-like species. The dark wing apex may also indicate the presence of sexual dimorphism or courtship behavior. These findings provide valuable insights into the habitats and ecological conditions in the area at that time, adding new information on Carboniferous tropical terrestrial environments of Spain.
Acknowledgements
Thanks to José Luis Garrido (Elche, Alicante, Spain) for the discovery of the specimen studied herein and its donation to the MUPE. We gratefully acknowledge the constructive comments to the editor and reviewers Jakub Prokop (Charles University, Prague, Czech Republic) and Andrew Ross (National Museums Scotland, Edinburgh, UK). Thanks also to the paleoartist Pollyanna von Knorring (Swedish Museum of Natural History, Stockholm, Sweden) for the scientific illustration. AS is supported by a postdoctoral fellowship funded from the Swedish Research Council Grant VR 2022-03920 managed by Stephen McLoughlin.
Editor: Krzysztof Hryniewicz.
References
Bashforth, A.R. and Zodrow, E.L. 2007. Partial reconstruction and palaeoecology of Sphenophyllum costae (Middle Pennsylvanian, Nova Scotia, Canada). Bulletin of Geosciences 82: 365–382. Crossref
Bashforth, A.R., Falcon-Lang, H.J., and Gibling, M.R. 2010. Vegetation heterogeneity on a Late Pennsylvanian braided-river plain draining the Variscan Mountains, La Magdalena Coalfield, northwestern Spain. Palaeogeography, Palaeoclimatology, Palaeoecology 292: 367–390. Crossref
Bechly, G. 1996. Morphologische Untersuchungen am Flügelgeäder der rezenten Libellen und deren Stammgruppenvertreter (Insecta; Pterygota; Odonata), unter besonderer Berücksichtigung der Phylogenetischen Systematik und des Grundplanes der *Odonata. Petalura Special Volume 2: 1–402.
Brauckmann, C. 1993. Notiz über insekten-Reste aus dem Ober-Karbon in Spanien. Jahresberichte des Naturwissenschaftlichen Vereins in Wuppertal 46: 115–119.
Brongniart, C. 1885. Les insectes fossiles des terrains primaires. Coup d’œil rapide sur la faune entomologique des terrains paléozoïques. Bulletin de la Société des Amis des Sciences Naturelles de Rouen 3: 50–68. Crossref
Brongniart, C. 1894. Recherches pour servir à l’histoire des insectes fossiles des temps primaires précédées d’une étude sur la nervation des ailes des insectes. Bulletin de la Société d’Industrie Minérale de Saint-Etienne 7: 1–491. Crossref
Carpenter, F.M. 1951. Studies on Carboniferous insects from Commentry, France: Part II. The Megasecoptera. Journal of Paleontology 25: 336–355. https://www.jstor.org/stable/1299927
Carpenter, F.M. 1963. A megasecopteron from Upper Carboniferous strata in Spain. Psyche: A Journal of Entomology 70: 44–49. Crossref
Carpenter, F.M. 1992. Superclass Hexapoda. In: R.L. Kaesler (ed.). Treatise on Invertebrate Paleontology, Part R, Arthropoda 3–4. 655 pp. Geological Society of America, Boulder, Colorado.
Castro, M.P. 2005a. La flora Estefaniense B de La Magdalena (León, España), un referente europeo. Tomo I: Antecedentes y análisis florístico. Cuadernos del Museo Geominero 4: 1–251.
Castro, M.P. 2005b. La flora estefaniense B de La Magdalena (León, España), un referente europeo. Tomo II: Descripción sistemática de las Gimnospermas. Cuadernos del Museo Geominero 4: 1–229.
Colmenero, J.R., Bahamonde, J.R., and Barba, P. 1996. Las facies aluviales asociadas a los depósitos de carbón en las cuencas estefanienses de León (borde sur de la Cordillera Cantábrica). Cuadernos de Geología Ibérica 21: 71–92.
Correia, P., Bashforth, A.R., Šimůnek, Z., Cleal, C.J., Sá, A.A., and Labandeira, C.C. 2020. The history of herbivory on sphenophytes: a new calamitalean with an insect gall from the Upper Pennsylvanian of Portugal and a review of arthropod herbivory on an ancient lineage. International Journal of Plant Sciences 181: 387–418. Crossref
Correia, P., Schubnel, T., and Nel, A. 2021. What is the roachoid genus Eneriblatta (Dictyoptera: Phyloblattidae) from the Carboniferous of Portugal. Historical Biology 33: 777–782. Crossref
DiMichele, W. A., Phillips, T. L., and Pfefferkorn, H.W. 2006 Paleoecology of late Paleozoic pteridosperms from tropical Euramerica. Journal of the Torrey Botanical Society 133: 83–118. Crossref
Engel, M.S., Davis, S.R., and Prokop, J. 2013. Insect wings: the evolutionary development of nature’s first flyers. In: A. Minelli, G. Boxshall, and G. Fusco, (Eds.). Arthropod Biology and Evolution: Molecules, Development, Morphology, 269–298. Springer, Berlin. Crossref
Garrouste, R., Azar, D., Loll, J.C., Vallois, B., and Nel, A. 2024. The first Carboniferous insects of the paleoforest of the Reyran Basin (Esterel Massif, South of France) with palaeoenvironnemental notes. Historical Biology [available online, https://doi.org/10.1080/08912963.2024.2370006] Crossref
Gastaldo, R.A., Bamford, M., Calder, J., DiMichele, W.A., Ianuzzi, R., Jasper, A., Kerp, H., McLoughlin, S., Opluštil, S., Pfefferkorn, H.W., Roessler, R., and Wang, J. 2020. The coal farms of the Late Paleozoic. In: E. Martinetto, E. Tschopp, and R.A. Gastaldo (eds.), Nature Through Time, 317–343. Springer, Berlin. Crossref
Gillespie, W.H., Clendening, J.A., and Pfefferkorn, H.W. 1978. Plant Fossils of West Virginia and Adjacent Areas. 172 pp. West Virginia Geological and Economic Survey, Morgantown, West Virginia.
Gravenhorst, J.L.C. 1843. Vergleichende Zoologie. xx+687 pp. Graß, Barth & Comp., Breslau. Crossref
Handlirsch, A. 1906. Revision of American Paleozoic insects. Proceedings of the United States National Museum 29: 661–820. Crossref
Handlirsch, A. 1919. Revision der paläozoischen Insekten. Denkschriften der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse in Wien 96: 511–592.
Heward, A.P. 1978. Alluvial fan and lacustrine sediments from the Stephanian A and B (La Magdalena, Ciñera-Matallana and Sabero) coalfields, northern Spain. Sedimentology 25: 451–488. Crossref
Huang, D.-Y., Fu, Y., Lian, X., Gao, J., and Nel, A. 2021. The first Chinese Protohymenidae (Palaeoptera: Megasecoptera). Historical Biology 34: 458–461. Crossref
Jouault, C., Tischlinger, H., Henrotay, M., and Nel, A. 2022. Wing coloration patterns in the Early Jurassic dragonflies as potential indicator of increasing predation pressure from insectivorous reptiles. Palaeoentomology 5: 305–318. Crossref
Kerp, H. and Barthel, M. 1993. Problems of cuticular analysis of pteridosperms. Review of Palaeobotany and Palynology 78: 1–18. Crossref
Knight, J.A., Cleal, C.J., and Álvarez-Vázquez, C. 2023. The challenge of relating the Kasimovian to West European chronostratigraphy: a critical review of the Cantabrian and Barruelian substages of the Stephanian Stage. In: S.G. Lucas, W.A. DiMichele, S. Opluštil, and X. Wang (ed.), Ice Ages, Climate Dynamics and Biotic Events: The Late Pennsylvanian World. Geological Society, London, Special Publications 535: 31–71. Crossref
Krings, M. and Kerp, H. 2000. A contribution to the knowledge of the pteridosperm genera Pseudomariopteris Danzé-Corsin nov. emend. and Helenopteris nov. gen. Review of Palaeobotany and Palynology 111: 145–195. Crossref
Kukalová-Peck, J. 1972. Unusual structures in the Paleozoic insect orders Megasecoptera and Palaeodictyoptera with description of a new family. Psyche: A Journal of Entomology 79: 243–268. Crossref
Kukalová-Peck, J. 1975. Megasecoptera from the Lower Permian of Moravia. Psyche: A Journal of Entomology 82: 1–19. Crossref
Kukalová-Peck, J. 1991a. Designation of type species for Hana (Hanidae, Megasecoptera). Psyche: A Journal of Entomology 98: 193. Crossref
Kukalová-Peck, J. 1991b. Fossil history and the evolution of hexapod structures. In: I.D. Naumann, P.B. Carne, J.F. Lawrence, E.S. Nielsen, J.P. Spradbery, R.W. Taylor, M.J. Whitten, and M.J. Littlejohn (eds.), Insects of Australia: A textbook for Students and Research Workers, 141–179. Melbourne University Press, Carlton, Victoria.
Labandeira, C.C. and Phillips, T.L. 1996. Insect fluid-feeding on Upper Pennsylvanian tree ferns (Palaeodictyoptera, Marattiales) and the early history of the piercing-and-sucking functional feeding group. Annals of the Entomological Society of America 89: 157–183. Crossref
Labandeira, C.C., Wilf, P., Johnson, K.R., and Marsh, F. 2007. Guide to insect (and other) damage types on compressed plant fossils. Version 3.01. 25 pp. Smithsonian Institution, National Museum of Natural History, Department of Paleobiology, Washington, DC.
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Laurentius Salvius, Stockholm. Crossref
Martynov, A.V. 1930. New Permian insects from Tikhie Gory, Kazan province. 1. Palaeoptera [in Russian]. Trudy Geologičeskogo Muzeâ, Akademii nauk SSSR 6: 69–86.
Martynov, A.V. 1937. Permian fossil insects from Kargala and their relationships [in Russian. Trudy Paleontologičeskogo Instituta Akademii nauk SSSR 7: 8–92.
McLoughlin, S. 2001. The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Australian Journal of Botany 49: 271–300. Crossref
Nel, A., Bechly, G., Prokop, J., Béthoux, O., and Fleck, G. 2012. Systematics and evolution of Paleozoic and Mesozoic damselfly-like Odonatoptera of the ‘Protozygopteran’ grade. Journal of Paleontology 86: 81–104. Crossref
Nel, A., Fleck, G., Garrouste, R., Gand, G., Lapeyrie, J., Bybee, S.M., and Prokop, J. 2009. Revision of Permo-Carboniferous griffenflies (Insecta: Odonatoptera: Meganisoptera) based upon new species and redescription of selected poorly known taxa from Eurasia. Palaeontographica A 289: 89–121. Crossref
Nel, A., Garrouste, R.E., Peñalver, E., Hernández-Orúe, A., and Jouault, C. 2022b. Discovery of the first blattinopsids of the genus Glaphyrophlebia Handlirsch, 1906 (Paoliida: Blattinopsidae) in the Upper Carboniferous of Southern France and Spain and hypothesis on the diversification of the family. Diversity 14: 1129. Crossref
Nel, A., Prokop, J., Pecharová, M., Engel, M.S., and Garrouste, R. 2018. Palaeozoic giant dragonflies were hawker predators. Scientific Reports 8: 12141. Crossref
Nel, A., Santos, A.A., Hernández-Orúe, A., Wappler, T., Diez, J.B., and Peñalver, E. 2022a. The first representative of the roachoid family Spiloblattinidae (Insecta, Dictyoptera) from the Late Pennsylvanian of the Iberian Peninsula. Insects 13: 1–14. Crossref
Novokshonov, V.G. 1995. New fossil insects from the Kungurian of Middle Urals. Paleontological Journal 29: 61–67.
Pecharová, M., Prokop, J., and Ren, D. 2015a. Early Pennsylvanian aykhalids from Xiaheyan, northern China and their palaeogeographical significance (Insecta: Megasecoptera). Comptes Rendus Palévol 14: 613–624. Crossref
Pecharová, M., Ren, D., and Prokop, J. 2015b. A new palaeodictyopteroid (Megasecoptera: Brodiopteridae) from the Early Pennsylvanian of northern China reveals unique morphological traits and intra-specific variability. Alcheringa 39: 236–249. Crossref
Pecharová, M., Sinitshenkova, N.D., and Prokop, J. 2020. On the morphology of the Late Paleozoic insect families Bardohymenidae and Aspidothoracidae (Palaeodictyopterida: Megasecoptera). Arthropod Structure and Development 55: 100916. Crossref
Pinto, I.D. 1986. Carboniferous insects from Argentina. 3. Family Xenopteridae Pinto nov. fam. (Megasecoptera). Pesquisas 18: 23–29. Crossref
Pinto, I.D. 1994. Sphecorydaloides lucchesei, a new Carboniferous megasecopteran insect from Argentina. Pesquisas 21: 85–89. Crossref
Prokop, J., Pecharová, M., and Ren, D. 2016. Hidden surface microstructures on Carboniferous insect Brodioptera sinensis (Megasecoptera) enlighten functional morphology and sensorial perception. Scientific Reports 6: 28316. Crossref
Prokop, J., Pecharová, M., Nel, A., Grey, M., and Hörnschemeyer, T. 2017. A remarkable insect from the Pennsylvanian of the Joggins Formation in Nova Scotia, Canada: Insights into unusual venation of Brodiidae and nymphs of Megasecoptera. Journal of Systematic Palaeontology 15: 1051–1065. Crossref
Ragot, L. 1955. Contribution à l’étude des formes carbonifères du genre Alethopteris. 76 pp. Thèse pour le Diplôme d’Études Supérieures, Faculté des Sciences de l’Université de Paris, Paris.
Rohdendorf, B.B. 1947. On fossil insects from the Vorkutsk coal field Basin. Doklady Akademii Nauk SSSR 57: 391–393 [in Russian].
Rohdendorf, B.B. 1961. Subclass Pterygota. In: B.B. Rohdendorf, E.E. Becker-Migdisova, O.M. Martynova, and A. Sharov (eds.), Palaeozoic insects of the Kuznetsk Basin. Trudy Paleontologičeskogo Instituta Akademii nauk SSSR 85: 69–88 [in Russian].
Ross, A.J., Nicholson, D.B., and Jarzembowski, E.A. 2013. Omaliidae Handlirsch, 1904 (Insecta, Archaeorthoptera) and Xenopteridae Pinto, 1986 (Insecta, Megasecoptera): Proposed emendation to Omaliaidae and Xenopteraidae respectively to remove homonymy with Omaliinae MacLeay, 1825 (Insecta, Coleoptera) and Xenopteridae Riek, 1955 (Insecta, Orthoptera). Bulletin of Zoological Nomenclature 70: 166–170. Crossref
Santos, A.A., Diez, J.B., and Nel, A. 2024. Wappleria tremoris gen. et sp. nov. the first representative of the insect order Caloneurodea in Spain (León, NW Spain) found in a Late Carboniferous forest. Historical Biology [available online, https://doi.org/10.1080/08912963.2024.2344799]. Crossref
Santos, A.A., Hernández-Orúe, A., Wappler, T., and Diez, J.B. 2022. Plant–insect interactions from the Late Pennsylvanian of the Iberian Peninsula (León, northern Spain). Review of Palaeobotany and Palynology 301: 104658. Crossref
Santos, A.A., Hernández-Orúe, A., Wappler, T., Peñalver, E., Diez, J.B., and Nel, A. 2023. Late Carboniferous insects from the Iberian Peninsula: state of the art and new taxa. Palaeontographica Abteilung A Palaozoologie Stratigraphie 326: 1–27. Crossref
Schubnel, T., Desutter-Grandcolas, L., Legendre, F., Prokop, J., Mazurier, A., Garrouste, R., Grandcolas, P., and Nel, A. 2020. To be or not to be: postcubital vein in insects revealed by microtomography. Systematic Entomology 45: 327–336. Crossref
Scotese, C.R. and Golonka, J. 1992. Palaeogeographic Atlas. Progress Report 20-0692. 34 pp. PALAEOMAP Project, Department of Geology. University of Texas, Arlington.
Šimůnek, Z. 1989. Stephanian and Permian species of Alethopteris from Bohemia and Moravia. Sborník geologických věd, Paleontologie 33: 5–37.
Sinitshenkova, N.D. 1993. A new insect family Aykhalidae from the Upper Palaeozoic of Yakutia-Sakha (Insecta: Mischopterida = Megasecoptera). Paleontological Journal 27 (1A): 131–134.
Sinitshenkova, N. D., Ponomareva, Y., and Aristov, S. 2020. A new megasecopteran species of the genus Aspidothorax Brongniart, 1893 (Insecta: Mischopterida = Megasecoptera: Aspidothoracidae) from the Middle Permian of Perm region, Russia. Paleontological Journal 54: 273–278. Crossref
Tabor, N.J. and Poulsen, C.J. 2008. Palaeoclimate across the Late Pennsylvanian–Early Permian tropical palaeolatitudes: a review of climate indicators, their distribution, and relation to palaeophysiographic climate factors. Palaeogeography, Palaeoclimatology, Palaeoecology 268: 293–310. Crossref
Tillyard, R.J. 1924. Kansas Permian insects. 3. The new order Protohymenoptera. American Journal of Science (Series 5) 8: 111–122. Crossref
Wagner, R.H. 1965. Stephanian B flora from the Ciñera-Matallana Coalfield (León) and neighbouring outliers. III: Callipteridium and Alethopteris. Notas y Comunicaciones Instituto Geológico y Minero de España 78: 5–70.
Wagner, R.H. 1968, Upper Westphalian and Stephanian species of Alethopteris from Europe, Asia Minor and North America. Mededelingen Rijks Geologische Dienst, Serie C III-1 (6): 1–188.
Wagner, R.H. and Álvarez-Vázquez, C. 2010. The Carboniferous floras of the Iberian Peninsula: A synthesis with geological connotations. Review of Palaeobotany and Palynology 162: 239–324. Crossref
Wagner, R.H. and Castro, M.P. 2011. Compositional changes in a mid-Stephanian (Kasimovian) flora in relation to alluvial plain deposits derived from westward-receding mountains and bordered by the Paleotethys: La Magdalena Coalfield, Northwestern Spain. Palaios 26: 33–54. Crossref
Weiss, C.E. 1869–1872. Fossile Flora der jüngsten Steinkohlenformation und des Rothliegenden im Saar-Rhein-Gebiete. Band I. A. 250 pp. Henry, Bonn.
Zalessky, G. 1937. Ancestors of some groups of the present-day insects. Nature 140: 847–848. Crossref
Zodrow, E.L. 2007. Reconstructed tree fern Alethopteris zeilleri (Carboniferous, Medullosales). International Journal of Coal Geology 69: 68–89. Crossref
Acta Palaeontol. Pol. 70 (1): 115–124, 2025
https://doi.org/10.4202/app.01203.2024