

First fossil representatives of the limoniid dipteran Rhabdomastix with extremely elongate antennae from Eocene ambers
WIKTORIA JORDAN-STASIŁO, IWONA KANIA-KŁOSOK, and WIESŁAW KRZEMIŃSKI
Jordan-Stasiło, W., Kania-Kłosok, I., and Krzemiński, W. 2025. First fossil representatives of the limoniid dipteran Rhabdomastix with extremely elongate antennae from Eocene ambers. Acta Palaeontologica Polonica 70 (1): 57–75.
The first representatives of the genus Rhabdomastix (Diptera, Limoniide) with extremely long antennae (much longer than the body) is discovered in the fossil record. The paper presents new data on Eocene species of the genus Rhabdomastix including a new species with surprisingly long and tiny antennae with characteristic almost black spots on individual flagellomeres from Bitterfeld amber: Rhabdomastix (Rhabdomastix) woottoni sp. nov. Two other species have been discovered and described based on inclusions in Baltic amber: Rhabdomastix (Rhabdomastix) setosa sp. nov. and Rhabdomastix (Rhabdomastix) rafali sp. nov. Antennae with similar morphology are characteristic for the American Recent species collectively known as “Rhabdomastix illudens”. New nomenclature decisions include a transfer of three species from Baltic amber with antenna longer than the body to the subgenus Rhabdomastix. These species: Rhabdomastix (Rhabdomastix) grussica Podenas, 2006 comb. nov., Rhabdomastix (Rhabdomastix) mastix Podenas, 2006 comb. nov. and Rhabdomastix (Rhabdomastix) setix Podenas, 2006 comb. nov., were not previously classified to any subgenus. New discovery of a species of Rhabdomastix with extremely elongate, very narrow antennae, sheds new light on the evolutionary history of this genus. The paper also presents interpretations of the ecological preferences of some modern representatives of the genus and their Eocene ancestors.
Key words: Insecta, Diptera, Limoniide, inclusions, taxonomy, evolution, Baltic amber, Bitterfeld amber, Eocene.
Wiktoria Jordan-Stasiło [wjordan@ur.edu.pl; ORCID: https://orcid.org/0000-0001-5621-6917 ] and Iwona Kania-Kłosok [ikania@ur.edu.pl; ORCID: https://orcid.org/0000-0002-2325-4308 ] (corresponding author), Institute of Biology, University of Rzeszów, Zelwerowicza 4, 35-601 Rzeszów, Poland.
Wiesław Krzemiński [wieslawk4@gmail.com; ORCID: https://orcid.org/0000-0001-5685-891X ], Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Sławkowska 17, 31-016 Kraków, Poland.
Received 19 September 2024, accepted 10 January 2025, published online 11 March 2025.
Copyright © 2025 W. Jordan-Stasiło et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The represetatives of the genus Rhabdomastix Skuse, 1890, are currently distributed worldwide and all its 131 known Recent species and subspecies are classified to two subgenera only: Rhabdomastix Skuse, 1890, and Lurdia Starý, 2003. Sixty eight species and subspecies of Rhabdomastix are known from the Holarctic region, 24 species occur in the Neotropic region, 23 in the Australasian/Oceanian region, 15 in the Oriental region, and only three species of the genus Rhabdomastix occur in the Afrotropic (Table 1).
Table 1. Species and subspecies diversity of the limoniid dipteran Rhabdomastix Skuse, 1890, in zoogeographical regions (Oosterbroek 2024; Jordan-Stasiło et al. 2023).
|
Zoogeographical regions |
Number |
Number of species and subspecies of subgenera |
||
|
Lurdia |
Rhabdomastix |
unplaced to subgenus |
||
|
Palaearctic |
39 |
10 |
28 |
1 |
|
Nearctic |
29 |
3 |
21 |
5 |
|
Neotropic |
24 |
0 |
24 |
– |
|
Afrotropic |
3 |
0 |
3 |
– |
|
Oriental |
15 |
0 |
15 |
– |
|
Australasian/Oceanian |
23 |
0 |
23 |
– |
Three subgenera of the genus Rhabdomastix are known from the fossil record: Lurdia Starý, 2003, with one extinct species (Podenas 2006), entirely extinct Myanmamastix Kania-Kłosok et al., 2021, with five species (Kania-Kłosok et al. 2021) and Rhabdomastix Skuse, 1890, with two species known only from the fossil record (Meunier 1906; Podenas 2006). All five species of the subgenus Myanmamastix are described from the Cretaceous Myanmar amber (Table 2) (Kania-Kłosok et al. 2021). Sixteen species of genus Rhabdomastix out of the 24 known from the fossil record, have not been classified into any subgenera (Table 2) (Cockerell 1920; Statz 1944; Scudder 1894; Meunier 1906; Alexander 1931; Evenhuis 1994; Podenas 2006; Kania-Kłosok et al. 2021; Jordan-Stasiło et al. 2023). Additionally, two groups without subgeneric attribution were dinstinguished by Starý (2003): “Rhabdomastix caudata” with species complex centred around Rhabdomastix caudata (Lundbeck, 1898). It’s interesting that this group is distinguished by its partially developed crossvein R2 (r-r), while “Rhabdomastix illudens”, is characterized by extremely elongate antenna, even five times longer than the body. The similar structure of the body occur in the Recent Bolivian Rhabdomastix (Rhabdomastix) illudens Alexander, 1914b. This is group which includes five Neotropical, five Oriental and one Nearctic species Rhabdomastix (Rhabdomastix) nuttingi Alexander, 1950 (Oosterbroek 2024). In 1914, Alexander described the first Neotropical species R. (R.) illudens, whose antenna were extremely elongate approximately three to five times longer than the body (Alexander 1914b; Starý 2003; Jordan-Stasiło et al. 2023), then Starý (2003) mentions a group of species centered around “R. illudens” with antennae much longer than the body.
Table 2. List of fossil species of the limoniid dipteran Rhabdomastix Skuse, 1890, known from the fossil record with the stratigraphic range, age, type of fossil and division into subgenera.
|
Species |
Age |
Type of fossil |
|
Subgenus Lurdia Starý, 2003 |
||
|
Rhabdomastix (L.) ratix Podenas, 2006 |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Subgenus: Myanmamastix Kania-Kłosok et al., 2021 |
||
|
Rhabdomastix (M.) asiatica Kania-Kłosok et al., 2021 |
Late Cretaceous; 98.79 ± 0.62 Ma |
inclusion/Burmese amber |
|
Rhabdomastix (M.) cretacica Kania-Kłosok et al., 2021 |
Late Cretaceous; 98.79 ± 0.62 Ma |
inclusion/Burmese amber |
|
Rhabdomastix (M.) jarzembowskii Krzemiński, 2004 |
Late Cretaceous; 98.79 ± 0.62 Ma |
inclusion/Burmese amber |
|
Rhabdomastix (M.) krzeminskae Kania-Kłosok et al., 2021 |
Late Cretaceous; 98.79 ± 0.62 Ma |
inclusion/Burmese amber |
|
Rhabdomastix (M.) myanmae Kania-Kłosok et al., 2021 |
Late Cretaceous; 98.79 ± 0.62 Ma |
inclusion/Burmese amber |
|
Subgenus Rhabdomastix Skuse, 1890 |
||
|
Rhabdomastix (R.) elegantula (Meunier, 1906) |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix (R.) grussica Podenas, 2006 comb. nov. |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix (R.) lutix Podenas, 2006 |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix (R.) mastix Podenas, 2006 comb. nov. |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix (R.) rafali sp. nov. |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix (R.) setix Podenas, 2006 comb. nov. |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix (R.) setosa sp. nov. |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix (R.) woottoni sp. nov. |
middle–late Eocene |
inclusion/Bitterfeld amber |
|
Unplaced to subgenus |
||
|
Rhabdomastix borussica (Meunier, 1906) |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix brevis Alexander, 1931 |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix frigida Scudder, 1894 |
Oligocene; 37.2–33.9 Ma |
imprint/Florrisant |
|
Rhabdomastix hoffeinsi Jordan-Stasiło et al., 2023 |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix klebsi Alexander, 1931 |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix labefactata Scudder, 1894 |
Oligocene; 37.2–33.9 Ma |
imprint/Florrisant |
|
Rhabdomastix oligocaenica Statz, 1944 |
Oligocene; 28.4–23.0 Ma |
imprint/Rott Formation |
|
Rhabdomastix praecursor Cockerell, 1927 |
Oligocene; 37.2–33.9 Ma |
imprint/Florrisant |
|
Rhabdomastix primogenitalis Scudder, 1894 |
Oligocene; 37.2–33.9 Ma |
imprint/Florrisant |
|
Rhabdomastix profundi Scudder, 1894 |
Oligocene; 37.2–33.9 Ma |
imprint/Florrisant |
|
Rhabdomastix pulcherrima (Meunier, 1906) |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix redophilax Podenas, 2006 |
middle Eocene (Lutetian); 47.8–41.3 Ma |
inclusion/Baltic amber |
|
Rhabdomastix scudderi Cockerell, 1920 |
Eocene; 50.3–46.2 Ma |
imprint/Rocky Mountains |
Hitherto, Rhabdomastix with this type of antennae was unknown from the fossil record. The new discovery of a new species with extremely elongate antenna (much longer than the body) in Eocene Bitterfeld amber is the first record of this type of antennae in fossil Rhabdomastix.
We report here three new species of Rhabdomastix from Eocene fossil resins displaying antennae longer than the body length, including one with extremely long antennae, and transfer to the subgenus Rhabdomastix three other already described species.
Institutional abbreviations.—SDEI, Senckenberg Deutsches Entomologisches Institut, Germany; GMUG, University of Gӧttingen, Germany; ISEA PAS MP, Natural History Museum of the Institute of Systematics and Evolution of Animals, PAS, Kraków, Poland.
Other abbreviations.—A1–A2, anal veins; C, costal vein; Cu, cubital vein; d, discal cell; h, humeral vein; I–IV, palpomeres 1–4; M1+2 to M4, medial veins; m-cu, medial-cubital crossvein; R1 –R5, radial veins; Rs, radial sector; Sc, subcostal vein; sc-r, subcostal radial crossvein.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in Zoobank: urn:lsid:zoobank.org:pub:45A50B90-72C3-44AF-B5F4-7FA090BF142A.
Material and methods
The study was based on ten specimens attributed to the genus Rhabdomastix Skuse, 1890, preserved as inclusions in Eocene Baltic amber (nine specimens) and Bitterfeld amber (one specimen). The examined material is deposited at the Institute of Systematics and Animal Evolution of the Polish Academy of Sciences Kraków, Poland (ISEA PAS) (seven specimens) and at the Senckenberg Deutsches Entomologisches Institut (SDEI) Müncheberg, Germany (the collection of Christel and Hans Werner Hoffeins) (two specimens) (Table 3). One specimen examined herein belongs to the Manfred Kutscher’s collection, stored at the University of Göttingen (GMUG).
Table 3. List of materials examined herein, with sex, type of resin and repository indicated.
|
Species |
Number of specimen |
Status of the material |
Sex |
Type of material |
Collection |
|
Rhabdomastix (R.) mastix Podenas, 2006 comb. nov. |
MP/3321 |
additional material |
♀ |
Baltic amber |
ISEA PAS |
|
Rhabdomastix (R.) rafali sp. nov. |
MP/3092 |
holotype |
♂ |
Baltic amber |
ISEA PAS |
|
MP/3948a |
additional material |
♂ |
Baltic amber |
ISEA PAS |
|
|
MP/3948b |
additional material |
♂ |
Baltic amber |
ISEA PAS |
|
|
MP/3948c |
additional material |
♂ |
Baltic amber |
ISEA PAS |
|
|
Rhabdomastix (R.) setosa sp. nov. |
CCHH 1462-2 |
holotype |
♂ |
Baltic amber |
SDEI/coll. Ch. and H.W. Hoffeins |
|
MP/3472 |
additional material |
♂ |
Baltic amber |
ISEA PAS |
|
|
CCHH 1471-2 |
additional material |
♂ |
Baltic amber |
SDEI/ coll. Ch. and H.W. Hoffeins |
|
|
MP/3945 |
additional material |
♂ |
Baltic amber |
ISEA PAS |
|
|
Rhabdomastix (R.) woottoni sp. nov. |
GMUG 80 |
holotype |
♂ |
Bitterfeld amber |
GMUG/coll. M. Kutscher |
The specimens were examined using a Nikon SMZ 1500 stereomicroscope equipped with a Nikon DS-Fi1 camera. The measurements were performed with NIS-Elements D 3.0 software in the University of Rzeszów. The drawings were made by tracing specimens and photographs by WJ-S. The map was built using the map Maps-For-Free (https:// maps-for free.com) and modified with the CorelDraw 2018 software. We follow the nomenclature of wing venation of Krzemiński (2002), the designations of hypopygium and ovipositor were adapted from McAlpine et al. (1981), Podenas (2006), Krzemiński and Krzemińska (2003).
Systematic palaeontology
Order Diptera Linnaeus, 1758
Infraorder Tipulomorpha Rohdendorf, 1961
Family Limoniidae Speiser, 1909
Subfamily Chioneinae Rondani, 1861
Genus Rhabdomastix Skuse, 1890
Subgenus Rhabdomastix Skuse, 1890
Type species: Rhabdomastix ostensackeni Skuse, 1890; Australian/Oceanian, extant; by monotypy.
Emended diagnosis.—Antenna of various length; tip of Sc in half or beyond half length of wing; r-r (R2) completly reduced; sc-r present or reduced; Rb bifurcation Rb before half length of wing; Rs elongate, usually longer than half of Mb; d-cell hexagonal, small, shorter or equal to length of M3; R3 more or less oblique; distal part of M1+2 and M3 clearly arched; outer gonostylus longer than inner one.
Rhabdomastix (Rhabdomastix) rafali sp. nov.
Figs. 1, 2.
ZooBank LSID: urn:lsid:zoobank.org:act:D8C77073-6D92-4683-A92C -173E623D5DE5.
Etymology: The name of the new species is dedicated to Rafał Stasiło, the husband of WJ-S.
Holotype: ISEA PAS MP/3092, well preserved male imago lacking some legs, the mouthparts not visible.
Type locality: Baltic area, the Blue Earth (Blaue Erde).
Type horizon: Secondary deposit found mainly in glauconitic marine sediments of middle Eocene age (Lutetian Stage; 47.8–41.3 Ma), deposited along the paleo-North Sea margin (Wolfe et al. 2016).
Material.—Holotype and ISEA PAS MP/3948a, male, well-preserved specimen, hypopygium poorly visible; ISEA PAS MP/3948b, male, well-preserved specimen, hypopygium poorly visible; ISEA PAS MP/3948c, male, well-preserved specimen, hypopygium poorly visible; all from Baltic amber (Lutetian, middle Eocene, 47.9–41.3 Ma).
Diagnosis.—Antenna elongate, thin, longer than body by about 0.3 times of its length, longer than wing, without color pattern; flagellomeres 1–13 narrow, elongate, first flagellomere 5 times as long as wide, flagellomeres 2–13 6, 7 times as long as wide, last flagellomere approximately 0.16 times of penultimate one, 2 times of its width; setae on antennae relatively sparsely distributed, shorter than length of segments bearing them; Sc terminating beyond half length of wing, just beyond half of Rs; Rs shorter than R2+3+4 and R4 combined, about 0.2 times of its length; tip of R1 beyond half length of d-cell, opposite approximately 0.75 times of R2+3+4; R4 3 times as long as R3, R4 shorter than R2+3+4; distance between tips of R1 and R3 longer than R3; M3 and d-cell of comparable length, m-cu situated just before half length of d-cell; d-cell 2.5 times its width, narrow at base, slightly expanded distally; anal angle unexpanded; distance between tips of A1 and A2 correspondence to approximately 2 times distance between tips of Cu and A1; tip of A2 before fork of Rb; tip of A1 before fork of Rs; haltere less than 0.25 times of wing length.
Description.—Body: 3.28–3.87 mm long, wing without color pattern, pterostigma absent, haltere slightly elongate (Fig. 1A1, A2).
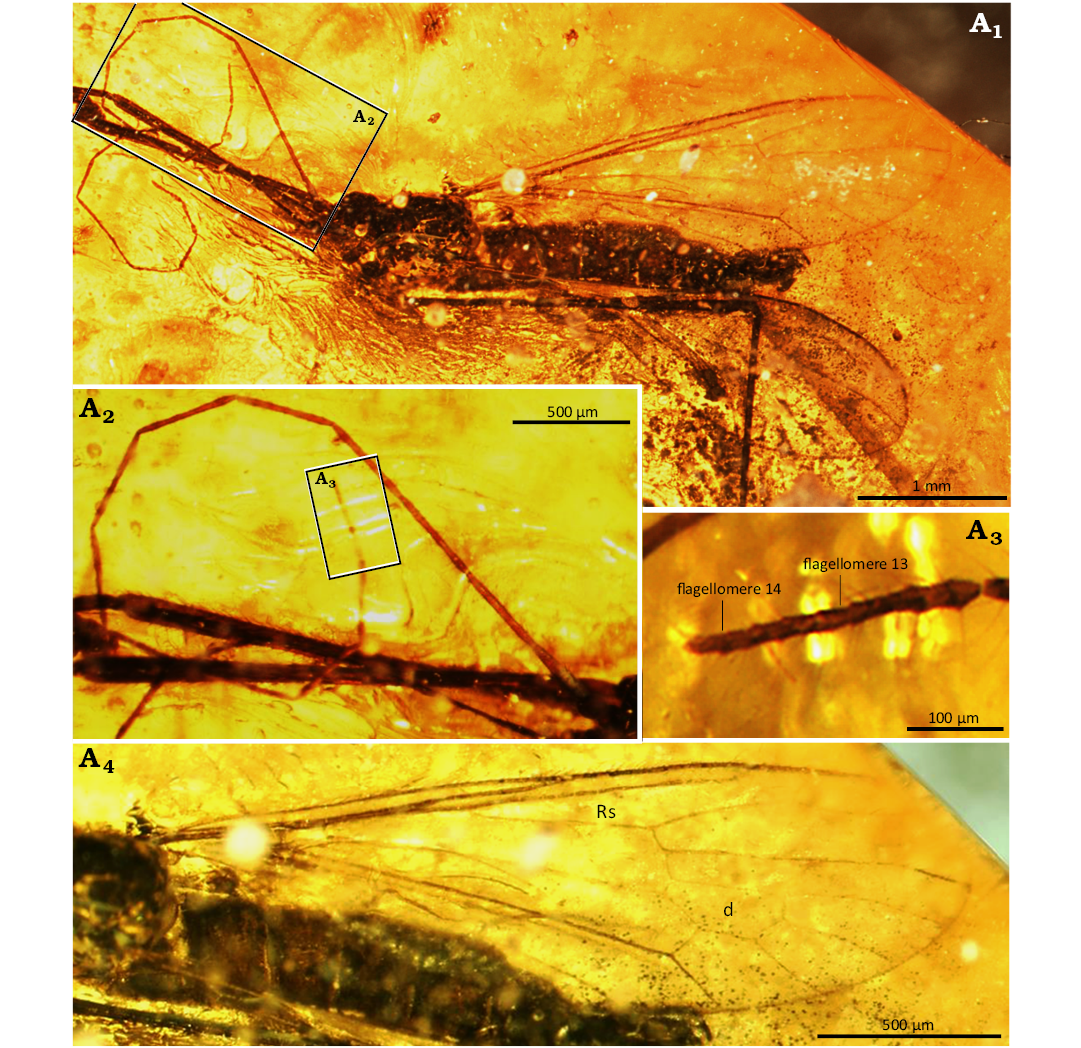
Fig. 1. Limoniid dipteran Rhabdomastix (Rhabdomastix) rafali sp. nov., holotype (male) ISEA PAS MP/3092 from Baltic amber, 47.8–41.3 Ma, Lutetian. Body in latero-dorsal view (A1), antenna in latero-dorsal view (A2), antenna, last flagellomere in lateral view (A3), wing (A4). Abbreviations: d, discal cell; Rs, radial sector.
Head: 0.30–0.41 mm wide; antenna (Figs. 1A1–A3, 2A2) with scape slightly elongate, scape 2 times as long as wide; pedicel narrow at the base, only slightly wider in the distal part, as long as wide; setae on the flagellomeres 3–15 times shorter than half the length of the segment bearing them, only slightly longer than its width; setae occur perpendicular to the surface of flagellomere; the last flagellomere with numerous setae at the tip; antenna 4.29 mm long: scape 0.07 mm long, pedicel 0.08 mm long; flagellomeres: 1, 0.26 mm; 2, 0.29 mm; 3, 0.34 mm; 4, 0.34 mm; 5, 0.34 mm; 6, 0.34 mm; 7, 0.34 mm; 8, 0.34 mm; 9, 0.35 mm; 10, 0.33 mm; 11, 0.29 mm; 12, 0.27 mm; 13, 0.27 mm; 14, 0.04 mm.
Thorax: Wing 3.30–3.50 mm long, 0.77–0.95 mm wide, 3.5 times as long as wide (Figs. 1A1, A4, 2A1); crossvein (h) situated at 0.2 times the length of the wing from its base; crossvein sc-r occurs at the distance from the tip of the vein Sc equal to 2 times its length; the distance between tips of Sc and R1 3.5 times longer than the distance between tips of R1 and R3; R3 short, slightly shorter than the distance between tips of R1 and R3; vein M1+2 1.3 times longer than d-cell; M4 short, shorter than d-cell; Mb 3 times as long as M3; Cu from the point of connection with crossvein m-cu to the edge of wing straight; the distance between the tips of M1+2 and M3 shorter than the distance between tips of M3 and M4; the distance between the tips of M3 and M4 shorter than the distance between tips of M4 and Cu; the distance between the tips of M4 and Cu and also the tips of Cu and A1 comparable; distance between the tips of A1 and A2 and also the tips of M4 and A1 comparable; A1 almost straight, A2 slightly sinusoidal.
Haltere: 0.68–0.72 mm long, stem short 1.5 times as long as wide, as massive knob.
Hypopygium: 0.63–0.88 mm long.
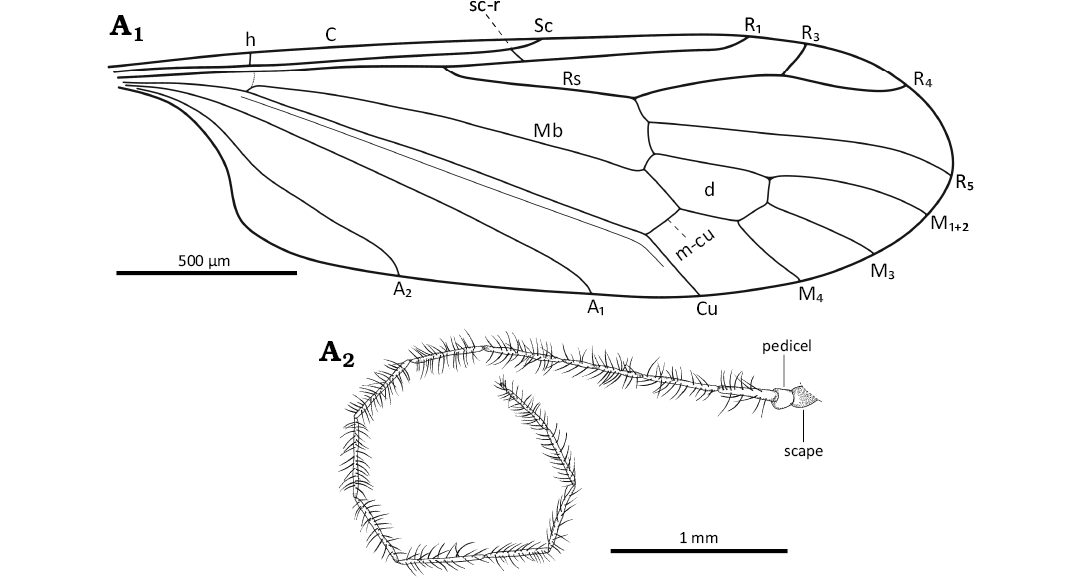
Fig. 2. Limoniid dipteran Rhabdomastix (Rhabdomastix) rafali sp. nov., holotype (male) ISEA PAS MP/3092 from Baltic amber, 47.8–41.3 Ma, Lutetian. Wing venation (A1), antenna (A2). Abbreviations: A1, A2, anal veins; C, costal vein; Cu, cubital vein; d, discal cell; h, humeral vein; M1+2 to M4, medial veins; Mb, medial-basal vein; m-cu, medial-cubital crossvein; R1–R5, radial veins; Rs, radial sector Sc, subcostal vein; sc-r, subcostal radial crossvein.
Remarks.—The features which allow to include the species within the subgenus Rhabdomastix are as follows: elongate antennae, longer than body length, crossvein well developed, d-cell hexagonal, distal part of M1+2 and M3 arched, outer gonostylus longer than inner gonostylus. Antenna in Rhabdomastix (R.) rafali sp. nov. are longer than the body length. Antenna longer than the body length also occur in the following species: Rhabdomastix (R.) grussica Podenas, 2006 comb. nov., Rhabdomastix (R.) mastix Podenas, 2006 comb. nov., Rhabdomastix (R.) setix Podenas, 2006 comb. nov., Rhabdomastix (R.) setosa sp. nov., and Rhabdomastix (R.) woottoni sp. nov. In the remaining species represented in Cenozoic fossil resins belonging to the subgenus Rhabdomastix, antennae are shorter than the body length. In contrast to R. (R.) woottoni sp. nov. a species with the antennae very thin and the very elongate flagellomeres with a few and short setae, antennae are longer than the length of the body by about half its length, in R. (R.) rafali sp. nov. the antennae are longer than the body by only 0.3 times of its length, they are not as thin as in R. (R.) woottoni sp. nov. and the setae are numerous, although they are relatively sparsely distributed on each segment and more elongate in contrast to those occurring in R. (R.) woottoni sp. nov. In R. (R.) setosa sp. nov. setae on each of the flagellomeres are much longer and more dense, often longer than half the length of the segment on which they occur, in R. (R.) rafali sp. nov. the setae are always shorter.
In R. (R.) rafali sp. nov. the vein M3 is as long as d-cell, unlike in R. (R.) grussica Podenas, 2006 comb. nov., R. (R.) mastix Podenas, 2006 comb. nov., R. (R.) setix Podenas, 2006 comb. nov., R. (R.) setosa sp. nov. and R. (R.) woottoni sp. nov., in which the vein M3 is much longer than d-cell. Moreover, the vein Sc in R. (R.) mastix Podenas, 2006 comb. nov. is very short, ending before half the length of Rs, and in R. (R.) rafali sp. nov. it ends just beyond half the length of Rs, measured from the fork of Rb. In contrast to R. (R.) grussica Podenas, 2006 comb. nov. the distance between the tips of A1 and A2 is 2.5 times longer than the distance between the tips of Cu and A1, in R. (R.) rafali sp. nov. this distance is 2 times longer than the distance between the tips of the Cu and A1. Moreover, in a species described from the Eocene sediments of the Green River, USA, Rhabdomastix scudderi Cockerell, 1920, the Sc vein is very elongate, ending just before the Rs bifurcation, and the vein A2 just before the fork of Rs, while in R. (R.) rafali sp. nov. Sc connect with C just behind half of Rs, vein A2 ends before the fork of Rb, measured from the base of the wing.
Stratigraphic and geographic range.—Baltic amber is dated by some authors to middle Eocene age (Lutetian Stage; 47.8–41.3 Ma) (Wolfe et al. 2016), but the age range of this fossil resin is still under debate, dated by Grimaldi and Ross (2017) as Priabonian, based on pollen, spores and phytoplankton of the amber-bearing layer, the so-called Blue Earth. The specimen was collected in the Baltic area from the Blue Earth (Blaue Erde) (Wolfe et al. 2016).
Rhabdomastix (Rhabdomastix) setosa sp. nov.
Figs. 3, 4.
ZooBank LSID: lsid:zoobank.org:act:E6B70F17-F77B-40DE-B319-13 B9E8DCA87E
Etymology: From Latin saetae, setaceous, bristly. Adjective.
Holotype: SDEI CCHH 1462-2, male, specimen well preserved, only some of the legs are not preserved (coll. Ch. and H.W. Hoffeins).
Type locality: Baltic area, the Blue Earth (Blaue Erde).
Type horizon: Secondary deposit found mainly in glauconitic marine sediments of middle Eocene age (Lutetian Stage; 47.8–41.3 Ma), deposited along the paleo-North Sea margin (Wolfe et al. 2016).
Material.—ISEA PAS MP/3472, male, specimen very poorly preserved; ISEA PAS MP/3945, male, specimen poorly preserved, only the wing and antennae were well visible; SDEI CCHH 1471-2, male, specimen well preserved, only some of the legs are not preserved; all from Baltic amber, Lutetian, middle Eocene, 47.9–41.3 Ma.
Diagnosis.—Antennae elongate, thin, longer than body by about 0.3 times of its length, longer than wing, without color pattern; flagellomeres elongate, first flagellomere almost 5 times as long as wide, flagellomeres 2–10, 7 times as long as wide, flagellomeres 11–13, 5 times as long as wide, last flagellomere half length of penultimate one; long setae on flagellomeres elongate, only slightly shorter than length of segments bearing them; tip of Sc beyond half length of wing, opposite 0.6 times of Rs; Rs shorter than R2+3+4 and R4 combined, by about 0.3 times of its length; R1 ends beyond half length of d-cell, opposite approximately 0.6 times of R2+3+4; R4 4 times as long as R3, R4 longer than R2+3+4; distance between tips of R1 and R3 longer than R3; M3 2 times longer than d-cell, m-cu situated beyond half length of d-cell, at level of approximately 0.6 times of d-cell length; d-cell 2 times as long as wide, narrow at base, widened in distal part; anal angle unexpanded; distance between tips of A1 and A2 2.5 times longer than distance between tips of Cu and A1; tip of A2 before Rb bifurcation; tip of A1 before Rs fork; outher gonostylus elongate, narrow, only slightly longer than inner gonostylus; haltere arrange less than 0.25 times of wing length.
Description.—Body: 3.12–3.36 mm long, dark brown (Fig. 3A1), wing without color pattern, pterostigma absent, haltere slightly elongate (Fig. 3A2).
Head: 0.24 mm wide (Fig. 3A1); antennae elongate (Figs. 3A1, A6, 4A4) scape narrower at the base, slightly widened distal; padicel 2 times as long as wide, in the middle of the pedicel there are single, relatively short setae; flagellomeres very narrow, thin, elongate. Antenna 3.61–4.23 mm long: scape 0.09–0.10 mm long; pedicel 0.10 mm long; flagellomeres: 1, 0.14–0.21 mm; 2, 0.27–0.32 mm; 3, 0.28–0.32 mm; 4, 0.28–0.34 mm; 5, 0.28–0.34 mm; 6, 0.28–0.34 mm; 7, 0.28–0.35 mm; 8, 0.28–0.37 mm; 9, 0.28–0.36 mm; 10, 0.26–0.32 mm; 11, 0.25–0.28 mm; 12, 0.25–0.26 mm; 13, 0.22–0.17 mm; 14, 0.05–0.07 mm. Palpus (Figs. 3A1, A5, 4A2) 0.32–0.47 mm long (1, 0.05–0.11 mm; 2, 0.08 mm; 3, 0.08–0.11 mm; 4, 0.11–0.17 mm); palpomeres elongate; the last palpomere 2 times as long as penultimate one, 6 times as long as wide, other palpomeres 2 times as long as wide; on each palpomeres several setae of varying length, 1.5– 3 times longer than the width of the segment on which they occur.
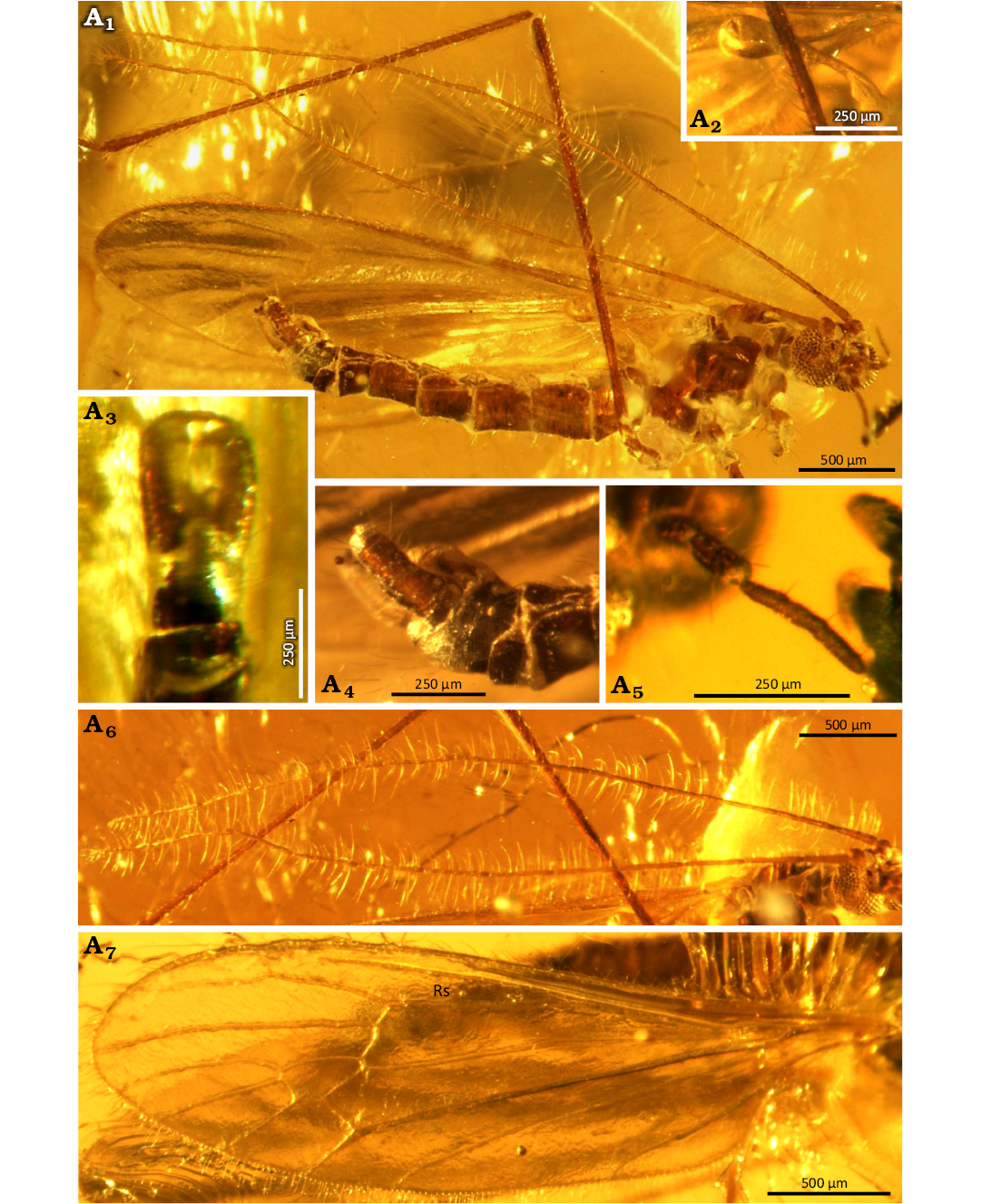
Fig. 3. Limoniid dipteran Rhabdomastix (Rhabdomastix) setosa sp. nov., holotype (male) SDEI CCHH 1462-2 from Baltic amber, 47.8–41.3 Ma, Lutetian. Body in lateral view (A1), haltere in ventral view (A2), hypopygium in ventral (A3) and lateral (A4) views, palpus in latero-ventral view (A5), antenna in lateral view (A6), wing (A7). Abbreviation: Rs, radial sector.
Thorax: Wing 3.23–3.50 mm long, 0.79–0.91 mm wide, 3.5 times as long as wide (Figs. 3A1, A7, 4A1); crossvein (h) positioned at 0.2 times the length of the wing from its base; crossvein sc-r terminating 2 times its own length from the tip of Sc; the distance between tips of Sc and R1 2.5 times longer than the tips of R1 and R3; M1+2 over 2 times longer than d-cell; M4 short, slightly longer than d-cell; Mb 3 times as long as M3; Cu from the point of connection with crossvein m-cu to the edge of wing straight; A1 slightly bent at the edge of the wing; A2 sinusoidal. The distance between the tips of M1+2 and M3 shorter than the distance between the tips of M3 and M4; the distance between the tips of M3 and M4 and the tips of M4 and Cu, and the distance between the tips of M4 and Cu and also the tips of Cu and A1 comparable; the distance between the tips of A1 and A2 slightly longer than the distance between the tips of M4 and A1; A1 almost straight, A2 slightly sinusoidal.
Haltere: 0.48–0.53 mm long, sterm long, narrow 2 times as long as knob (Fig. 3A2).
Hypopygium: elongate, 0.63 mm long, gonocoxites very elongate, rather narrow, outer gonostylus elongate, narrow, inner gonostylus slightly widened at the base, narrowed distally, numerous setae on the outer and inner gonostylus (Figs. 3A1, A3–A5, 4A3).
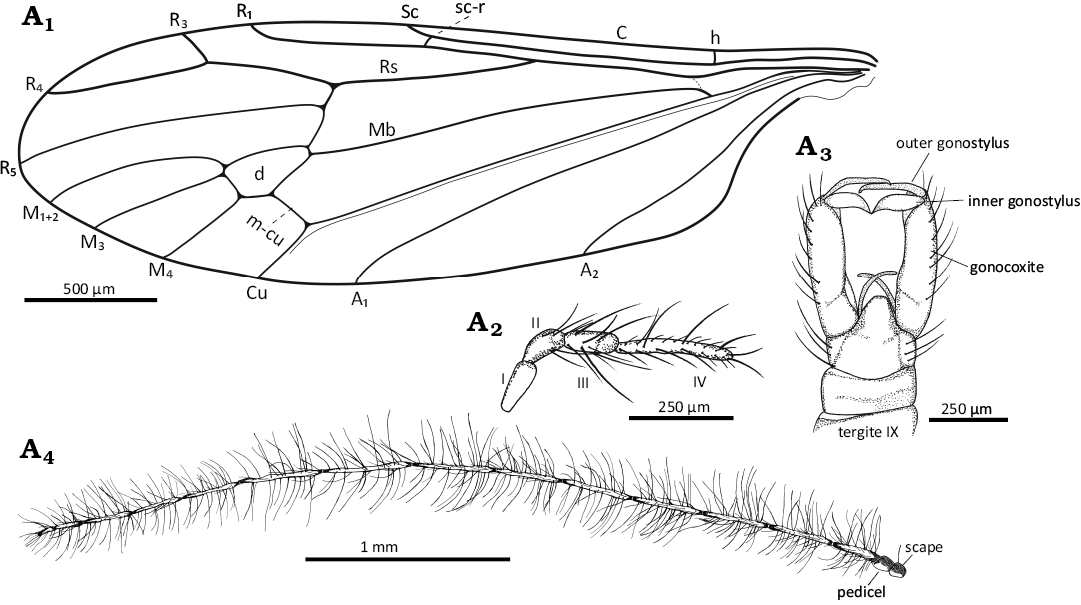
Fig. 4. Limoniid dipteran Rhabdomastix (Rhabdomastix) setosa sp. nov., hololtype (male) SDEI CCHH 1462-2 from Baltic amber, 47.8–41.3 Ma, Lutetian. Wing venation (A1), palpus (A2), hypopygium in ventral view (A3), and antenna (A4). Abbreviations: A1, A2, anal veins; C, costal vein; Cu, cubital vein; d, discal cell; h, humeral vein; I–IV, palpomeres 1–4; M1+2 to M4, medial veins; Mb, medial-basal vein; m-cu, medial-cubital crossvein; R1–R5, radial veins; Rs, radial sector; sc-r, subcostal radial crossvein.
Remarks.—The features which allow to include the species within the subgenus Rhabdomastix are: elongate antennae, longer than body length, crossvein well developed, d-cell hexagonal, distal part of M1+2 and M3 arched, outer gonostylus longer than inner gonostylus.
The antennae in Rhabdomastix (R.) setosa sp. nov. are longer than the body length. Antennae longer than the body length also occur in the following species: Rhabdomastix (R.) grussica Podenas, 2006 comb. nov., Rhabdomastix (R.) mastix Podenas, 2006 comb. nov., Rhabdomastix (R.) rafali sp. nov., Rhabdomastix (R.) setix Podenas, 2006 comb. nov., and Rhabdomastix (R.) woottoni sp. nov. In other species known from Cenozoic resins, belonging to the subgenus Rhabdomastix, the antennae are shorter than the body length. In R. (R.) woottoni sp. nov. antennae are 1.5 times as long as the body and are very narrow, flagellomeres are very elongete with numerous, short setae. In R. (R.) setosa sp. nov. antenna are not so elongate, flagellomeres are much shorter than their length, only 7 times as long as wide. The setae on each flagellomere are much longer and more dense. Moreover, the R. (R.) setosa sp. nov. differs from other Cenozoic species within the subgenus Rhabdomastix in its wing venation and morphology of the hypopygium. Unlike the R. (R.) grussica Podenas, 2006 comb. nov., setae occur on the antennae constitute even more than half the length of the segment bearing them, in R. (R.) grussica Podenas, 2006 comb. nov. they are never longer than half the length of the flagellomeres bearing them. The d-cell in R. (R.) setosa sp. nov. is small, 1.5 times as long as wide, vein M3 is 2 times as long as the d-cell. In R. (R.) grussica Podenas, 2006 comb. nov. d-cell is 2 times as long as wide, vein M3 is only slightly longer (1.5 times). In R. (R.) mastix Podenas, 2006 comb. nov. the setae are shorter than half the length of the flagellomeres on which they occur, d-cell is 2 times as long as wide. Vein Sc in R. (R.) mastix Podenas, 2006 comb. nov. is very short, ending before half the length of Rs, while in R. (R.) setosa sp. nov. this vein ending just beyond half the length of Rs, measured from the fork of Rb. In contrast to R. (R.) setix Podenas, 2006 comb. nov. in R. (R.) setosa sp. nov. crossvein m-cu is situated beyond half the length of the d-cell, measured from the fork of Mb, R3 is shorter than the distance between the tips of R1 and R3, almost straight vein R4 is 4 times as long as R3. R. (R.) setix Podenas, 2006 comb. nov. crossvein m-cu is situated beyond half the length of d-cell, R3 is shorter than the distance between the tips of R1 and R3, almost straight vein R4 is 4 times as long as R3. In R. (R.) setix Podenas, 2006 comb. nov. m-cu situated before d-cell, R3 is longer than the distance between the tips of R1 and R3, R4 is arched and is only 3 times longer than R3. Moreover, in a species described from the Eocene of the Green River, USA Rhabdomastix scudderi vein Sc is very elongate, ending just before the bifurcation of Rs, tip of vein A2 is just before the bifurcation of Rs, while in R. (R.) setosa sp. nov. Sc ending just beyond half the length of Rs, and vein A2 terminating before fork of Rb.
Stratigraphic and geographic range.—Baltic amber is dated by some authors to middle Eocene age (Lutetian Stage; 47.8–41.3 Ma) (Wolfe et al. 2016), but the age range of this fossil resin is still under debate, dated by Grimaldi and Ross (2017) as Priabonian, based on pollen, spores and phytoplankton of the amber-bearing layer, the Blue Earth. The specimen was collected at the Baltic area, the Blue Earth (Blaue Erde) (Wolfe et al. 2016).
Rhabdomastix (Rhabdomastix) woottoni sp. nov.
Figs. 5, 6.
ZooBank LSID: lsid:zoobank.org:act:92A02AC5-1693-4BC0-AB01-CBE1560931CD
Etymology: Dedicated to the outstanding invertebrate researcher, Robin J. Wootton (Department of Biological Sciences, University of Exeter, UK).
Holotype: GMUG 80, male, the specimen is poorly preserved, the head is not well visible, partially damaged. (coll. Manfred Kutscher).
Type locality: Bitterfeld, Upper Saxony (Sachsen-Anhalt), Germany.
Type horizon: Cottbus Formation, “Glimmersand”, middle–upper Eocene.
Material.—Holotype only.
Diagnosis.—Antennae very elongate, thin, much longer than body, 1.5 times longer; almost black, small spots usually present at base and distal part of flagellomeres, longer than wing; flagellomeres very narrow, significantly elongate, length of flagellomeres 1–3 corresponding to approximately 5 times (first flagellomere) or 6 times (second and third flagellomere) width of each of them, with numerous short setae, flagellomeres 4–9 elongate, corresponding to approximately 26 times their width, flagellomeres 10–14 shorter than others, length of last segment corresponds to half of penultimate one and 3 times its width, the setae of flagellomeres 4–9 very few and short, much shorter than length of segment on which they occur; tip of Sc beyond half length of wing, beyond half of Rs; Rs shorter than R2+3+4 and R4 combined by about 0.2 times of its length; tip of R1 opposite half of d-cell, opposite approximately half length of R2+3+4; length of R4 4 times length of R3, R4 only slightly shorter than R2+3+4; distance between tip of R1 and R3 longer than R3; M3 1.5 times longer than d-cell; m-cu just behind half of d-cell; d-cell length corresponding to 1.5 times its width, only slightly narrowed at base, slightly widened in distal part; anal angle expanded; distance between the tips of A1 and A2 corresponds to almost 5 times distance between tips of Cu and A1; tip of A2 before Rb fork; tip of A1 beyond Rs fork; outer gonostylus elongate, narrow, sharply tipped, with one thick setae in apical part, only slightly longer than inner gonostylus, pointed at the end, with two thick setae at the end; haltere less than 0.25 times of wing length.
Description.—Body: 2.54 mm long, dark brown, wing without color pattern, pterostigma absent, haltere significantly elongate.
Head: 0.35 mm wide (Fig. 5A4); antenna elongate (Figs. 5A1, A3, 6A1–A4), very thin, scape pear-shaped, elongate, longer than its width, significantly expanded distally; pedicel short and very wide, wider than its length, a few elongate setae in the distal part of the scape and pedicel; the last flagellomere bluntly ended with two setae on the tip; antenna 4.40 mm long: scape 0.12 mm long; pedicel 0.09 mm long; flagellomeres: 1/0.22 mm; 2/0.27 mm; 3/0.36 mm; 4/0.46 mm; 5/0.45 mm; 6/0.45 mm; 7/0.41 mm; 8/0.41 mm; 9/0.40 mm; 10/0.36 mm; 11/0.17 mm; 12/0.09 mm; 13/0.08 mm; 14/0.06 mm; palpus (Fig. 6A6) 0.55 mm long (1/0.09 mm; 2/0.11 mm; 3/0.13 mm; 4/0.22 mm); palpomeres narrow and elongate, first and second palpomere of comparable length, approximately 2 times as long as wide, slightly expanded distally, the third palpomere longer than the first and the second but shorter than the last one, third palpomere 4 times as long as wide, the last palpomere 6 times as long as wide; each palpomeres with a few setae, slightly longer than the width of segments bearing them and much shorter than the length of the segments on which they occur.
Thorax: Wing 4.45 mm long, 1.52 mm wide, almost 3 times as long as wide (Figs. 5A2, 6A5); crossvein (h) positioned at the distance of 0.1 times of the length of the wing, measured from its base; crossvein sc-r occurs at the distance from the tip of the vein Sc corresponding to 3 times of its length; the distance between the tips of Sc and R1 slightly longer than the distance between the tips of R1 and R3; R3 short, shorter than half the distance between the tips of R1 and R3; M1+2 more than 2 times as long as the d-cell; M4 short, as long as d-cell; Mb 3 times as long as M3; the section of the Cu from the point of connection of the Cu with crossvein m-cu to the edge of the wing almost straight; the distance between the tips of M1+2 and M3 shorter than the distance between the tips of M3 and M4; distances between the tips of M3 and M4 and the tips of M4 and Cu comparable; the distance between the tips of M4 and Cu 0.3 times longer than the distance between the tips of Cu and A1; the distance between the tips of A1 and A2 2 times longer than the distance between the tips of M4 and A1; A1 slightly curved, A2 sinusoidal.
Haltere: 0.57 mm long, stem massive, only slightly longer than knob.
Hypopygium: 0.84 mm long, gonocoxites massive, elongate 2 times as long as wide with numerous long and thick setae (Figs. 5A5, 6A7, A8).

Fig. 5. Limoniid dipteran Rhabdomastix (Rhabdomastix) woottoni sp. nov., holotype (male) GMUG 80 from Bitterfeld amber, middle–upper Eocene. Antenna (A1), wing (A2), middle flagellomeres (A3), head detail in lateral view (A4), hypopygium in ventral view (A5). Arrows indicate dark spots on flagellomeres. Abbreviations: d, discal cell; Rs, radial sector.
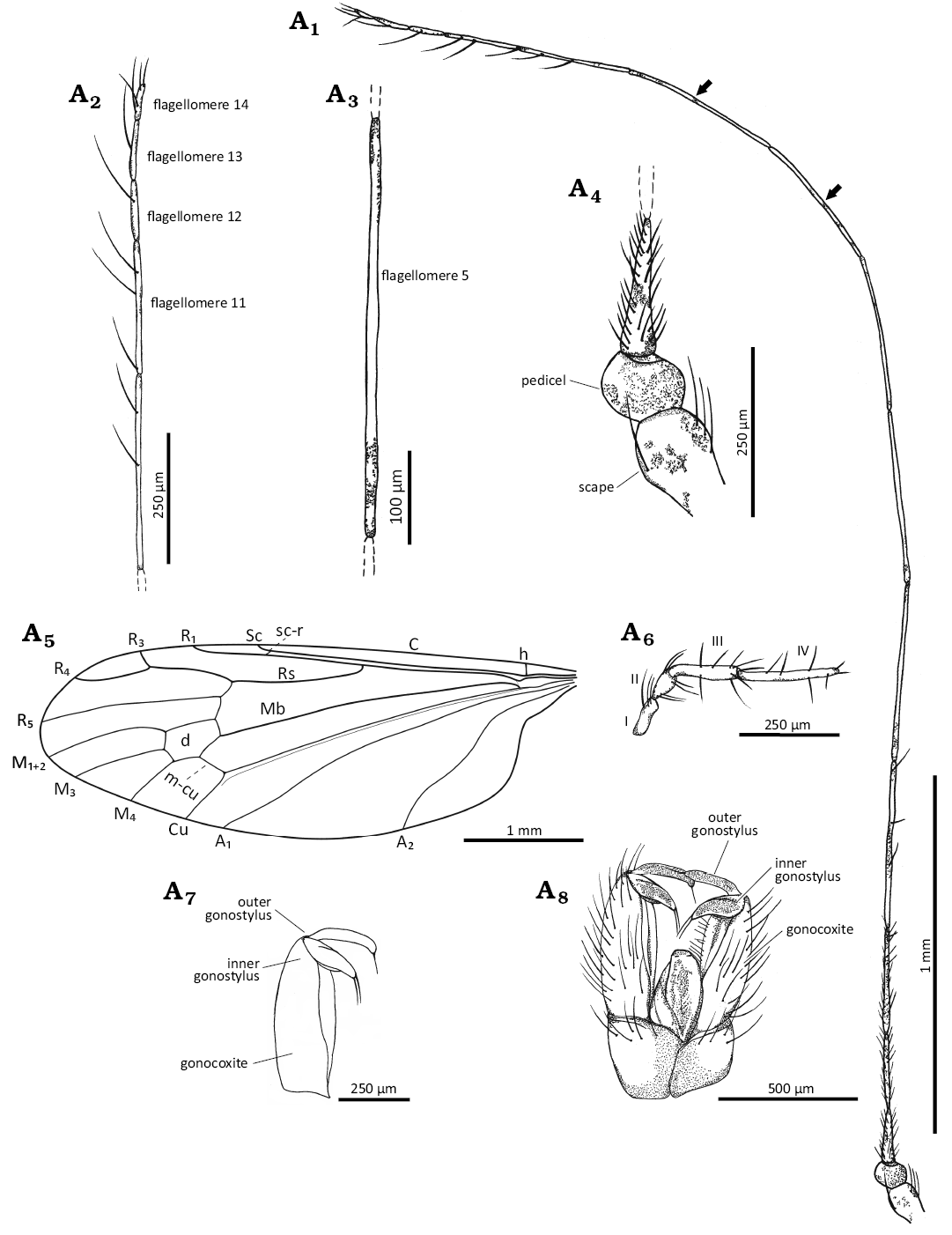
Fig. 6. Limoniid dipteran Rhabdomastix (Rhabdomastix) woottoni sp. nov., holotype holotype (male) GMUG 80 from Bitterfeld amber, Middle–Late Eocene. Antenna (A1), the last flagellomeres (A2), middle flagellomere (A3), scape and pedicel (A4), wing venation (A5), palpus (A6), gonocoxite and gonostylus (A7), and hypopygium (A8). Arrows indicate dark spots on flagellomeres. Abbreviations: A1, A2, anal veins; C, costal vein; Cu, cubital vein; d, discal cell; h, humeral vein; I–IV, palpomeres 1–4; M1+2 to M4, medial veins; Mb, medial-basal vein; m-cu, medial-cubital crossvein; R1–R5, radial veins; Rs, radial sector; sc-r, subcostal radial crossvein.
Remarks.—The features which allow to include the species within the subgenus Rhabdomastix are: elongate antennae, much longer than body length, crossvein well developed, d-cell hexagonal, distal part of M1+2 and M3 arched, outer gonostylus longer than inner gonostylus.
Rhabdomastix (R.) woottoni sp. nov. differs from the other species belonging to the subgenus Rhabdomastix represented in Cenozoic fossil resins by the proportions of the length of the antennae in relation to the length of the body. The antennae of this species are much longer than the body, about half its length, very elongate and thin. The individual flagellomeres are very elongate and narrow, the scape and pedicel are wide, massive, much wider than the other flagellomeres. In addition, there are single, clear, almost black color spots on the antenna and relatively short, sparse setae. So far, no fossil species of the Rhabdoamastix genus have had this type of antennae. In other species belonging to the subgenus Rhabdomastix, represented in Cenozoic fossil resins, no color spots are observed on flagellomeres, the setae of this part of the body are more pronounced, usually numerous and more elongate. The shape of the scape and pedicel are also slightly different in the remaining species within the subgenus Rhabdomastix represented in Cenozoic fossil resins, they are not so massive in relation to the flagellomeres, unlike in R. (R.) woottoni sp. nov. Moreover, R. (R.) woottoni sp. nov. differs from the species described from Eocene sediments from Green River, USA R. scudderi in the wing venation. Vein Sc in R. scudderi is very elongate, ending just before the Rs fork, and the vein A2 achieves the edge of the wing just before the Rs fork, while in R. (R.) woottoni sp. nov. it is much shorter, ended in half the length of Rs, and the end of the A2 vein is situated far before the fork of Rb, measured from the base of the wing.
Stratigraphic and geographic range.—Bitterfeld amber is believed to be approximately the same age as Baltic amber (Szwedo and Sontag 2013), but it came probably from distinct ecosystems. Biterfeld amber belongs to the same group of ambers as Baltic amber, with an age similar to that of Baltic amber, as indicated by the dipteran species similarity preserved in these fossil resins. The similarity of the species preserved as an inclusions in this fossil resin contradicts the large age difference of these fossil resins. The differences found seem to reflect differences ecological conditions, not age (Standtke 2008). Some recent geochronological efforts (Knuth et al. 2002; Blumenstengel 2004) date deposits of Bitterfeld amber as the late Oligocene (Chattian; 23.0–28.1 Ma), some authors date the deposits of this fossil resin as Early Miocene (e.g., Barthel and Hetzer 1982; Rikkinen and Poinar 2000). But, some have argued that Bitterfeld amber being merely a younger redeposited fraction of primary Eocene Baltic amber (Szwedo and Sontag 2013; Wolfe et al. 2016), the resin can be dated on middle–late Eocene (Drohojowska et al. 2024). The specimen was collected at the Cottbus Formation near the town of Bitterfeld in Upper Saxony (Sachsen-Anhalt) (Wolfe et al. 2016).
Some species were previously described by Podenas (2006) and have not been classified into any of the subgenera. Features such as the complete atrophy of r-r (R2), presence of sc-r, the bifurcation of Rb before half the length of the wing, the presence of small, hexagonal d-cell shorter than the length of the vein M3, a cleary arched distal part of part M1+2 and M3, and the inner gonostylus shorter than the outher gonostylus, allow the following three species: Rhabdomastix (R.) grussica Podenas, 2006 comb. nov., Rhabdomastix (R.) setix Podenas, 2006 comb. nov., Rhabdomastix (R.) mastix Podenas, 2006 comb. nov, all from the Baltic amber, to be included into the subgenus Rhabdomastix.
Rhabdomastix (Rhabdomastix) grussica Podenas, 2006 comb. nov.
2006 Rhabdomastix (?) grussica; Podenas 2006: 46.
Remarks.—The species is characterized by elongate antennae, elongate flagellomeres with numerous setae and a very small apical segment, antennae longer than the body and wings. The wings of this species are typical for the genus. Hypopygium is characterized by straight elongated gonocoxites and long, slender outer gonostylus, many setae occur on inner gonostylus, interbase are long, with a straight aedeaus ending before tip of interbase and by slightly extended at mid-posterior margin ninth sternite (after Podenas 2006).
Rhabdomastix (Rhabdomastix) setix Podenas, 2006 comb. nov.
2006 Rhabdomastix (?) setix; Podenas 2006: 50.
Remarks.—The species is characterized by long antennae, longer than the body, with many long setae and a small apical segment, much smaller than the previous one. The wings are typical for the genus. Hypopygium of this species is characterized by long, slender bare outer gonostylus, without apical spine, fleshy inner gonostylus with straight posterior margin and slightly curved apex (after Podenas 2006).
Rhabdomastix (Rhabdomastix) mastix Podenas, 2006 comb. nov.
Figs. 7, 8.
2006 Rhabdomastix (?) mastix sp. nov.; Podenas 2006: 47, figs. 27–30.
Material.—ISEA PAS MP/3321, female from Baltic amber, Lutetian, middle Eocene, 47.9–41.3 Ma.
Emended diagnosis.—Antennae longer than body, shorter than wings, without color pattern; flagellomers 1–13 narrow of length corresponding to approximately 7 times their width, last flagelomere approximately 0.3 times shorter than penultimate one; setae shorter than flagellomeres bearing them, densely spaced; Sc terminating before half length of wing, opposite 0.3 times of Rs; Rs shother than R2+3+4 and R4 combined, approximately 0.2 times of its length; R1 terminating opposite end of d-cell, opposite of 0.8 times of R3+4; length of R4 corresponds to 4 times length of R3; R4 longer than R2+3+4 by about 0.2 times of its length; distance between tips of R1 and R3 longer than R3; M3 1.5 times longer than d-cell; m-cu situated in the middle of length of d-cell, d-cell length corresponding to 2 times its width, narrow at base, extended in the distal part; anal angle unexpanded; distance beetween tips of A1 and A2 correspond to almost 2.5 times distance between tips of Cu and A1; tip of A2 before Rb fork; A1 before Rs fork; posterior edge of outer gonostylus serrated; haltere elongate, as long as 0.2 times wing length.
Description.—Body: 3.39 mm long, brown (Fig. 7A1), wing without color pattern, pterostigma absent, haltere significantly elongate (Fig. 7A2).
Head: 0.30 mm wide (Fig. 7A1); antenna with cylindrical scape, narrowed at the base, slightly wider in the distal part (Figs. 7A1, 8A2), scape and pedicel slightly longer than their width; pedicel with a few setae; flagellomeres 1–13 of comparable length; the last flagellomere with two setae at the tip, penultimate one 3 times longer than last one; setae on flagellomers 3–15 longer than the width of the flagellomeres bearing them, shorter than half the length of these segments; antenna 1.09 mm long: scape 0.06 mm; pedicel 0.09 mm; flagellomeres: 1, 0.07 mm; 2, 0.07 mm; 3, 0.07 mm; 4, 0.07 mm; 5, 0.07 mm; 6, 0.07 mm; 7, 0.07 mm; 8, 0.07 mm; 9, 0.07 mm; 10, 0.07 mm; 11, 0.07 mm; 12, 0.07 mm; 13, 0.07 mm; 14, 0.03 mm).
Palpus: 0.36 mm long (1, 0.06 mm; 2, 0.08 mm; 3, 0.08 mm; 4, 0.14 mm, Figs 7A1, 8A3), palpomeres elongate, palpomeres 1–3 of comparable length, the last palpomere 2 times longer than penulitmate one; first palpomere 3 times as long as wide, second palpomere 2 times as long as wide, third palpomere 2.5 times as long as wide; the last palpomere elongate, 5 times as long as wide; each palpomere with a few elongate setae, longer than wide but shorter than palpomeres bearing them.
Thorax: Wing 3.91 mm long, 0.79 mm wide, 3.5 times as long as wide (Figs. 7A1, 8A1); the distance between tips of Sc and R1 5 times longer tan the distance between the tips of R1 and R3; R3 short, slightly shorter than the distance between the tips of R1 and R3; M1+2 almost 2 times longer than length of d-cell; vein Mb approximately 2.5 times longer than the M3; Cu from the point of connection with crossvein m-cu to the edge of wing slightly curved; the distance between the tips of M1+2 and M3 shorter than 0.25 times of its length, shorter than the distance between the tips of M3 and M4; the distance between the tips of M3 and M4 slightly longer than the distance between the tips of M4 and Cu; the distance between the tips M4 and Cu and the tips Cu and A1 comparable length; the distance between the tips of A1 and A2 longer than 0.2 times the distance between the tips of M4 and A1; A1 and A2 almost straight.
Haltere: 0.60 mm long, sterm narrow, 2 times longer than knob (Fig. 7A2).
Ovipositor: 1.13 mm long, strongly sclerotized; cerci elongate, narrow and sharply ended; valves relatively short, narrow, 5 times as long as wide (Figs. 7A1, A3, 8A4).

Fig. 7. Limoniid dipteran Rhabdomastix (Rhabdomastix) mastix Podenas, 2006 comb. nov., (female) ISEA PAS MP/3321 from Baltic amber, 47.8–41.3 Ma, Lutetian. Body in latero-dorsal view (A1), haltere in lateral view (A2), ovipositor in lateral view (A3).
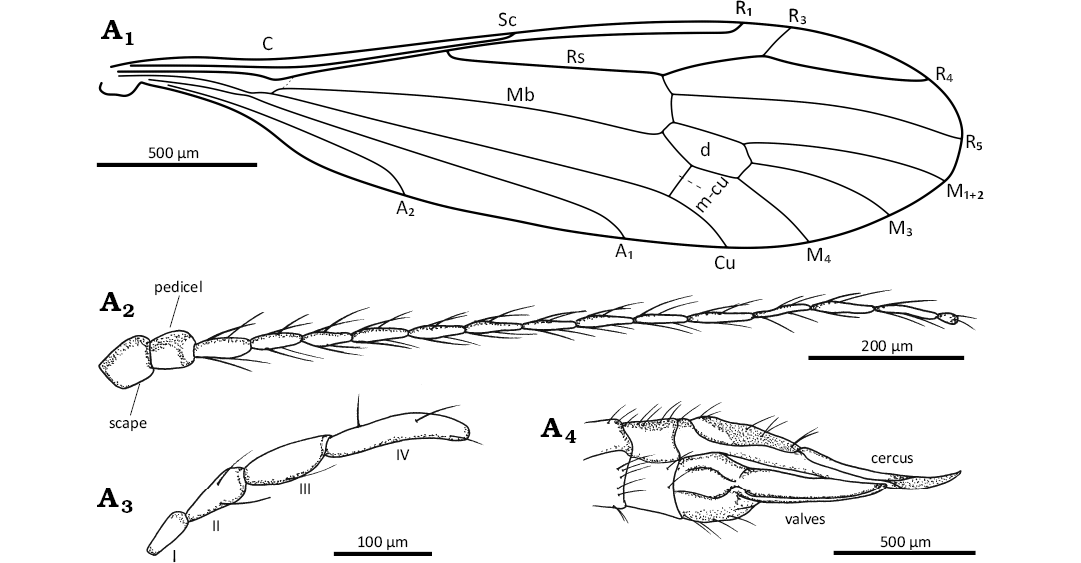
Fig. 8. Limoniid dipteran Rhabdomastix (Rhabdomastix) mastix Podenas, 2006 comb. nov., (female) ISEA PAS MP/3321 from Baltic amber, 47.8–41.3 Ma, Lutetian. Wing venation (A1), antenna (A2), palpus in lateral view (A3), ovipositor in lateral view (A4). Abbreviations: A1, A2, anal veins; C, costal vein; Cu, cubital vein; d, discal cell; I–IV, flagellomeres 1–4; M1+2 to M4, medial veins; Mb, medial-basal vein; m-cu, medial-cubital crossvein; R1–R5, radial veins; Rs, radial sector; Sc, subcostal vein; sc-r, subcostal radial crossvein.
Remarks.—Characters of wing morphology (female wing venation is almost identical compared to the male’s wing venation), as well as the ratio of the length of the hatltere to the length of the wings of the first pair allowed to classify female as Rhabdomastix (R.) mastix Podenas, 2006 comb. nov. In the female, just like in the male, the halteres constitutes 0.25 times of the length of the wings and are very elongate.
In contrast to the other species within the subgenus Rhabdomastix represented in Cenozoic fossil resins in R. (R.) mastix Podenas, 2006 comb. nov. the haltere is as much as 0.25 times of the length of the wing, in other species haltere is shorter than 0.25 times of the wing length.
Stratigraphic and geographic range.—Baltic amber is dated by some authors to middle Eocene age (Lutetian Stage; 47.8–41.3 Ma) (Wolfe et al. 2016), but the age range of this fossil resin is still under discussion, dated by Grimaldi and Ross (2017) as Priabonian, based on pollen, spores and phytoplankton of the amber-bearing layer, the so-called Blue Earth. The specimen was collected at the Baltic area, the Blue Earth (Blaue Erde) (Wolfe et al. 2016).
Discussion
The internal classification system of the genus Rhabdomastix used by Alexander (1931), Savchenko (1976), Savchenko et al. (1992) and Krzemiński (2004) was based on differences related mainly to the proportion of the length of male antennae to the length of the body. It included three subgenera: Rhabdomastix sensu stricto, with antennae two to three times longer than the body, Paleogonomyia Meunier, 1899, with antennae reaching the length of the body or half of it’s length, and Sacandaga Alexander, 1911, where the antennae were much shorter than the body length.
According to results of the latest analysis of the genus Rhabdomastix the latter is divided into three subgenera, two of them represented in fossil and recent fauna: Lurdia Starý, 2003, Rhabdomastix Skuse, 1890, and one known only from the fossil record, i.e., Myanmamastix Kania et al., 2021, from the Cretaceous of Myanmar. Moreover, Starý (2003) distinguished two morphological groups of species “Rhabdomastix caudata” and “Rhabdomastix illudens” without including them into any of the subgenera. The “Rhabdomastix caudata” group is centred around the species of Rhabdomastix caudata Lundbeck, 1898, with six representatives recovered from Nearctic region: Rhabdomastix leptodoma Alexander, 1943, Rhabdomastix monticola Alexander, 1916, Rhabdomastix subarctica Alexander, 1933, Rhabdomastix subcaudata Alexander, 1927a, one Westpalaearctic species Rhabdomastix parva Siebke, 1863 and known from the fossil record Rhabdomastix hoffeinsi Jordan-Stasiło et al., 2023. All these species are characterized by more or less developed crossvein r-r (R2) (Starý 2003; Oosterbroek et al. 2007; Jordan-Stasiło et al. 2023). The second group is represented by species centered around Neotropical Rhabdomastix illudens Alexander, 1914b, from Bolivia. The group comprises 11 Neotropical and Oriental species, and one from the Southern Nearctic, include also some species from Mexico like Rhabdomastix isabella Alexander, 1927b, Rhabdomastix mexicana Alexander, 1938 and also Rhabdomastix longiterebrata Alexander, 1938, from Costa Rica, such as Rhabdomastix septentrionalis Alexander, 1914b. Only one species classified to this group is recorded from Nearctic, i.e., Rhabdomastix nuttingi Alexander, 1950 from Arizona, USA (Alexander 1914a, b, 1927b, 1938, 1950; Starý 2003). All of these species are characterized by extremely long male antennae which is three to five times longer than the body and much longer than wings. Scape of these very elongate antennae is enlarged in contrast to small and short pedicel, last flagellomere is longer than the panultimate one. Other differences in body structure, especially differences in wing venation, coloration of the body, trichation of the wings are also well visible, but the most characteristic feature of this group of insects are the proportion o male antennae relative to body and wing length respectively.
Although all species belonging to the group called “Rhabdomastix illudens” are classified in the subgenus Rhabdomastix sensu stricto, the taxonomic status of this extralimital group still remains unresolved. As Starý (2003) emphasizes, this group is more closely related to Rhabdomasix sensu stricto than with the subgenus Lurdia. Moreover, it is considered to be a highly apomorphic evolutionary line of Rhabdomastix sensu stricto without subgeneric status (Starý 2003).
Starý (2003) proposed the extremely elongate antennae to be an apomorphic feature of the species belonging to “Rhabdomastix illudens” group, and we have an evidence that this feature appeared in the evolution of the group already in the Eocene. The antennae of all Cretaceous representatives of the genus Rhabdomastix are not so elongate, only slightly longer than the combined length of the head and body. The species Rhabdomastix (Myanmamastix) jarzembowskii Krzemiński, 2004, Rhabdomastix (Myanmamastix) asiatica Kania-Kłosok et al., 2021, Rhabdomastix (Myanmamastix) cretacica Kania-Kłosok et al., 2021, Rhabdomastix (Myanmamastix) krzeminskae Kania-Kłosok et al., 2021, and Rhabdomastix (Myanmamastix) myanmae Kania-Kłosok et al., 2021, belongs to one subgenus (Kania-Kłosok et al. 2021).
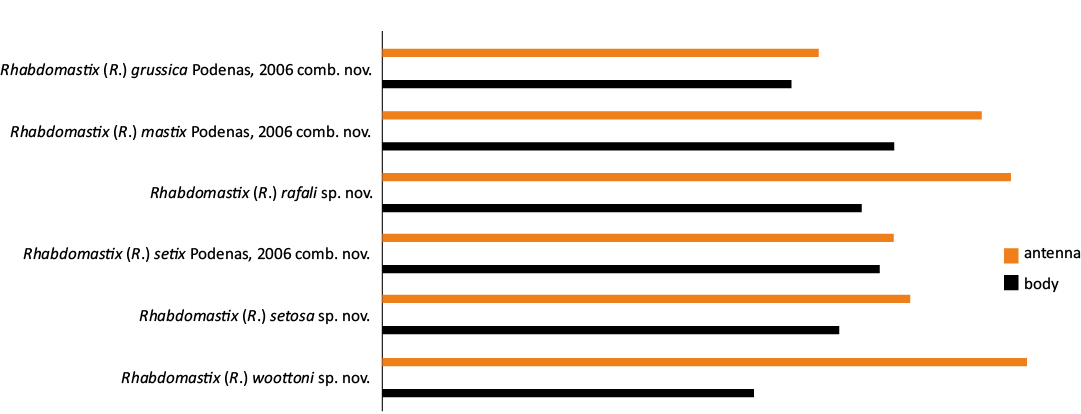
Fig. 9. Diagram showing the ratio of the length of the antennae to the body length of fossil representatives of subgenus Rhabdomastix Skuse, 1890, with the antenna longer than body.
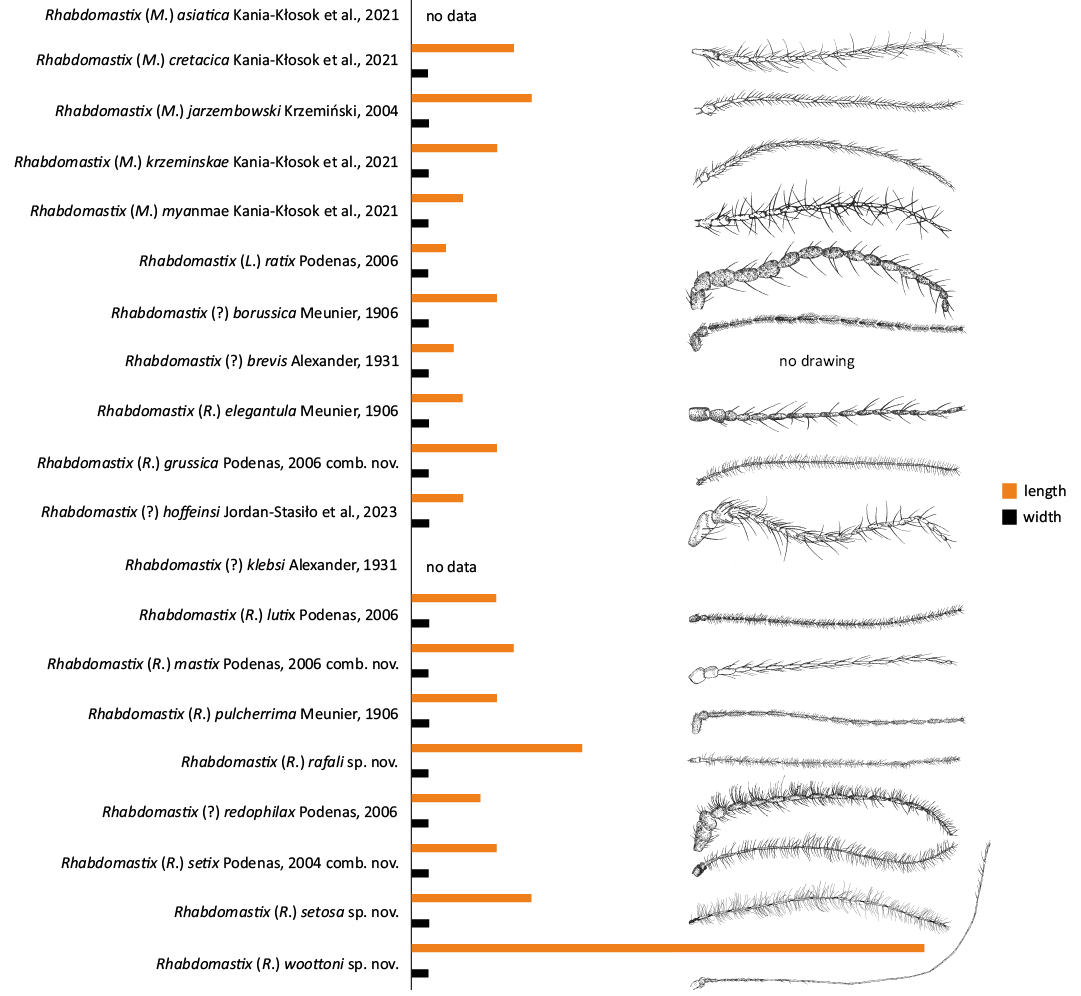
Fig. 10. Diagram showing the ratio of length to width of the flagellar antenna (F10). Antenna of Rhabdomastix (Rhabdomastix) rafali sp. nov., reconstructed; antennae of Rhabdomastix (Rhabdomastix) grussica Podenas, 2006 comb. nov., Rhabdomastix (Rhabdomastix) setix Podenas, 2006 comb nov., redrawn after Podenas (2006).
Between the Cretaceous and today, a group with this type of antennae morphology could have emerged, possibly as a result of the adaptation of these insects to the changing environment. The new discovery in Eocene certainly sheds new light on the evolution of this group of insects. The finding of representatives of the genus Rhabdomastix with very narrow and elongated antennae in fossil resins is the first such evidence of their occurrence in the fossil record (Figs. 9, 10).
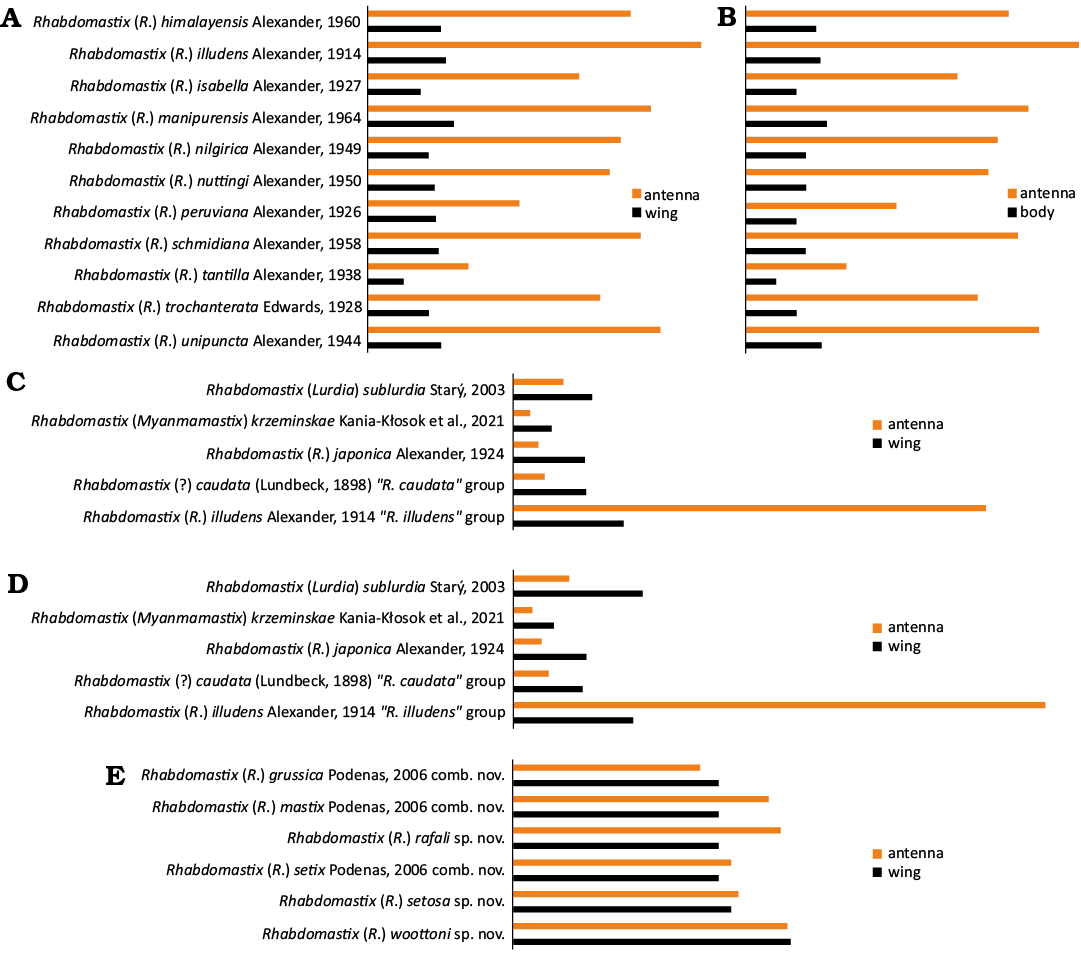
Fig. 11. Diagrams showing the ratio of the length of the antennae to the length of the body (B) and the length of the antennae to the length of the wing (A, C–E) of Recent representatives of the “Rhabdomastix illudens” group (A, B); representatives of subgenera and representatives of the groups “Rhabdomastix caudata” and “Rhabdomastix illudens”(C, D); fossil representatives of the “Rhabdomastix illudens” group (E).
The representative of the genus Rhabdomastix, R. (R.) woottoni sp. nov. found in the Bitterfeld amber is very similar in morphology of antenna to the Bolivian “R. illudens” group, distinguished mainly on the basis of the criterion related to the proportions of the length of the antennae and the body, extremely elongate male antenna, even five times as long as the body (Figs. 10, 11). The newly described species is characterized by very narrow and elongate male antenna, which leads to the conclusion that it probably forms one group with modern American species with similar morphological characters. On the other hand, the elongate antennae may have appeared many times in evolution. It is also worth mentioning that the genus Rhabdomastix is more abundant in colder parts of the world, while fewer species of this genus occur in warmer zones (Fig. 12).
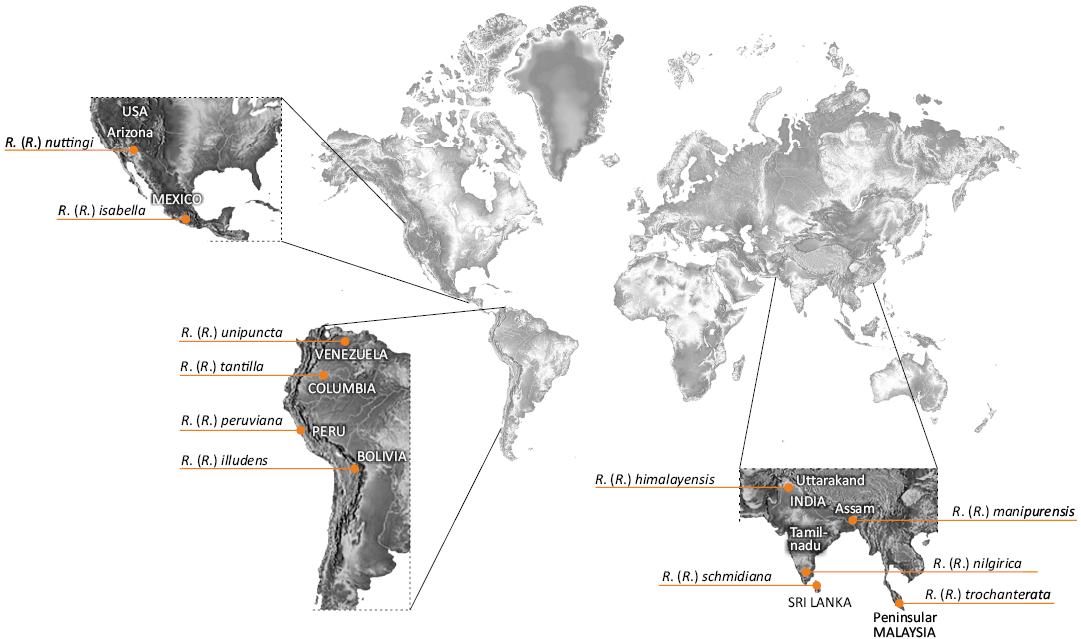
Fig. 12. Map of the distribution of representatives of the “Rhabdomastix illudens” group (the data used after Oosterbroek 2024).
The group of species named by Starý (2003) “R. illudens”, which include species from Mexico, Bolivia, and Arizona in the USA, apparently prefer is a warmer climate than majority of other species in Rhabdomastix.
Although both Baltic amber and Bitterfeld amber are believed to be approximately the same age (Szwedo and Sontag 2013), they came probably from distinct ecosystems. The differences found seem to reflect differences ecological conditions, not age (Standtke 2008).
Baltic and Bitterfeld ambers are compositionally distinct since each of them originates from different area of the Eocene North Sea, i.e., Baltic amber from the north while Bitterfeld from the south (Wolfe et al. 2016). Generally, during the Eocene in northern Europe tropical and subtropical elements were gradually replaced by boreal forms (Weitschat 2008), and it is suggested that the Bitterfeld amber could have been deposited during a warm phase just after the Paleocene–Eocene Thermal Maximum (Westerhold et al. 2009) with mean annual temperatures in Central Europe of ~22 °C (Grein et al. 2011; Dunlop et al. 2018).
It is plausible that, similarly to modern species from the “R. illudens” group, occurring in warmer climates, the extinct species documented from Bitterfeld amber also preferred warm climatic conditions. This group is known not only from Bitterfeld amber, but also from all fossil resins known from the Baltic region. Nevertheless, further research is necessary to fully corroborate this hypothesis.
The discovery of a representative of the group “R. illudens” in Bitterfeld amber is important for the interpretation of the evolution of this group of insects, as well as their detailed bionomics and environmental preferences in the Eocene.
Conclusions
Although there is observed morphological diversity and disparity of extant and extinct representatives of the genus Rhabdomastix, specimens with features such as very elongated antennae, much longer than body length, have not been known from the fossil record until now.
The new discovery shed new light on the evolution of this group of insects, indicating similarity to some modern representatives of the genus which occcur in Nearctic, Neotropic and Oriental regions. Therefore, these and subsequent new discoveries may help resolving some taxonomic problems of this group of insects as the internal classification of the genus Rhabdomastix is not fully assessed, and the taxonomic position of the groups of “Rhabdomastix illudens” and “Rhabdomastix caudata” remains still undefined.
Ackowledgements
We are deeply grateful to Christel and Hans Werner Hoffeins from Hamburg, Germany (the donor of the collection of specimens currently deposited SDEI) for making the Rhabdomastix specimens available for our study. We would like to acknowledge the editors and two reviewers: Katarzyna Kopeć (ISEA PAS) and Antonio Arillo (Departamento de Biodiversidad, Ecología y Evolución, Facultad de Biología, Universidad Complutense, Madrid, Spain) for valuable comments and suggestions. This research was realized within the project of the National Science Centre, Poland, Grant No. 2020/37/B/NZ8/03042.
Authors’ contributions
WJ-S. conceived and designed the study, lead and performed the data analysis, interpretations and analysis, prepared photographs and drawings, wrote the manuscript and corrected the manuscript. IK-K conceived and designed the study, lead and performed the data analysis, interpretations and analysis, wrote the manuscript and corrected the manuscript. WK provided specimens, analyzed the data and corrected the manuscript. All authors edited and reviewed final version of the manuscript and agreed to the submitted version.
Editor: Krzysztof Hryniewicz.
References
Alexander, C.P. 1911. Notes on two Tipulidae (Diptera). Entomological News 22: 349–354.
Alexander, C.P. 1914a. The Neotropical Tipulidae in the Hungarian national museum (Diptera). III. Entomological News 25: 205–215.
Alexander, C.P. 1914b. The craneflies collected in Costa Rica by Dr. P.P. Calvert (Tipulidae, Diptera). Journal of the New York Entomological Society 22: 116–124.
Alexander, C.P. 1916. New or little-known crane-flies from the United States and Canada: Tipulidae, Ptychopteridae, Diptera. Part 3. Proceedings of the Academy of Natural Sciences of Philadelphia 68: 486–549.
Alexander, C.P. 1924. Undescribed species of Japanese crane-flies. Part IV. Annals of the Entomological Society of America 17: 59–74. Crossref
Alexander, C.P. 1926. New species of crane-flies from South America. Part I. (Family Tipulidae, Order Diptera). Annals of the Entomological Society of America 19: 378–394. Crossref
Alexander, C.P. 1927a. New Nearctic crane-flies (Tipulidae, Diptera). Part XII. Canadian Entomologist 59: 184–193. Crossref
Alexander, C.P. 1927b. Studies on the crane-flies of Mexico. Part III (order Diptera, superfamily Tipuloidea). Annals of the Entomological Society of America 20: 301–318. Crossref
Alexander, C.P. 1931. Crane-flies of the Baltic amber (Diptera). Bernstein-Forschung 2: 1–135. Crossref
Alexander, C.P. 1933. Undescribed species of Eriopterine crane-flies from the United States and Canada (Tipulidae, Diptera). Part II. Journal of the New York Entomological Society 41: 91–100.
Alexander, C.P. 1938. Studies on the crane-flies of Mexico. Part V (order Diptera, superfamily Tipuloidea). Annals of the Entomological Society of America 31: 393–412. Crossref
Alexander, C.P. 1938. Records and descriptions of Tipulidae from tropical America (Diptera). Part I. Revista de Entomología 9: 426–441.
Alexander, C.P. 1943. Records and descriptions of North American crane-flies (Diptera). Part III. Tipuloidea of the Upper Gunnison Valley, Colorado. American Midland Naturalist 29: 147–179. Crossref
Alexander, C.P. 1944. New or little-known Tipulidae from Venezuela (Diptera). Part IV. Boletin Entomología Venezolana 3: 143–160.
Alexander, C.P. 1949. New or little-known Tipulidae (Diptera). LXXXII. Oriental-Australasian species. Annals and Magazine of Natural History 12: 639–663. Crossref
Alexander, C.P. 1950. New or insufficiently-known crane-flies from the Nearctic region (Diptera, Tipulidae). Part XII. Bulletin of the Brooklyn Entomological Society 45: 41–47.
Alexander, C.P. 1958. New or little-known Tipulidae (Diptera). CV. Oriental-Australasian species. Annals and Magazine of Natural History 13 (1): 213–231. Crossref
Alexander, C.P. 1960. Undescribed species of crane-flies from the Himalaya mountains (Tipulidae, Diptera). V. Journal of the New York Entomological Society 68: 135–144.
Alexander, C.P. 1964. New or little-known species of Asiatic Tipulidae (Diptera). I. Transactions of the American Entomological Society 90: 205–234.
Barthel, M. and Hetzer, H. 1982. Bernstein Inklusen aus dem Miozän des Bitterfelder Raumes. Zeitschrift für Angewandte Geologie 28: 314–336.
Blumenstengel, H. 2004. Zur Palynologie und Stratigraphie der Bitterfelder Bernsteinvorkommen (Tertiär). Exkursionsführer Veröffentlichungen Gesellschaft Geowissenschaften 224: 17.
Cockerell, T.D.A. 1920. Eocene insects from the Rocky Mountains. Proceeding of the United States National Museum 57: 233–260. Crossref
Cockerell, T.D.A. 1927. Fossil insects from the Miocene of Colorado. Annals and Magazine of Natural History 19 (9): 161–166. Crossref
Dunlop, J.A., Kotthoff, U., Hammel, J.U., Ahrens, J., and Harms, D. 2018. Arachnids in Bitterfeld amber: A unique fauna of fossils from the heart of Europe or simply old friends? Evolutionary Systematics 2: 31–44. Crossref
Drohojowska, J., Zmarzły, M.A., and Szwedo, J. 2024 The discovery of a fossil whitefly from Lower Lusatia (Germany) presents a challenge to current ideas about Baltic amber. Scientific Reports 14 (1): 1–9. Crossref
Edwards, F.W. 1928. Diptera Nematocera from the Federated Malay States museums. Journal of the Federated Malay States Museums 14: 1–139.
Evenhuis, N.L. 1994. Catalogue of the Fossil Flies of the World (Insecta: Diptera). 672 pp. Backhuys Publishers, Leiden.
Grein, M., Utescher, T., Wilde, V., and Roth-Nebelsick, A. 2011. Reconstruction of the middle Eocene climate of Messel using palaeobotanical data. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 260: 305–318. Crossref
Grimaldi, D.A. and Ross, A.J. 2017. Extraordinary Lagerstätten in amber, with particular reference to the Cretaceous of Burma. In: N.C. Fraser and H.-D. Sues (eds.), Terrestrial Conservation Lagerstätten: Windows into the Evolution of Life on Land, 287–342. Dunedin Academic Press Ltd, Edinburgh. Crossref
Jordan-Stasiło, W., Kania-Kłosok, I., Kopeć, K., Tuchowski, M., and Krzemiński, W. 2023. A typical wing venation of some fossil and Recent species of Rhabdomastix Skuse, 1890 (Diptera: Limoniidae), new data. Annales Zoologici 73: 683–694. Crossref
Kania-Kłosok, I., Jordan-Stasiło, W., Kopeć, K., Janiszewska, K., and Krzemiński, W. 2021. The first stage of the evolution of Rhabdomastix (Diptera, Limoniidae) and the taxonomic implications of genus. The European Zoological Journal 88: 1152–1169. Crossref
Knuth, G., Koch, T., Rappsilber, I., and Volland, I. 2002. Concerning amber in the Bitterfeld region—geological and genetic aspects. Hallesches Jahrbuch für Geowissenschaften 24: 35–46.
Krzemiński, W. 2002. Three new species of the genus Helius Lepeletier & Serville (Diptera, Limoniidae) from the Middle Miocene of Stavropol (norther Caucasus, Russia). Acta Zoologica Cracoviensia 35: 597–601.
Krzemiński, W. 2004. Fossil Limoniidae (Diptera, Tipulomorpha) from Lower Cretaceous amber of Myanmar. Journal of Systematic Palaeontology 2: 123–125. Crossref
Krzemiński, W. and Krzemińska, E. 2003. Triassic Diptera: descriptions, revisions and phylogenetic relations. Acta Zoologica Cracoviensia 46 (Supplement): 153–184.
Linnaeus, C. 1758. Systema nature per regna trianaturae, secundum classes, ordines, genera, species, cum caracteribus, differentiis, synonymi, locis. Tomus I. Editiodecima, reformata. 824 pp. L. Salvii, Holmiae [= Stockholm]. Crossref
Lundbeck, W. 1898. Diptera groenlandica. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Kjobenhavn 1900: 236–314.
McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J., Vockeroth, J.R., and Wood, D.M. 1981. Manual of Nearctic Diptera. Volume 1. Research Branch. 674 pp. Research Branch Agriculture Canada, Ottawa.
Meunier, F. 1899. Études de quelques Diptères de l’ambre tertiaire. Bulletin de la Société entomologique de France 4 (17): 334–335. Crossref
Meunier, F. 1906. Monographie des Tipulidae et Dixidae de l’ambre de la Baltique. Annales des Sciences Naturelles (Zoologie) 4 (9): 349–401.
Oosterbroek, P. 2024. Catalouge of the Crane-flies of the World. (Diptera: Tipuloidea: Pedicidae, Limoniidae, Cylindrotomidae, Tipulidae). [available online, https://ccw.naturalis.nl/ Last update: 15 Jul 2024].
Oosterbroek, P., Brodo, F., Lantsov, V.I., and Starý, J. 2007. The Tipulidae and Limoniidae of Greenland (Diptera, Nematocera, Craneflies). Entomologiske Meddelelser 75: 3–33.
Podenas, S. 2006. Rhabdomastix crane flies (Diptera: Limoniidae) in Baltic amber (Eocene). Proceedings of the Academy of Natural Sciences of Philadelphia 155: 41–66. Crossref
Rikkinen, J. and Poinar, G.O., Jr. 2000 A new species of resinicolous Chaenothecopsis (Mycocaliciaceae, Ascomycota) from 20 million year old Bitterfeld amber, with remarks on the biology of resinicolous fungi. Mycological Research 104: 7–15. Crossref
Rohdendorf, B.B. 1961. The oldest infraorders of Diptera from the Triassic of Middle Asia [in Russian]. Palaeontologičeskij žurnal 1961 (2): 90–100.
Rondani, C. 1861. Dipterologiae italicae prodromus. Vol. IV. Species italicae ordinis dipterorum in genera characteribus definita, ordinatim collectae, methodo analatica distinctae, et novis vel minus cognitis descriptis. Pars tertia: Muscidae, Tachininarum complementum. 174 pp. A. Stocchi, Parmae [= Parma].
Savchenko, E.N. 1976. On finding limoniid-flies (Diptera, Limoniidae) from the Palaeogonomyia Meunier subgenus of the Rhabdomastix Skuse genus in the USSR fauna [in Ukrainian]. Dopovidi Akademii Nauk Ukrainskoj RSR (B): 561–564.
Savchenko, E.N., Oosterbroek, P., and Starý, J. 1992. Family Limoniidae. Catalogue of Palaearctic Diptera 1: 183–369.
Scudder, S.H. 1894. Tertiary Tipulidae, with special reference to those of Florissant, Colorado. Proceedings of the American Philosophical Society 32: 163–245.
Siebke, H. 1863. Beretning om en i Sommeren 1861 foretagen entomologisk Reise. The New York Times Magazin for Naturvidenskapene 12: 105–192.
Skuse, F.A.A. 1890. Diptera of Australia. Part VII. The Tipulidae brevipalpi. Proceedings of the Linnean Society of New South Wales 4: 757–892. Crossref
Speiser, P. 1909. 4 Orthoptera. Orthoptera Nematocera. Wissenschaftliche Ergebnisse der Schwedischen Zoologische Expededition nach Kilimandjaro, Meru 10 (Diptera), 31–65.
Standke, G. 2008. Bitterfelder Bernstein gleich Baltischer Bernstein? – Eine geologische Raum-Zeit-Betrachtung und genetische Schlussfolgerungen. Exkursionsfuhrer der Deutschen Gesellschaft für Geowissenschaften 236: 11–33.
Starý, J. 2003. Revision of European species of the genus Rhabdomastix (Diptera: Limoniidae). Part 1: Introduction and subgenus Lurdia subgen. n. European Journal of Entomology 100: 587–608. Crossref
Statz, G. 1944. Neue Dipteren (Nematocera) aus dem Oberoligocän von Rott. III. Familie Limnobiidae (Stelzmücken). IV. Familie: Tipulidae (Schnaken). V. Familie: Culicidae (Stechmücken). Palaeontographica A 95: 93–120.
Szwedo, J. and Sontag, E. 2013. The flies (Diptera) say that amber from the Gulf of Gdańsk, Bitterfeld and Rovno is the same Baltic amber. Polish Journal of Entomology 82: 379–388. Crossref
Weitschat, W. 2008. Bitterfelder und Baltischer Bernstein aus Paläoklimatischer und Paläontologischer Sicht. In: J. Rascher, R. Wimmer, G. Krumbiegel, and S.Schmiedel (eds.), Bitterfelder Bernstein versus Baltischer Bernstein: Hypothesen, Fakten, Fragen. II. Bitterfelder Bernsteinkolloquium, 88–97. Exkursionsführer der Deutschen Gesellschaft für Geowissenschaften 236. Mecke Druck und Verlag, Duderstadt.
Westerhold, T., Röhl, U., McCarren, H.K., and Zachos, J.C. 2009 Latest on the absolute age of the Paleocene–Eocene Thermal Maximum (PETM): New insights from exact stratigraphic position of key ash layers +19 and -17. Earth and Planetary Science Letters 287: 412–419. Crossref
Wolfe, A.P., McKellar, R.C., Tappert, R., Sodhi, R.N.S., and Muehlenbachs, K. 2016. Bitterfeld amber is not Baltic amber: Three geochemical tests and further constraints on the botanical affinities of succinite. Review of Palaeobotany and Palynology 225: 21–32. Crossref
Acta Palaeontol. Pol. 70 (1): 57–75, 2025
https://doi.org/10.4202/app.01210.2024