


Advanced stereopsis and predatory adaptation in a Cretaceous mantis
RYO TANIGUCHI, YUKI FUKUDA, KANTA SUGIURA, and YASUHIRO IBA
Taniguchi, R., Fukuda, Y., Sugiura, K., and Iba, Y. 2025. Advanced stereopsis and predatory adaptation in a Cretaceous mantis. Acta Palaeontologica Polonica 70 (1): 1–6.
Visual systems have been crucial for animals to detect light signals. Binocular stereopsis has affected prey-predator relationships throughout animal evolution by providing depth perception, among others. However, it has been difficult to reconstruct extinct binocular functions due to a lack of suitable fossil material. Here, we show, based on morphological analysis of well-preserved eyes, that an extinct mantis (Ambermantis wozniaki Grimaldi, 2003) in the Cretaceous New Jersey amber developed an advanced visual system as a predator. We found that A. wozniaki possesses large compound eyes with numerous, ca. 12 000 ommatidia. The interocular distance is narrower than the eyes, and the estimated binocular visual field is broader than in the typical extant basal and derived taxa. The large number of ommatidia indicates that the compound eyes of A. wozniaki achieved high spatial resolution to capture objects visually. The broad binocular field supports that A. wozniaki increased the stereoscopic area and developed an advanced prey-recognition system. These findings suggest that the Cretaceous basal mantises were highly adaptive visual predators, implying the ecological domination of mantises as visual specialists for 90 million years.
Key words: Insecta, Mantodea, palaeobiology, predatory behaviour, visual system, binocular vision, New Jersey amber.
Ryo Taniguchi [ryoxtaniguchi@eis.hokudai.ac.jp; ORCID: https://orcid.org/0000-0001-6287-3817 ] and Kanta Sugiura [sugiura.kanta.u9@elms.hokudai.ac.jp; ORCID: https://orcid.org/0009-0007-1673-1691], Department of Natural History Sciences, Graduate School of Science, Hokkaido University, Sapporo, Hokkaido 060-0810, Japan.
Yuki Fukuda [huyk51@student.univet.hu; ORCID: https://orcid.org/0009-0009-2337-6821 ], University of Veterinary Medicine Budapest, István utca 2, Budapest 1078, Hungary.
Yasuhiro Iba [iba@sci.hokudai.ac.jp; ORCID: https://orcid.org/0009-0004-7163-5641 ], Department of Earth and Planetary Sciences, Hokkaido University, Sapporo, Hokkaido 060-0810, Japan.
Received 27 November 2024, accepted 23 January 2025, published online 25 February 2025.
Copyright © 2025 R. Taniguchi et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The eyes of animals detect light stimulation, and they are essential primary sensory organs responsible for simply perceiving environmental brightness to communicating complex social information (Land and Fernald 1992; Emery 2000; White et al. 2015). Although even one eye can achieve a broad visual field (e.g., nearly 180° in some vertebrates and insects) (Stevens 2006; Borst 2009; Bergman et al. 2021), most animals have two eyes and see objects “binocularly”. Stereopsis is a binocular visual function that extracts depth information based on different images recognised by the right and left eyes (binocular disparity) (Parker 2007; Read 2021). Depth perception by binocular stereopsis is crucial, especially for predatory animals, since it is advantageous for successful visual hunting, such as judging the distance to prey and breaking camouflage (Nityananda and Read 2017; Adams et al. 2019). Therefore, the evolution of stereopsis can be regarded as a critical adaptation that has strongly affected prey-predator relationships in the deep-time evolution of animals. Although some previous studies reconstructed the binocular visual function of extinct vertebrates, they lack robust bases such as soft tissue: the eyes themselves (Stevens 2006; Marinho et al. 2013; Konishi et al. 2016). Extinct binocular systems have remained hard to access using direct fossil evidence despite their evolutionary significance.
Praying mantises (order Mantodea) include about 2500 extant species, all predatory with spiny raptorial forelegs (Izquierdo‐López et al. 2024). Mantises were the first invertebrates proven to possess stereopsis based on neurobiological analysis, and they have been traditional models of binocular visual studies (Rossel 1983; Kral and Poteser 2009; Rosner et al. 2020). When prey-capturing, mantises need to recognise an appropriate distance to prey and judge whether to strike based on their stereoscopic ability with a large binocular visual field and complex information process. Their visual ability has been investigated by combining behavioural experiments and neurophysiological and morphological analyses (Rossel 1979; Yamawaki and Toh 2003; Rosner et al. 2019). Since compound eyes of arthropods have chitinous exoskeletons, their morphological characteristics, such as micron-scale ommatidia, are potentially preserved as fossils (Lee et al. 2011; Lindgren et al. 2019). In some cases of insect eyes fossilised in amber, their three-dimensional structures are exceptionally preserved with almost their original morphology (Lin et al. 2019; Kundrata et al. 2020; Vršanský et al. 2021b), although some taphonomic biases need to be noted (Koubová and Mlynský 2020). Fossilised mantises in amber can be therefore examined using the same morphological analysis as their extant counterparts. Fossil records of mantodeans are relatively rare: 38 species have been described, 25 from the Mesozoic and 13 from the Cenozoic (Delclòs et al. 2016; Li and Huang 2018; Terríquez-Beltrán et al. 2023; Vršanský 2024; Vršanský et al. 2025). All Mesozoic taxa, mostly from the Cretaceous and a few from the Late Jurassic, show plesiomorphic characters represented by short prothoraxes and forelegs (Vršanský 2002, 2024; Grimaldi 2003; Wieland 2013), and thus, the binocular vision of such basal taxa potentially can reveal the early evolution and ecology of the visual system of mantises.
Here, we analysed the exceptionally well-preserved compound eyes of a fossilised mantis Ambermantis wozniaki in mid-Cretaceous amber and compared the morphological property to extant relatives. Based on the comparison, we reconstructed the binocular visual system and prey-recognition/capture ability of the primitive mantis.
Institutional abbreviations.—AMNH, American Museum of Natural History, New York, USA.
Data availability.—The fossil material is deposited in the American Museum of Natural History under the assigned number AMNH NJ1085. The original CT slice images of the studied specimen are available from the Figshare Data Repository: https://doi.org/10.6084/m9.figshare.27916509.
Material and methods
The fossilised adult male mantis derived from the Raritan Formation (Turonian: 90–94 Ma) in central New Jersey (Gandolfo et al. 2018; Delclòs et al. 2023) and was described as Ambermantis wozniaki (Grimaldi 2003), deposited in the American Museum of Natural History (AMNH NJ1085). The specimen is three-dimensionally well preserved and shows nearly complete body remains except for some parts of the antennae and legs (Fig. 1).
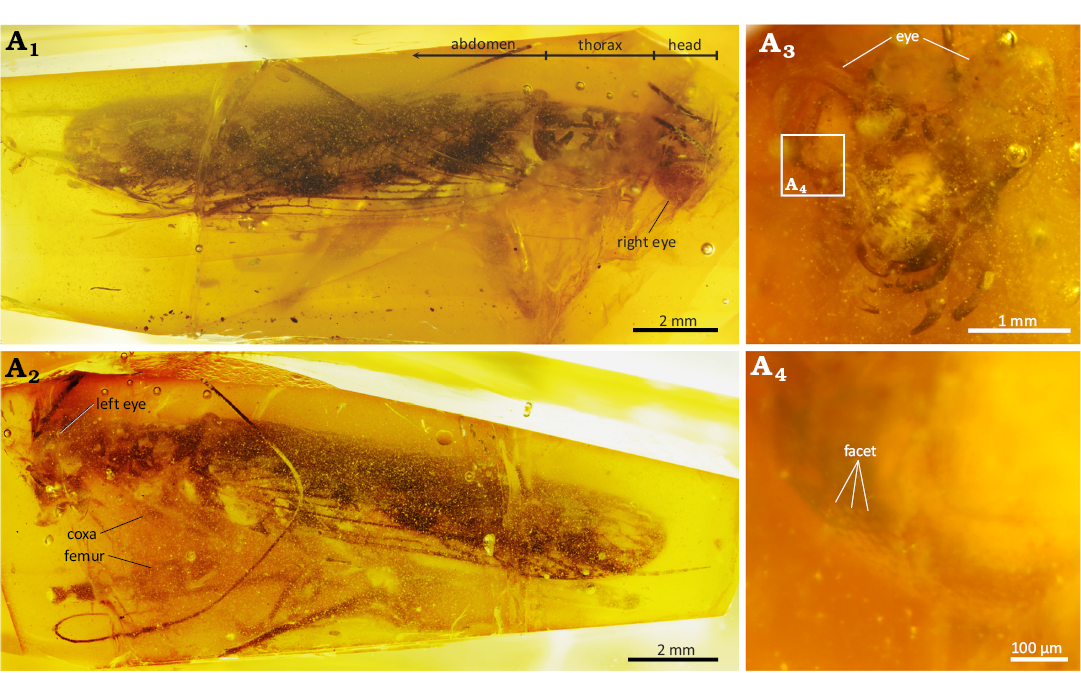
Fig. 1. Neomantodean mantis Ambermantis wozniaki Grimaldi, 2003 (AMNH NJ1085), New Jersey amber, Sayreville, Middlesex County (Raritan Formation, Turonian, 90–94 Ma); A1, specimen in dorsal view. The thorax is short, 1.8× the head in length; A2, specimen in left lateral view, showing the spiny raptorial foreleg; A3, head with large compound eyes; A4, enlarged image, showing many facets on the eye.
The fossil macrophotos were taken using a Canon EOS 5DS R (8688×5792 pixels) with a Canon MP-E 65 mm macro lens (F2.8, 1–5×) and a ZEISS LED Cold Light Source Dual Pipe Light System. We also used a Canon Extender EF 2× III for higher-magnified images. The amber was immersed in clove oil (Wako Pure Chemical Industries) to improve optical resolution by covering the surface cracks. The focus-stacked images of the specimen were obtained via Helicon Focus 8.2.3 (Helicon Soft). Facet diameters on the compound eyes were measured from these images, and the area of each facet was calculated by being regarded as a regular hexagon to estimate the ommatidium number explained below. The head was scanned with a microfocus X-ray CT system (60 kV, 3.5 µm3/voxel; Bruker SKYSCAN 2214) at Tomakomai Industrial Technology Center (Hokkaido, Japan). The CT data were visualised as a 3D model, and its surface data were created with Amira 3D 2023.2 (Thermo Fisher Scientific) after being converted to 8-bit images. The surface data were imported into Blender 4.3.2 (Blender Foundation), and the surface area of the compound eyes was digitally measured on the software. The number of ommatidia was estimated by dividing this surface area by the facet area.
We measured the binocular visual field angle to estimate the stereoscopic and prey-recognition ability of A. wozniaki from the 3D model, following the methodology for extant mantis species (Prete et al. 2011; Fig. 2). Frontal, lateral and rear parts of the compound eye were determined in the dorsal view by drawing a line from the frontal-medial-most to rear-most edge and dividing the straight angle into three 60° parts. The curvature of the frontal part was represented by the osculating circle passing the edges, and the angle between the two lines from the circle centres to the medial edges of each eye was regarded as the maximum binocular visual field angle. The drawing and measurement were performed on Serif Affinity Designer 2.5.5.
Results
Many facets are visible on the compound eyes (Fig. 1A4). The diameter of each facet is ~30 µm, and its area was calculated as 5.85×10-4 mm2. The total surface area of both compound eyes was measured at 7.26 mm2 from the 3D model. The compound eyes were estimated to comprise 12 410 ommatidia from these data.
The compound eyes of Ambermantis wozniaki are well-developed and slightly protrude anteriorly (Figs. 1A3, 2A1–A3). They are distributed with an anterior tilt from the dorsal view and have an enlarged frontal area of the eyes. (Fig. 2A1, A3). The head width and interocular distance were 2.87 mm and 0.96 mm, respectively. The ratio of interocular distance to head width of A. wozniaki is 0.34; i.e., from the frontal view, the eyes of A. wozniaki occupy about two-thirds of the head in their width. By geometrical drawing of osculating circles on the frontal parts, the maximum binocular visual field angle of A. wozniaki is estimated at 90.5° (Fig. 2A4).
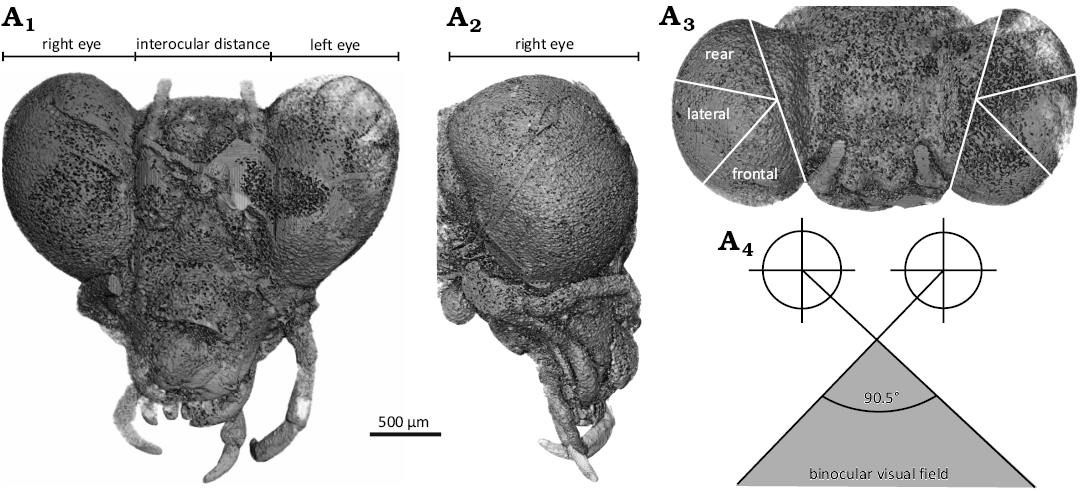
Fig. 2. Neomantodean mantis Ambermantis wozniaki Grimaldi, 2003 (AMNH NJ1085), New Jersey amber, Sayreville, Middlesex County (Raritan Formation, Turonian, 90–94 Ma). A three-dimensional model of the head from CT data (A1–A3) and estimated binocular visual field angle (A4) of A. wozniaki; A1, in frontal view; A2, in right lateral view; A3, in dorsal view, showing three divided parts on the eyes; A4, schematics of the binocular visual field reconstructed from the osculating circles of frontal parts on each eye.
Discussion
Insects recognise objects as images subdivided via ommatidia, and many ommatidia achieve a high spatial resolution (Buschbeck and Friedrich 2008). The spatial resolution is also restricted by body size, limiting the number of ommatidia (Streinzer et al. 2016). The number of ommatidia in the specimen of A. wozniaki was estimated at ca. 12 000. The extant mantises generally used as a model for visual function, whose body lengths are 8–10 mm in early nymphs and 50–80 mm in adults, increase their ommatidia ontogenetically from 8000 to 18000 ommatidia (e.g., Tenodora aridifolia, Mantis religiosa, and Polyspilota sp.) (Kral 2012, 2014). Considering the small adult body length (13 mm), A. wozniaki was equipped with a large amount of ommatidia relative to the body size, indicating high visual resolution.
We calculated the ratio of interocular distance and head width of A. wozniaki at 0.34. This value is significantly smaller than for extant mantises; basal and small species: 0.44 in Chaeteessa valida, 0.38 in Metallyticus splendidus, 0.45 in Mantoida maya, 0.44 in Amorphoscelis hamata; derived and large species: 0.35 in Tenodora aridifolia, 0.41 in Mantis religiosa, 0.52 in Clinia humeralis (Roy and Stiewe 2009; Prete et al. 2011; Wieland 2013) and for other Cretaceous mantises recorded an adult with the complete and clearly observable heads/eyes; 0.41 in Burmantis hexispinea, 0.42 in Santanmantis axelrodi (Hörnig et al. 2017; Li and Huang 2018). The short interocular distance to head width indicates that compound eyes occupy a large proportion of the head. A. wozniaki possessed relatively large eyes, even compared to modern eumantodeans. The interocular distance to head value 0.34 of A. wozniaki is also smaller than for extinct Cretaceous cockroaches, closely related to mantodeans in phylogeny and ecology: 0.53 in Stavba babkaeva (Liberiblattindiae), 0.56 in Pseudomantina occisor (Blattulidae), 0.48 in Manipulatoides obscura (Manipulatoridae), 0.52 in Caputoraptor elegans (Alienopteridae) (Vršanský et al. 2019, 2021a; Wipfler et al. 2019; Li and Huang 2022; Luo et al. 2022; Sendi et al. 2023; Vršanský 2024). These comparisons imply that A. wozniaki evolved the broad frontal visual field after splitting from the cockroach-like ancestral stage.
Mantises utilise their stereoscopic ability to perceive the distance to prey and strike them at a critical distance (Nityananda et al. 2016; Read 2023). They detect the kinetic disparity of images between right and left compound eyes for the stereopsis (Nityananda et al. 2018; Read 2021); thus, large binocular visual field angles enlarge the range of depth perception. The potential binocular visual field angle of Ambermantis is 90.5°, considerably broader than extant derived species (ca. 70° in Tenodora australasiae, Tenodora aridifolia, Mantis religiosa and 44.0° in Clinia humeralis) (Rossel 1983; Prete et al. 2011) (Fig. 2A4). This suggests that A. wozniaki increased the area where distance can be measured through binocular stereopsis and search their prey in a broad range. Behavioural experiments with extant species have shown that mantises cannot define the target as prey if it is too large to capture enough within the binocular visual field (Prete and McLean 1996; Prete et al. 2011, 2012). In other words, the broader the binocular field, the greater the opportunity to recognise prey. A. wozniaki likely developed a broad binocular visual field and effectively captured their prey.
The extant derived mantises (most species are larger than 50 mm) are top predators among terrestrial invertebrates, and their predators are often also vertebrates (Yamawaki 2017). In contrast, A. wozniaki is very small (13 mm in body length), meaning the genus occupied middle-rank predatory status. They were likely preyed on by various higher predators, including insects or other arthropods. The large and high-resolution eyes of A. wozniaki may have also helped detect predators and escape from them, considering that extant species rely on visual stimulation in their defensive behaviour (Yamawaki 2011, 2017). Our lines of evidence suggest that A. wozniaki was a highly adaptive visual animal, enhancing the ability to search and recognise prey by equipping numerous ommatidia into the compound eyes and broadening its binocular stereoscopic visual field. The ancient tiny mantis from the Cretaceous period may have occupied the ecological niche as an adaptive predator comparable to modern derived relatives. The advanced visual ability of praying mantises seems to have supported their role as principal predators for 90 million years.
Conclusions
In this study, the compound eyes of a fossilised mantis, Ambermantis wozniaki, were analysed morphologically and compared to extant species. The A. wozniaki specimen showed exceptionally preserved eyes in 3D, and we could directly apply the analytical method established in extant mantises. Our results revealed that the compound eyes were composed of ca. 12 000 ommatidia, the ratio of interocular distance to head width was 0.34, and the binocular visual field angle was 90.5° in A. wozniaki. These data indicate that Ambermantis had advanced visual abilities supported by large, high-resolution eyes and broad binocular stereoscopic field comparable to extant relatives. Although it is considerably small and has a basal position in the mantodean phylogeny, Ambermantis likely occupied an adaptive predatory niche with a superior visual system, suggesting a long evolutionary history and ecological success of mantis hunting style relying on their vision on a geological time scale.
Acknowledgements
We are grateful for the support of David A. Grimaldi (AMNH) in accessing the specimen and helpful comments on the research; Agnieszka Pierwola (AMNH) in curating the specimen; Noritaka Saito and Junya Morimoto (both Tomakomai Industrial Technology Center, Japan) in CT analysis. We also thank André Nel (Muséum National d’Histoire Naturelle, Paris, France) and Peter Vršanský (Slovak Academy of Sciences, Bratislava, Slovakia) for the manuscript review and constructive suggestions. This work was supported by the Japan Society for the Promotion of Science KAKENHI, 22KJ0139 (RT), 19H02010 and 23H02544 (YI), and Hokkaido University 2023 HU Researcher Overseas Travel Grant Program (RT).
Authors’ contributions
Conceptualisation: RT; Methodology: RT and YF; Investigation: RT, YF and KS; Visualisation: RT and KS; Funding acquisition: RT and YI; Supervision: YI; Writing—original draft: RT; Writing—review and editing: All authors.
Editor: Andrzej Kaim.References
Adams, W.J., Graf, E.W., and Anderson, M. 2019. Disruptive coloration and binocular disparity: breaking camouflage. Proceedings of the Royal Society B: Biological Sciences 286 (1896): 20182045. Crossref
Bergman, M., Smolka, J., Nilsson, D.-E., and Kelber, A. 2021. Seeing the world through the eyes of a butterfly: visual ecology of the territorial males of Pararge aegeria (Lepidoptera: Nymphalidae). Journal of Comparative Physiology A 207: 701–713. Crossref
Borst, A. 2009. Drosophila’s view on insect vision. Current Biology 19: R36–R47. Crossref
Buschbeck, E.K. and Friedrich, M. 2008. Evolution of insect eyes: tales of ancient heritage, deconstruction, reconstruction, remodeling, and recycling. Evolution: Education and Outreach 1: 448–462. Crossref
Delclòs, X., Peñalver, E., Arillo, A., Engel, M.S., Nel, A., Azar, D., and Ross, A. 2016. New mantises (Insecta: Mantodea) in Cretaceous ambers from Lebanon, Spain, and Myanmar. Cretaceous Research 60: 91–108. Crossref
Delclòs, X., Peñalver, E., Barrón, E., Peris, D., Grimaldi, D.A., Holz, M., Labandeira, C.C., Saupe, E.E., Scotese, C.R., Solórzano-Kraemer, M.M., Álvarez-Parra, S., Arillo, A., Azar, D., Cadena, E.A., Dal Corso, J., Kvaček, J., Monleón-Getino, A., Nel, A., Peyrot, D., Bueno-Cebollada, C.A., Gallardo, A., González-Fernández, B., Goula, M., Jaramillo, C., Kania-Kłosok, I., López-Del Valle, R., Lozano, R.P., Meléndez, N., Menor-Salván, C., Peña-Kairath, C., Perrichot, V., Rodrigo, A., Sánchez-García, A., Santer, M., Sarto I Monteys, V., Uhl, D., Viejo, J.L., and Pérez-de La Fuente, R. 2023. Amber and the Cretaceous resinous interval. Earth-Science Reviews 243: 104486. Crossref
Emery, N.J. 2000. The eyes have it: the neuroethology, function and evolution of social gaze. Neuroscience & Biobehavioral Reviews 24: 581–604. Crossref
Gandolfo, M.A., Nixon, K.C., Crepet, W.L., and Grimaldi, D.A. 2018. A late Cretaceous fagalean inflorescence preserved in amber from New Jersey. American Journal of Botany 105: 1424–1435. Crossref
Grimaldi, D. 2003. A revision of Cretaceous mantises and their relationships, including new taxa (Insecta: Dictyoptera: Mantodea). American Museum Novitates 3412: 1–47. Crossref
Hörnig, M.K., Haug, J.T., and Haug, C. 2017. An exceptionally preserved 110 million years old praying mantis provides new insights into the predatory behaviour of early mantodeans. PeerJ 5: e3605. Crossref
Izquierdo‐López, A., Kiesmüller, C., Gröhn, C., Haug, J.T., Haug, C., and Hörnig, M.K. 2024. Patterns of morphological evolution in the raptorial appendages of praying mantises. Insect Science [available online, https://doi.org/10.1111/1744-7917.13423]. Crossref
Konishi, T., Caldwell, M.W., Nishimura, T., Sakurai, K., and Tanoue, K. 2016. A new halisaurine mosasaur (Squamata: Halisaurinae) from Japan: the first record in the western Pacific realm and the first documented insights into binocular vision in mosasaurs. Journal of Systematic Palaeontology 14: 809–839. Crossref
Koubová, I. and Mlynský, T. 2020. Two new mid-Cretaceous dictyopterans (Umenocoleidae: Vitisminae) from northern Myanmar exemplify taphonomic bias. AMBA projekty 10 (1): 1–16.
Kral, K. 2012. The functional significance of mantis peering behaviour. European Journal of Entomology 109: 295–301. Crossref
Kral, K. 2014. Orientation behavior with and without visual cues in newly hatched and adult praying mantis. Journal of Insect Behavior 27: 192–205. Crossref
Kral, K. and Poteser, M. 2009. Relationship between body size and spatial vision in the praying mantis—an ontogenetic study. Journal of Orthoptera Research 18: 153–158. Crossref
Kundrata, R., Bukejs, A., Prosvirov, A.S., and Hoffmannova, J. 2020. X-ray micro-computed tomography reveals a unique morphology in a new click-beetle (Coleoptera, Elateridae) from the Eocene Baltic amber. Scientific Reports 10 (1): 20158. Crossref
Land, M.F. and Fernald, R.D. 1992. The evolution of eyes. Annual Review of Neuroscience 15: 1–29. Crossref
Lee, M.S.Y., Jago, J.B., García-Bellido, D.C., Edgecombe, G.D., Gehling, J.G., and Paterson, J.R. 2011. Modern optics in exceptionally preserved eyes of Early Cambrian arthropods from Australia. Nature 474: 631–634. Crossref
Li, X.-R. and Huang, D. 2018. A new praying mantis from middle Cretaceous Burmese amber exhibits bilateral asymmetry of forefemoral spination (Insecta: Dictyoptera). Cretaceous Research 91: 269–273. Crossref
Li, X.-R and Huang, D. 2022. Predators or herbivores: cockroaches of Manipulatoridae revisited with a new genus from Cretaceous Myanmar amber (Dictyoptera: Blattaria: Corydioidea). Insects 13 (8): 732. Crossref
Lin, X., Labandeira, C.C., Shih, C., Hotton, C.L., and Ren, D. 2019. Life habits and evolutionary biology of new two-winged long-proboscid scorpionflies from mid-Cretaceous Myanmar amber. Nature Communications 10 (1): 1235. Crossref
Lindgren, J., Nilsson, D.-E., Sjövall, P., Jarenmark, M., Ito, S., Wakamatsu, K., Kear, B.P., Schultz, B.P., Sylvestersen, R.L., Madsen, H., LaFountain, J.R., Alwmark, C., Eriksson, M.E., Hall, S.A., Lindgren, P., Rodríguez-Meizoso, I., and Ahlberg, P. 2019. Fossil insect eyes shed light on trilobite optics and the arthropod pigment screen. Nature 573: 122–125. Crossref
Luo, C., Beutel, R.G., Engel, M.S., Liang, K., Li, L., Li, J., Xu, C., Vršanský, P., Jarzembowski, E.A., and Wang, B. 2022. Life history and evolution of the enigmatic Cretaceous–Eocene Alienopteridae: A critical review. Earth-Science Reviews 225: 103914. Crossref
Marinho, T.D.S., Iori, F.V., Carvalho, I.D.S., and De Vasconcellos, F.M. 2013. Gondwanasuchus scabrosus gen. et sp. nov., a new terrestrial predatory crocodyliform (Mesoeucrocodylia: Baurusuchidae) from the Late Cretaceous Bauru Basin of Brazil. Cretaceous Research 44: 104–111. Crossref
Nityananda, V. and Read, J.C.A. 2017. Stereopsis in animals: evolution, function and mechanisms. Journal of Experimental Biology 220: 2502–2512. Crossref
Nityananda, V., Bissianna, G., Tarawneh, G., and Read, J. 2016. Small or far away? Size and distance perception in the praying mantis. Philosophical Transactions of the Royal Society B: Biological Sciences 371: 20150262. Crossref
Nityananda, V., Tarawneh, G., Henriksen, S., Umeton, D., Simmons, A., and Read, J.C.A. 2018. A novel form of stereo vision in the praying mantis. Current Biology 28: 588–593.e4. Crossref
Parker, A.J. 2007. Binocular depth perception and the cerebral cortex. Nature Reviews Neuroscience 8: 379–391. Crossref
Prete, F.R. and McLean, T. 1996. Responses to moving small-field stimuli by the praying mantis, Sphodromantis lineola (Burmeister). Brain, Behavior and Evolution 47: 42–54. Crossref
Prete, F.R., Komito, J.L., Dominguez, S., Svenson, G., López, L.Y., Guillen, A., and Bogdanivich, N. 2011. Visual stimuli that elicit appetitive behaviors in three morphologically distinct species of praying mantis. Journal of Comparative Physiology A 197: 877–894. Crossref
Prete, F.R., Theis, R., Komito, J.L., Dominguez, J., Dominguez, S., Svenson, G., and Wieland, F. 2012. Visual stimuli that elicit visual tracking, approaching and striking behavior from an unusual praying mantis, Euchomenella macrops (Insecta: Mantodea). Journal of Insect Physiology 58: 648–659. Crossref
Read, J.C.A. 2021. Binocular vision and stereopsis across the animal kingdom. Annual Review of Vision Science 7: 389–415. Crossref
Read, J.C.A. 2023. Stereopsis without correspondence. Philosophical Transactions of the Royal Society B: Biological Sciences 378: 20210449. Crossref
Rosner, R., Tarawneh, G., Lukyanova, V., and Read, J.C.A. 2020. Binocular responsiveness of projection neurons of the praying mantis optic lobe in the frontal visual field. Journal of Comparative Physiology A 206: 165–181. Crossref
Rosner, R., Von Hadeln, J., Tarawneh, G., and Read, J.C.A. 2019. A neuronal correlate of insect stereopsis. Nature Communications 10 (1): 2845. Crossref
Rossel, S. 1979. Regional differences in photoreceptor performance in the eye of the praying mantis. Journal of Comparative Physiology A 131: 95–112. Crossref
Rossel, S. 1983. Binocular stereopsis in an insect. Nature 302: 821–822. Crossref
Roy, R. and Stiewe, M.B.D. 2009. Contribution to the knowledge of Eastern African Amorphoscelis Stål, 1871, with description of two new species (Dictyoptera, Mantodea, Amorphoscelidae). Bulletin de la Société entomologique de France 114: 195–209. Crossref
Sendi, H., Vršanský, P., and Azar, D. 2023. Jordanian-Lebanese-Syrian cockroaches s.s. from Lower Cretaceous amber—Monograph. Biologia 78: 1447–1541. Crossref
Stevens, K.A. 2006. Binocular vision in theropod dinosaurs. Journal of Vertebrate Paleontology 26: 321–330. Crossref
Streinzer, M., Huber, W., and Spaethe, J. 2016. Body size limits dim-light foraging activity in stingless bees (Apidae: Meliponini). Journal of Comparative Physiology A 202: 643–655. Crossref
Terríquez-Beltrán, J., Riquelme, F., and Varela-Hernández, F. 2023. A new species of mantis (Insecta: Mantodea: Amelidae) from the Miocene Amber-Lagerstätte in Mexico. Historical Biology 35: 2127–2134. Crossref
Vršanský, P. 2002. Origin and the early evolution of mantises. AMBA Projekty 6 (1): 1–16.
Vršanský, P. 2024. Late Mesozoic cockroaches s.l. from the Karabastau Formation in Kazakhstan. AMBA Projekty 14 (1): 1–700.
Vršanský, P., Hinkelman, J., Koubová, I., Sendi, H., Kúdelová, T., Kúdela, M., and Barclay, M. 2021a. A single common ancestor for praying mantids, termites, cave roaches and umenocoleoids. AMBA Projekty 11 (1): 1–16.
Vršanský, P., Kováčová, Z., Vasilenko, D.V., Pálková, H., Nagy, Š., Kosnáč, D., Vidlička, Ľ., and Martin, S.K. 2025. Systematics of Mesozoic ‘Arctic’ polar cockroaches. Biologia 80: 51–77. Crossref
Vršanský, P., Sendi, H., Hinkelman, J., and Hain, M. 2021b. Alienopterix Mlynský et al., 2018 complex in North Myanmar amber supports Umenocoleoidea/ae status. Biologia 76: 2207–2224. Crossref
Vršanský, P., Vršanská, L., Beňo, M., Bao, T., Lei, X., Ren, X., Wu, H., Šmídová, L., Bechly, G., Jun, L., Yeo, M., and Jarzembowski, E. 2019. Pathogenic DWV infection symptoms in a Cretaceous cockroach. Palaeontographica Abteilung A 314 (1–3): 1–10. Crossref
White, T.E., Zeil, J., and Kemp, D.J. 2015. Signal design and courtship presentation coincide for highly biased delivery of an iridescent butterfly mating signal. Evolution 69: 14–25. Crossref
Wieland, F. 2013. The phylogenetic system of Mantodea (Insecta: Dictyoptera). Species, Phylogeny and Evolution 3: 3–222. Crossref
Wipfler, B., Kočárek, P., Richter, A., Boudinot, B., Bai, M., and Beutel, R.G. 2019. Structural features and life habits of †Alienoptera (Polyneoptera, Dictyoptera, Insecta). Palaeoentomology 2: 465–473. Crossref
Yamawaki, Y. 2011. Defence behaviours of the praying mantis Tenodera aridifolia in response to looming objects. Journal of Insect Physiology 57: 1510–1517. Crossref
Yamawaki, Y. 2017. Decision‐making and motor control in predatory insects: a review of the praying mantis. Ecological Entomology 42 (S1): 39–50. Crossref
Yamawaki, Y. and Toh, Y. 2003. Response properties of visual interneurons to motion stimuli in the praying mantis, Tenodera aridifolia. Zoological Science 20: 819–832. Crossref
Acta Palaeontol. Pol. 70 (1): 1–6, 2025
https://doi.org/10.4202/app.01226.2024