

First evidence of attached juveniles in the solutan echinoderm Pahvanticystis from the middle Cambrian Weeks Formation (Utah, USA)
HARRY J. SAVAGE and IMRAN A. RAHMAN
The fossil record of the Palaeozoic echinoderm class Soluta suggests they originated in the Miaolingian (middle Cambrian) of Laurentia as permanently attached suspension feeders, demonstrating a stepwise shift towards vagility in successive strata. Here, we report a new specimen of Pahvanticystis cf. utahensis associated with three putative juveniles interpreted as belonging to the same species. We interpret this as evidence of facultative attachment in juveniles of Pahvanticystis, which had not previously been reported in this taxon, but is known in the earlier genus Castericystis. Our findings indicate that attachment as a juvenile was more widespread in solutans than previously thought.
Introduction
Solutans are an extinct class of non-radial echinoderms known from the middle Cambrian to the Middle Devonian, which are characterized by the presence of a short anterior feeding appendage, a polyplated theca, and a long posterior appendage (Caster 1968). To date, three species have been formally described from the middle Cambrian, all of which come from Utah in the western United States: Coleicarpus sprinklei Ubaghs & Robison, 1988, from the Wheeler Formation (Ubaghs and Robison 1988; Daley 1996), Castericystis vali Ubaghs & Robison, 1985, from the Marjum Formation (Ubaghs and Robison 1985; Daley 1995), and Pahvanticystis utahensis Lefebvre & Lerosey-Aubril, 2018, from the Weeks Formation (Ubaghs and Robison 1985; Lefebvre and Lerosey-Aubril 2018). These taxa apparently varied in the extent to which they were attached during life. Both juvenile and adult specimens of C. sprinklei are preserved attached to hard substrates (e.g., skeletal fragments) by the distal end of the posterior appendage, strongly suggesting it was permanently attached throughout life (Daley 1996). In contrast, while juveniles of C. vali have been found attached to adults, adult specimens are thought to have been free-living, indicative of facultative attachment (Ubaghs and Robison 1985; Daley 1995; Smith 2008). However, until now, there was no evidence for attachment in any known specimens of Pahvanticystis (Lefebvre and Lerosey-Aubril 2018). Here, we report three juveniles of Pahvanticystis directly associated with an adult specimen, which we interpret as attachment. Considering the scarcity of known juveniles for most solutan taxa, this is consistent with the suggestion that facultative attachment as juveniles was more common across the group than previously thought (Lefebvre and Lerosey-Aubril 2018).
Material and methods
Our study is based on a specimen of Pahvanticystis cf. utahensis (formerly identified as “Castericystis sp. nov.”) housed at the Natural History Museum, London (NHMUK EE 5769). This specimen comes from the upper Weeks Formation (Guzhangian, Miaolingian, Cambrian) in the North Canyon in the Sawtooth Mountains, Central House Range, Millard County, Utah, USA, about 200 m above the underlying Marjum Formation. It was collected by E. Cole and donated to the Natural History Museum by K.D. Kehrberg in 1993. The specimen is preserved in a light grey shale matrix, different to all previously reported specimens of Pahvanticystis utahensis. It consists of three putative juvenile solutans preserved on top of an adult. It was photographed with a Canon EOS 90D camera using a Canon EF-S 18–135 mm lens and Canon EF 100mm macro lens.
Institutional abbreviations.—NHMUK, Natural History Museum, London, UK.
Results
The adult solutan has a pear-shaped theca, measuring ~17 mm in maximum length and ~9 mm in maximum width. It is made up of numerous small, tessellated, polygonal plates, with a well-developed preanal lobe (Figs. 1A1, 2). The theca shows signs of post-mortem flattening (i.e., raised thecal margins) indicating that it was probably inflated in life. Many small, unorganised plates (adsteleals) border the insertion of the posterior appendage (homoiostele). The periproct is located on the lateral margin of the theca to the right of the homoiostele (Figs. 1A1, A3, 2). It consists of a small pyramid (~0.7 mm in diameter) of elongate plates.
The feeding appendage is inserted laterally towards the left margin of the theca (Figs. 1A1, 2). It is composed of two sets of opposing flooring plates and two sets of cover plates, which are partially exposed and apparently aligned with the flooring plates (Figs. 1A8, 2). The feeding appendage is incomplete in the middle, with around 30 rows of plates evident in the preserved part. It is ~7 mm in length and decreases in width distally. The number and arrangement of thecal plates surrounding the feeding appendage are unclear. No hydropore or gonopore is apparent, perhaps because they are obscured by the feeding appendage.
The homoiostele is ~17 mm in length and can be divided into proximal (proxistele) and distal (dististele) regions (Figs. 1A1, 2). The proxistele is ~3.5 mm at its widest, tapering distally, and comprises about 38% (~6.5 mm) of the total length of the homoiostele. It appears to be largely unorganised, consisting of numerous scale-like plates; however, these plates are apparently arranged into at least three rings at the distal end of the proxistele (Figs. 1A6, 2). An intermediate region (mesistele) between the proxistele and dististele may be present but is poorly preserved, with the structure of the plating unclear (Figs. 1A6, 2). The dististele is largely missing, but impressions in the matrix indicate that it was strongly curved and flattened (Fig. 1A1). An elongate spike-like structure measuring ~1.5 mm, is located at the distal end of the dististele (Figs. 1A1, A2, 2). This is composed of six plates ~0.25 mm long, with width slightly tapering from ~0.2 mm to 0.1 mm distally.
The morphology of this specimen strongly suggests that it is a species of Pahvanticystis rather than a new species of Castericystis. In particular, the laterally displaced feeding appendage, flattened, curved dististele, and pear-shaped theca are diagnostic characters shared with Pahvanticystis utahensis. The specimen differs from C. vali in two main ways: (i) the feeding appendage is laterally displaced to a greater extent than in C. vali, (ii) there is a large preanal lobe, which is not present in C. vali. The proxistele rings in this specimen do not appear to be tetrameric, as previously reported for P. utahensis (Lefebvre and Lerosey-Aubril 2018) and instead consist of numerous unorganised scales. However, specimens of C. vali exhibit similar variation in the structure of the proxistele (Ubaghs and Robison 1985; Daley 1995); and so we consider this difference as insufficient to erect a new species. Instead, this morphological variation probably corresponds to intraspecific or ontogenetic variation, as previously reported in the Ordovician solutan Dendrocystites Barrande, 1887 (Noailles et al. 2014). Nevertheless, the alternative proxistele organization prevents confident identification of the specimen at the species level. We therefore refer to it herein as Pahvanticystis cf. utahensis.
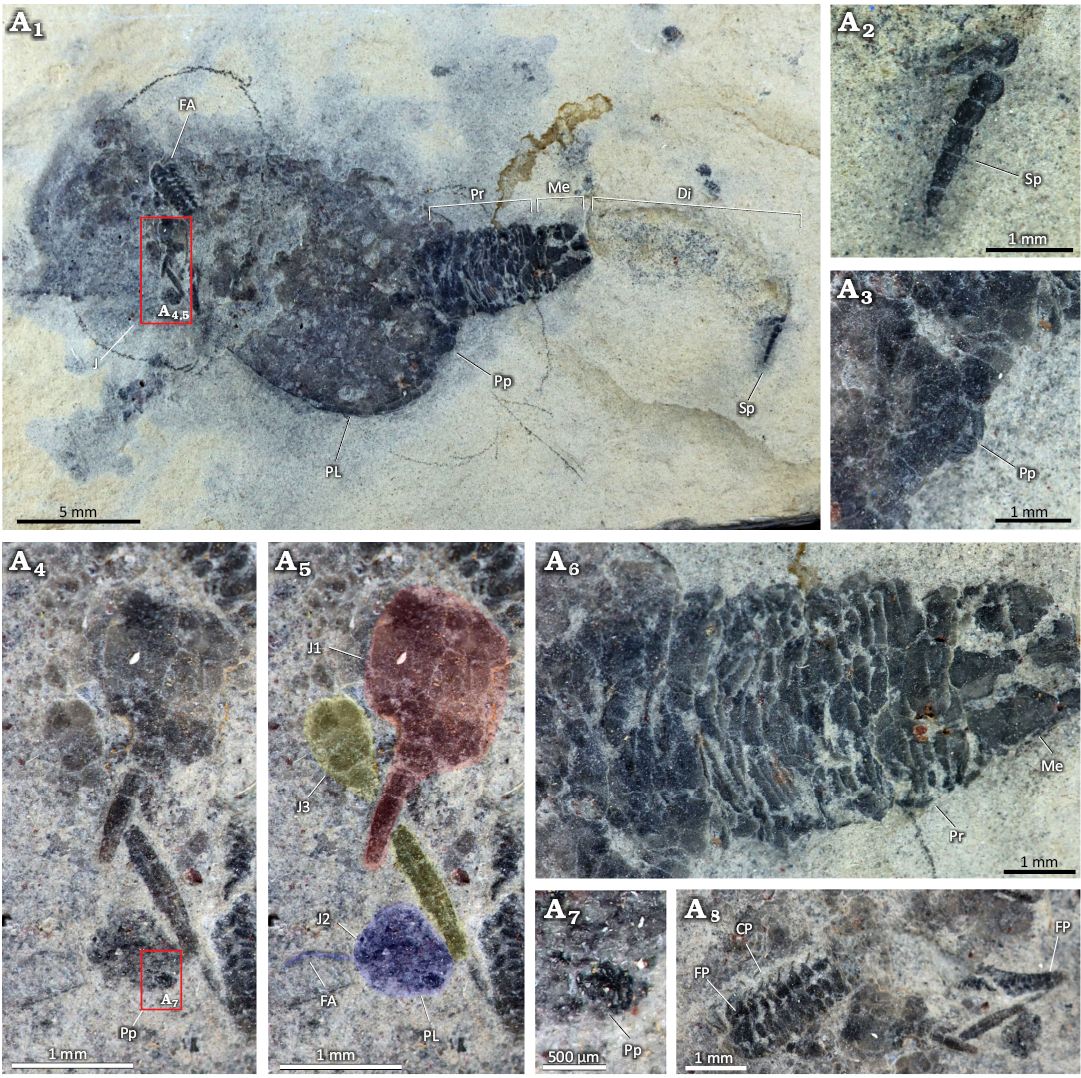
Fig. 1. Solutan echinoderm Pahvanticystis cf. utahensis Lefebvre & Lerosey-Aubril, 2018 (NHMUK EE 5769) from the upper Weeks Formation, Guzhangian, Miaolingian, Cambrian, House Range, Utah, USA. A1, overview of complete specimen; A2, close-up of spike-like structure at the distal end of the dististele; A3, close-up of periproct in the adult; A4, A5, close-ups of three juveniles of Pahvanticystis cf. utahensis; red, blue, and yellow overlays highlight the three juveniles. A6, close-up of proxistele in the adult; A7, close-up of periproct in juvenile 2; A8, Close-up of feeding appendage in the adult. Abbreviations: FA, feeding appendage, J, juveniles, J1–3, juvenile 1–3, Pr, proxistele, Me, mesistele, Di, dististele, PL, preanal lobe, Sp, spike, Pp, periproct, CP, cover plates, FP, flooring plates.
Three smaller echinoderms are preserved on top of the adult towards the anterior margin of the theca (Fig. 1A4, A5). Owing to the very small size of these individuals (at least 10× smaller than the adult), they are best interpreted as juveniles. The juveniles are partly disarticulated and, in some cases, it is difficult to unambiguously determine which fragments belong to which specimen. Our interpretation (Fig. 1A5) is as follows. Juvenile 1 has a polyplated, unorganised theca comprised of at least eight polygonal plates. It measures ~1.5 mm in length and has a posterior appendage measuring ~1 mm in length (presumably incomplete), in which at least three plates are visible. Juvenile 2 has a theca measuring ~0.75 mm in length, with no visible plate boundaries but exhibiting lobation at the posterior part of the theca. An apparently radially plated structure ~0.2 mm wide located near the lobed region is inferred to be the periproct (Fig. 1A7). A curved elongate structure ~0.6 mm long appears to protrude from the anterior of the theca and is interpreted as the feeding appendage. Two posterior appendage plates are preserved close to the theca, but they may not have belonged to this individual. Juvenile 3 has a smaller theca (~0.6 mm long) than the others, comprised of at least three rounded polygonal plates. Its posterior appendage is ~2 mm long and is comprised of two longitudinal series of plates. At least three elongate plates can be seen on one side of the appendage, but plate sutures are not apparent on the other side.
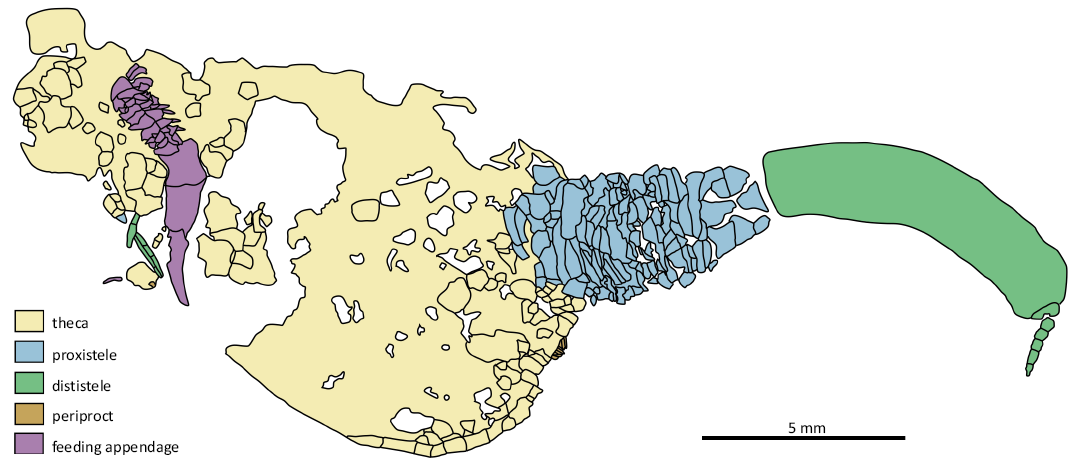
Fig. 2. Line drawing of solutan echinoderm Pahvanticystis cf. utahensis Lefebvre & Lerosey-Aubril, 2018 (NHMUK EE 5769) from the upper Weeks Formation, Guzhangian, Miaolingian, Cambrian, House Range, Utah, USA.
Discussion
The presence of a posterior appendage and a single anterior feeding appendage clearly demonstrate the putative juveniles are solutans. We interpret them as belonging to the genus Pahvanticystis cf. utahensis for three main reasons: (i) the two series of homoiostele plates have a left-right arrangement in juvenile 3, similar to “syringocrinid” solutans (Noailles et al. 2014), (ii) the thecal lobation in juvenile 2 resembles the preanal lobe of adult Pahvanticystis, which is further supported by the position in this specimen, and (iii) Pahvanticystis is the only solutan genus known from the upper Weeks Formation (Lefebvre and Lerosey-Aubril 2018). Moreover, in Coleicarpus sprinklei and Castericystis vali, juveniles associated with adults are always of the same species (Ubaghs and Robison 1985; Daley 1995, 1996), and we therefore suggest the same was true for Pahvanticystis cf. utahensis.
The three juvenile Pahvanticystis cf. utahensis are located near the anterior end of the theca of the underlying adult Pahvanticystis cf. utahensis, situated on the oral surface of the adult, i.e., the surface bearing the ambulacral food groove, and we infer that they were attached to the adult in life, as in the solutans C. sprinklei (Ubaghs and Robison 1988; Daley 1996) and C. vali (Ubaghs and Robison 1985; Daley 1995). There is no evidence of a holdfast structure at the termination of the homoiostele in any of the juveniles described herein, but this part is poorly preserved in all cases. Moreover, the presence of a spike-like structure at the end of the dististele in adults of Pahvanticystis, which resembles that of C. vali, could indicate that such a structure was also present in juveniles, where it played a role in attachment (Lefebvre and Lerosey-Aubril 2018). However, we cannot categorically rule out the alternative interpretation that the association of juveniles with the adult could be due to post-mortem transportation.
If the juveniles were attached to a free-living adult while it was alive, this would necessarily imply that they were associated with either the upper or lateral surfaces of an inflated theca (Noailles et al. 2014). If the juveniles were attached to the lateral surface, their position would provide no indication of the animal’s life orientation. However, if they were attached to the upper surface of the associated adult, this would have meant the food groove must have faced away from the substrate, indicating that Pahvanticystis cf. utahensis was likely a suspension feeder (an upward-facing food groove could not have reached the substrate unless highly contorted). The latter would differ from the situation in C. vali, in which juveniles are preserved attached to the upper surface (dorsal surface of Daley 1995) with the food groove facing downwards, consistent with the interpretation of C. vali as a deposit feeder (at least as an adult) (Daley 1995; Noailles et al. 2014). An alternative possibility is that the juveniles were attached to a dead adult, in which case the orientation of the adult would not necessarily inform on their likely feeding mode, but this is very unlikely considering the relatively good preservation of the adult specimen (owing to the weak suturing between thecal plates in solutans, very rapid post-mortem disarticulation would be expected (Brett et al. 1997).
Conclusions
In conclusion, we hypothesise that Pahvanticystis cf. utahensis displayed a similar (facultative) attachment strategy to Castericystis vali, attaching to hard substrates as a juvenile before developing into a free-living adult. If juveniles did indeed attach to the upper surface of a free-living adult, this would imply a suspension feeding strategy for Pahvanticystis , albeit we cannot rule out the alternative possibility that they were attached to the lateral margin of an inflated theca. Lastly, our findings strengthen support for the suggestion that attachment as juveniles was widespread across the class Soluta (Lefebvre and Lerosey-Aubril 2018), at least in the earliest solutan taxa.
Acknowledgements.—We thank E.Cole (Thermopolis, Wyoming, USA), and K. D. Kehrberg for collecting and donating the specimen described in this paper. We also thank Timothy Ewin (NHMUK) for facilitating access to their fossil echinoderm collections, Bertrand Lefebvre (University of Lyon, France) and an anonymous reviewer for their constructive and critical comments on an earlier version of the manuscript, and Frankie Dunn (Oxford University Museum of Natural History, UK) for helpful discussions of solutan functional morphology.
Editor: Andrzej Kaim.
References
Brett, C.E., Moffat, H.A., Taylor, W. 1997. Echinoderm taphonomy, taphofacies, and Lagerstätten. In: J.A. Waters and C.G. Maples (eds.), Geobiology of Echinoderms. Paleontological Society Papers 3: 147–190. Crossref
Caster, K.E. 1968. Homoiostelea. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology. Part S, Echinodermata, 581–627. Geological Society of America & University of Texas Press, Austin.
Daley, P.E.J. 1995. Anatomy, locomotion and ontogeny of the solute Castericystis vali from the Middle Cambrian of Utah. Geobios 28: 585–614. Crossref
Daley, P.E.J. 1996. The first solute which is attached as an adult: a Mid-Cambrian fossil from Utah with echinoderm and chordate affinities. Zoological Journal of the Linnean Society 117: 405–440. Crossref
Lefebvre, B. and Lerosey-Aubril, R. 2018. Laurentian origin of solutan echinoderms: new evidence from the Guzhangian (Cambrian Series 3) Weeks Formation of Utah, USA. Geological Magazine 155: 1190–1204. Crossref
Noailles, F., Lefebvre, B., and Kašička, L. 2014. A probable case of heterochrony in the solutan Dendrocystites Barrande, 1887 (Echinodermata: Blastozoa) from the Upper Ordovician of the Prague Basin (Czech Republic) and a revision of the family Dendrocystitidae Bassler, 1938. Bulletin of Geosciences 89: 451–476. Crossref
Smith, A. B. 2008. Deuterostomes in a twist: the origins of a radical new body plan. Evolution & Development 10: 493–503. Crossref
Ubaghs, G. and Robison, R.A. 1985. A new homoiostelean and a new eocrinoid from the Middle Cambrian of Utah. The University of Kansas Paleontological Contributions 115: 1–24.
Ubaghs, G. and Robison, R.A. 1988. Homalozoan echinoderms of the Wheeler Formation (Middle Cambrian) of Western Utah. The University of Kansas Paleontological Contributions 120: 1–24.
Harry J. Savage [savage_harryjames@hotmail.com; ORCID: https://orcid.org/0000-0003-3610-5114 ], Oxford University Museum of Natural History, Oxford OX1 3PW, UK.
Imran A. Rahman [imran.rahman@nhm.ac.uk; ORCID: https://orcid.org/0000-0001-6598-6534 ], The Natural History Museum, London SW7 5BD, UK; Oxford University Museum of Natural History, Oxford OX1 3PW, UK.
Received 4 December 2024, accepted 15 February 2025, published online 24 March 2025.
Copyright © 2025 H.J. Savage and I.A. Rahman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Acta Palaeontol. Pol. 70 (1): 175–178, 2025
https://doi.org/10.4202/app.01228.2024