

The record of cricetid rodents across the Eocene–Oligocene transition in Transylvania, Romania: implications for the “Grande Coupure” at European scale
OLIVIER MARIDET, VLAD A. CODREA, CRISTINA FĂRCAȘ, ALEXANDRU A. SOLOMON, MÁRTON VENCZEL, and JÉRÉMY TISSIER
Maridet, O., Codrea, V.A., Fărcaș, C., Solomon, A.A., Venczel, M., and Tissier, J. 2025. The record of cricetid rodents across the Eocene–Oligocene transition in Transylvania, Romania: implications for the “Grande Coupure” at European scale. Acta Palaeontologica Polonica 70 (2): 291–327.
A number of localities in Transylvania (Romania) have yielded vertebrate microfossil remains. Two localities have been stratigraphically and biochronologically dated to the late Eocene: i.e., Treznea and Bociu. The remaining three localities are dated to the early Oligocene: Mera, Cetățuie, and Suceag. The study of cricetid rodents corroborates the presence of this family in Eastern Europe during the late Eocene, as evidenced by the species Witenia sp., Bustrania cf. B. dissimile, and Eocricetodon cf. Eo. meridionalis. The cricetids identified in the sites of the early Oligocene age show a complete turnover and a notable increase in species richness following the Eocene/Oligocene boundary, with: Eucricetodon aff. Eu. huerzeleri, Tenuicricetodon arcemis gen. et sp. nov., Pseudocricetodon cf. Ps. montalbanensis, Paracricetodon cf. Pa. walgeri, Paracricetodon kavakderensis, Paracricetodon aff. Pa. stojonovici, and Paracricetodon wentgesi. In the context of the wider biogeographic history of Europe, these new discoveries indicate that Cricetidae arrived in Europe during at least two successive migrations from Asia in the late Eocene and earliest Oligocene. These migrations may have occurred via two different migration pathways through the north and south of Europe. In a second phase, Cricetidae arriving by the northern passway spread throughout Europe, whereas Cricetidae that arrived by the southern passway remained restricted to the central and southeastern Europe. The observations made on the Cricetidae allow for the proposal of a new, more general, scenario for the Eocene–Oligocene transition on a European scale, which is more complex than the “Grande Coupure” sensu stricto as initially proposed by Stehlin in 1909.
Key words: Rodentia, Eocene–Oligocene transition, Grande Coupure, Eastern Europe.
Olivier Maridet [olivier.maridet@jurassica.ch; ORCID: https://orcid.org/0000-0002-0956-0712 ] (corresponding author), Jurassica Museum, Rte de Fontenais 21, Porrentruy, Switzerland; Department of Geosciences, University of Fribourg, Fribourg, Switzerland.
Vlad A. Codrea [codrea_vlad@yahoo.fr; ORCID: https://orcid.org/0000-0002-3992-955X ] (corresponding author), Babeş-Bolyai University, STAR Institute, Laboratory of Paleotheriology and Quaternary Geology, Str. Mihail Kogălniceanu 1, Cluj-Napoca, Romania; Mureș County Museum, Department of Natural Sciences, Strada Horea 24, Târgu Mureş, Romania; Țării Crișurilor Museum, Department of Natural History, Str. Armatei Române 1/A, Oradea, Romania; Institute of Speleology Emil Racoviță, Calea 13 Septembrie 13, Bucharest, Romania.
Cristina Fărcaș [farcas2002@yahoo.com; ORCID: https://orcid.org/0009-0003-5618-1509 ], Babeş-Bolyai University, STAR Institute, Laboratory of Paleotheriology and Quaternary Geology, Str. Mihail Kogălniceanu 1, Cluj-Napoca, Romania.
Alexandru A. Solomon [alex_solomon88@yahoo.com; ORCID: https://orcid.org/0000-0002-7264-6527 ], Babeş-Bolyai University, STAR Institute, Laboratory of Paleotheriology and Quaternary Geology, Str. Mihail Kogălniceanu 1, Cluj-Napoca, Romania; Mureș County Museum, Department of Natural Sciences, Târgu Mureş, Romania.
Márton Venczel [mvenczel@gmail.com; ORCID: https://orcid.org/0000-0003-2200-3619 ], Babeş-Bolyai University, STAR Institute, Laboratory of Paleotheriology and Quaternary Geology, Str. Mihail Kogălniceanu 1, Cluj-Napoca, Romania; Țării Crișurilor Museum, Department of Natural History, Oradea, Romania.
Jérémy Tissier [jeremy.tissier123@gmail.com; ORCID: https://orcid.org/0000-0002-8517-1612 ], Jurassica Museum, Rte de Fontenais 21, Porrentruy, Switzerland; O.D. Earth and History of Life, Royal Belgian Institute of Natural Sciences, Vautierstreet 29, Brussels, Belgium.
Received 20 December 2024, accepted 10 April 2025, published online 16 June 2025.
Copyright © 2025 O. Maridet et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The late Eocene–early Oligocene history of mammals in Europe is characterised by a radical turnover in the fauna. Swiss palaeontologist Hans Georg Stehlin (1909) first described this event, suggesting a correlation between European terrestrial data and the changes characterising the Eocene/Oligocene boundary in the marine domain. He designated this event as the “Grande Coupure”, a term that is still used today to describe the terrestrial transition between the Eocene and Oligocene. This event is one of the most significant turnovers in mammalian faunas, including synchronous extinctions and origins generated by dispersal. This marks a sudden change from the endemic European faunas to faunas with major components of Asian origin (e.g., Brunet 1979; Remy et al. 1987; Legendre 1989; Blondel 2001; Hooker et al. 2004). A number of recent studies have indicated that the earliest Oligocene glaciation may have triggered the turnover (e.g., Legendre 1989; Hooker et al. 2004; Sheldon et al. 2016). This suggests a correlation between the “Grande Coupure” and the earliest Oligocene oxygen isotope step (EOIS), designated as “Oi-1 Glaciation event” (ca. 33.65 Ma; Miller et al. 1991; Zachos et al. 2001; Jovane et al. 2006; Ladant et al. 2014; Hutchinson et al. 2021). This initial glaciation event is part of the the Eocene–Oligocene climatic transition (EOT), period of transition, which also includes the beginning of the Oligocene (ca. 33.9 Ma; Coxall and Pearson 2007).
Since its publication by Stehlin (1909), researchers have sought to identify a comparable event in other regions of the world (e.g., Pascual et al. 1985; Marshall and Cifelli 1989; Rasmussen et al. 1992; Stucky 1992; Prothero and Heaton 1996; Meng and McKenna 1998). In general, where the fossil record is sufficiently comprehensive, the results demonstrate a notable faunal change in proximity to the Eocene/Oligocene boundary (e.g., Marshall and Cifelli 1989; Rasmussen et al. 1992; Stucky 1992; Meng and McKenna 1998). However, these changes differ from those described by Stehlin (1909) in terms of both the nature of the change, which may be less drastic (Rasmussen et al. 1992; Stucky 1992), and the lack of a recognised associated migratory event (e.g., Hartenberger 1998; Meng and McKenna 1998), or because it is not synchronous with the Eocene/Oligocene boundary (e.g., Prothero and Heaton 1996; Sun et al. 2014). The aforementioned differences have led Meng and McKenna (1998) to refer to the change observed on the Mongolian plateau as “Mongolian Remodelling”, thereby emphasising the significant distinction in the fundamental nature of the observed change when compared to Europe. In a more general sense, the term “Grande Coupure” sensu lato can be applied to the changes observed in different regions of the world, as opposed to the “Grande Coupure” sensu stricto as described by Stehlin (1909) in Europe. However, even in Europe, the discoveries made over the last 20 years in Eastern Europe suggest a more complex scenario for the Eocene–Oligocene transition on a European scale, in particular with the arrival of taxa of Asian origin already in the late Eocene (e.g., Ünay-Bayraktar 1989; Baciu and Hartenberger 2001; de Bruijn et al. 2003, 2018, 2019; Delfino et al. 2003; Codrea et al. 2011; Grandi and Bona 2017; Mennecart et al. 2018; Tissier et al. 2018; van de Weerd et al. 2018; Licht et al. 2022; Lihoreau et al. 2023).
Cricetid rodents are emblematic taxa of Stehlin’s (1909) “Grande Coupure” due to their Asian origin and their rapid arrival and diversification in Europe from the Oligocene onwards. The discovery of Cricetidae in well-dated small mammal assemblages from the upper Eocene and lower Oligocene of Transylvania (Romania) has enabled a critical revision of the “Grande Coupure” on a European scale. A systematic study of these new specimens is shown below together with their analysis in the wider context of the Eocene–Oligocene transition on a European scale, leading to the proposal of a new scenario for the “Grande Coupure” in Europe.
Institutional abbreviations.—MPSUBB, Museum of Paleontology-Stratigraphy, Babeş-Bolyai University, Cluj-Napoca, Romania.
Other abbreviations.—EOT, Eocene–Oligocene transition; m/M, lower/upper molars; L, maximal length; W, maximal width.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in Zoobank LSID: urn:lsid:zoobank.org:pub:08C36725-96EB-4DDD-B 546-6231DB80E574.
Geological setting
The three mountain ranges of the Romanian Carpathians (eastern, southern, and western) encircle an area designated as the Transylvanian Depression (Fig. 1). Geologically, this region comprises portions of several superposed sedimentary basins, as evidenced by outcrops and borehole data (Balintoni et al. 1998; Krézsek and Bally 2006). The pre-Cenozoic units are not the focus of the present study, which will concentrate on the upper Eocene to lower Oligocene units (Fig. 2). The history of these deposits begins in the Maastrichtian, as evidenced by the presence of terrestrial deposits in the northwestern and southwestern areas of the depression (i.e., the Jibou Formation and the Șard Formation; Codrea and Dica 2005; Codrea and Godefroit 2008). The sedimentary history continued during the Paleogene, with clear documentation in the northwest area (Gilău, Meseș, and Preluca sedimentary areas; Rusu 1970, 1987; Popescu 1976). In the southwest, the Metaliferi sedimentary area and the Ighiu Formation exhibit terrestrial and marine interbeddings and marine transgressions (Codrea and Dica 2005). These discordantly cover older sedimentary deposits.
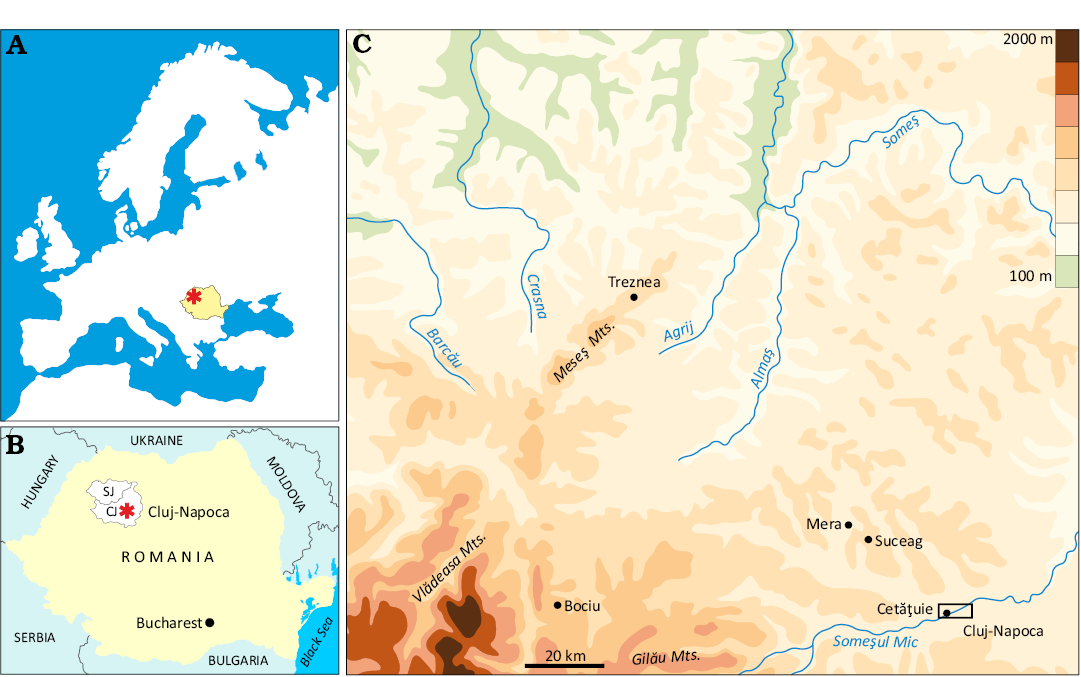
Fig. 1. Geographic and administrative context of the investigated area. A. Position of Romania within Europe. B. Sălaj (SJ) and Cluj (CJ) counties in Romania. C. Reliefs of Transylvania in Sălaj and Cluj counties.
Paleogene fossil-bearing localities with microvertebrates of interest for this paper are situated in the Gilău and Meseș sedimentary areas (Fig. 3). The oldest of them, Treznea (Sălaj County; Codrea and Fărcaș 2002; Fărcaș and Codrea 2004; Codrea and Venczel 2020; Codrea et al. 2022; Venczel 2023) is late Eocene (Priabonian) in age and is located in the Meseș sedimentary area, about 54 km NW of Cluj-Napoca (Fig. 3). From this location, Baciu and Hartenberger (2001) mentioned an isolated tooth assigned to Pseudocricetodon sp. and a charophyte flora. The rocks of interest are those belonging to the Turbuța Formation, which represents a transition from marine sedimentary environments to a short deltaic episode, which is then completed by fluvial plain deposits. These deposits are characterised by frequent interbedding of flooding that led to the formation of episodic palustrine ponds where thin coal strata occurred. However, these strata never acquired a consistent extension, with the thickness rarely exceeding a few centimetres. From such an intercalation, a faunal association of microvertebrates including, among other taxa (gar-fish, crocodilians, marsupials, etc.), cricetids, was recovered by sediment sieving-washing technique.
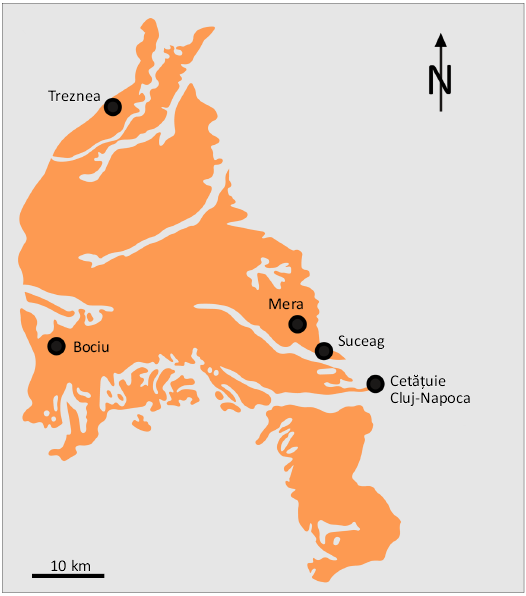
Fig. 2. Position of the fossil-bearing localities from this study, in Cenozoic sedimentary deposits near Cluj-Napoca.
The locality of Bociu, situated in the Gilau sedimentary area approximately 60 km west of Cluj-Napoca, is located at the contact between the Apuseni Mountains and the Transylvanian Depression (Fig. 4). The area is characterised by marine rocks of the Jebucu Formation, which date to the late Eocene, Priabonian period. These rocks are dominated by gypsum, which formed in lagoonal environments. The presence of the cricetid Atavocricetodon cf. nanoides was documented by an isolate tooth collected as a result of sediment washing in order to recover charophytes (Baciu and Hartenberger 2001). Despite the extensive washing of sedimentary rocks, subsequent attempts to enrich the microvertebrate sample yielded only limited results. This leads us to conclude that the terrestrial vertebrate fossils found in this locality are of a fortuitous context, rather than a common occurrence.
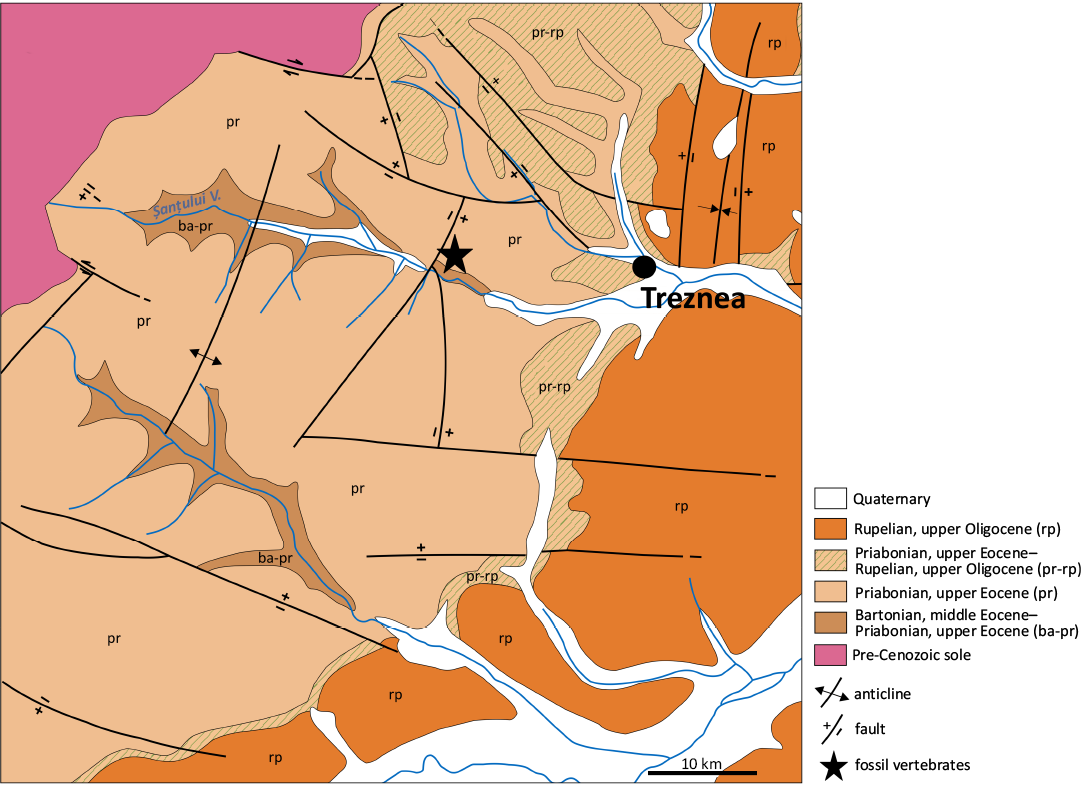
Fig. 3. Position of the Treznea locality (star), near Treznea village, Sălaj County, Romania (after the geological map 1: 50000 Meseș by Rusu et al. 1977, redrawn).
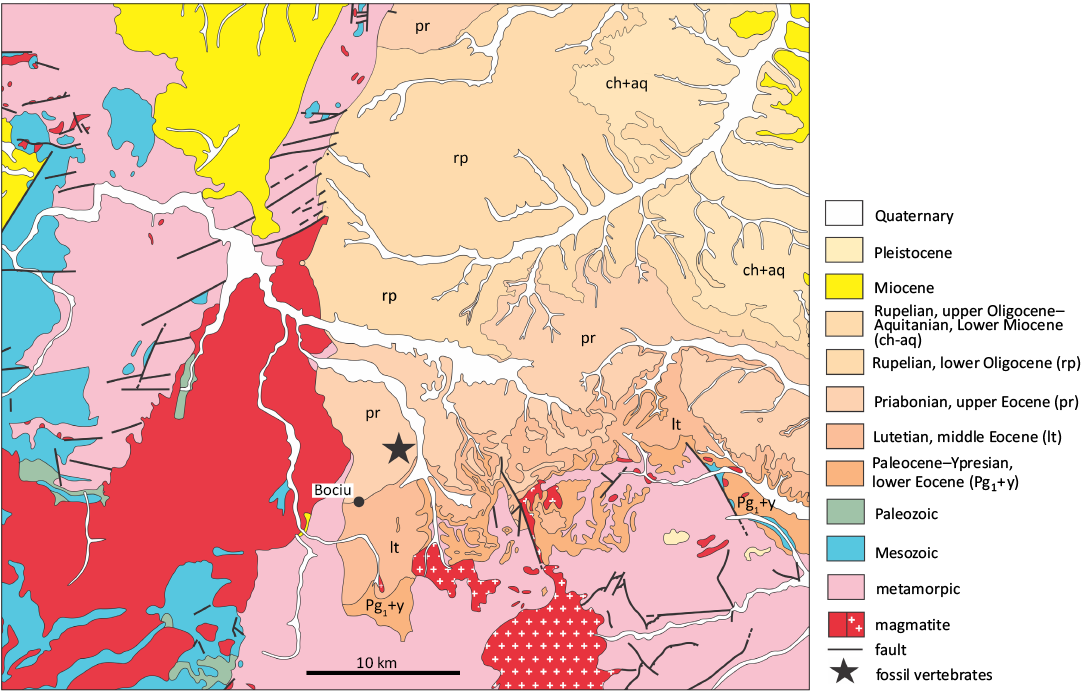
Fig. 4. Position of the Bociu locality (star), near Bociu village, Cluj County, Romania (after the geological map 1: 50000 Răchițele by Mantea et al. 1987, redrawn).
The Turbuța Formation is stratigraphically situated beneath the Jebucu Formation (Fig. 5). The latter ends by a phase of evaporation, which is overlain by an alternation of marine, clastic or calcareous sediments. This alternation is known in the literature as the “Călata Group” (Rusu 1995) or “lower marine series” (Răileanu and Saulea 1956). This unit can be placed between the Lutetian and the lower Priabonian. Indeed, two benthic foraminifera, Nummulites fabianii (Prever in Fabiani, 1905) and Alveolina elongata d’Orbigny, 1828, were reported in the last layers of this group, together with numerous other fossils, including molluscs and echinoderms (Popescu et al. 1978; Baciu and Hartenberger 2001). The benthic foraminifera indicate a correlation between the biozones SBZ 17 and 19 (between the Bartonian and Priabonian; Vandenberghe et al. 2012), whereas the rest of the fossils, including the nannoplankton, suggest a correlation with the biozone NP 18 (Priabonian, Vandenberghe et al. 2012). The fossil assemblage as a whole thus unequivocally establishes that both the Treznea and Bociu localities and the micromammals they yielded are unquestionably late Eocene.
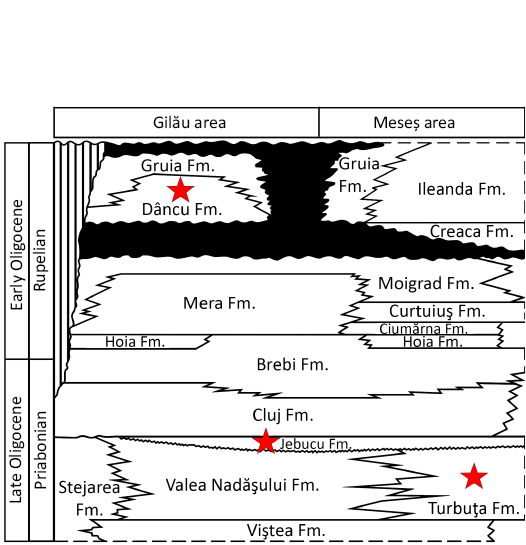
Fig. 5. Synthetic stratigraphic units in Gilău and Meseș sedimentary areas (from Codrea and Dica 2005 and related references; red stars indicate: Treznea 1 locality in Turbuța Formation, Bociu in Jebucu Formation, Cluj-Napoca-Cetățuie, Suceag 1, and Mera in Dâncu Formation.
The findings from the Cluj-Napoca-Cetățuie, Suceag sites (approximately 10 km to the west-north-west of Cluj-Napoca) and Mera on the Berecoaia valley (approximately 12 km from Cluj-Napoca in the same direction) are all from the Dâncu Formation (Rupelian) (Fig. 6). The formation is becoming progressively thicker as it progresses from Cluj-Napoca westward, towards Aghireș-Tămașa. The formation is initially observed to be only a few centimetres thick in the H2 Transgex borehole (Petrescu et al. 2002 and personal observations of VAC). However, it becomes more consistent (of metric order) in the Cetățuia Hill at Cluj-Napoca, continuing this trend at Suceag (Cipcheș Creek) and Mera (Berecoaia Valley). In the two final localities, clayey coal strata are present. Towards the west, at Aghireș, two brown coal strata (referred to as Francisc and Rozalia) are developing. These were mined through underground galleries in the past century, but are now abandoned. The early Oligocene age (MP 23) was established based on an association of micro- and macroflora (Petrescu 2003 and related references), invertebrates (Moisescu 1963, 1972, 1975, 1989) and vertebrates such as fishes (Osmeridae, Atherinidae, Ambassidae, Moronidae, Eleotridae, Gobiidae documenting a brackish low salinity environment, Reichenbacher and Codrea [1999] and three species of Dasyatidae, Trif and Codrea [2019]), amphibians (the frog Albionbatrachus oligocenicus, Venczel et al. [2013] and the salamander Mioproteus gardneri, Venczel and Codrea [2018]), crocodiles (Diplocynodon sp., Codrea and Venczel [2020]), birds (Anserinae indet., Kessler et al. [1998]) and mammals (anthracotheres, Codrea and Șuraru [1989]; Rădulescu and Samson [1989]; Fărcaș and Codrea [2005]).
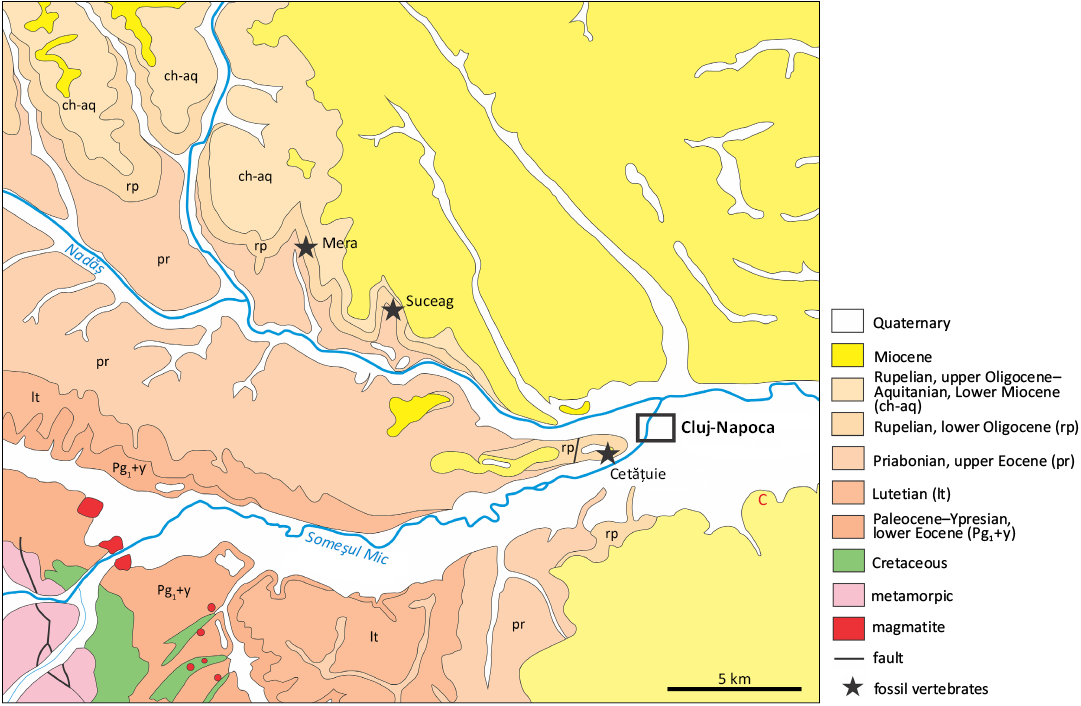
Fig. 6. Positions of the Mera, Suceag, and Cluj-Napoca-Cetățuie localities, Cluj County, Romania (after the geological map 1: 50000 Răchițele by Mantea et al. 1987, redrawn).
European chronological frameworks: questioning biochronological dogmas
There is a debate concerning the timing of taxa migrations into Europe, which could be either synchronous in all parts of Europe or not (e.g., Rage 1984; Legendre 1987a, b; Hooker et al. 2004; Costa et al. 2011). If the “Grande Coupure” remains better documented in Western Europe, a number of studies have been focusing on central and eastern parts of the continent during the last 20 years leading to new discoveries and demonstrating that some taxa previously thought to be post-“Grande Coupure” were already present in the late Eocene of Eastern Europe (e.g., Ünay-Bayraktar 1989; Baciu and Hartenberger 2001; de Bruijn et al. 2003, 2018, 2019, Delfino et al. 2003; Codrea et al. 2011; Grandi and Bona 2017; Mennecart et al. 2018; Tissier et al. 2018; van de Weerd et al. 2018; Licht et al. 2022; Lihoreau et al. 2023). The age of the different localities referred to in the present study is not based on the Paleogene European biochronological units (MP reference levels; Biochrom’97). Indeed, as previously discussed, the taxa that are typically considered biochronological markers, particularly the arrival of cricetids at the beginning of the Oligocene, are likely to be asynchronous at the European scale (de Bruijn et al. 2003, 2018, 2019; Ünay-Bayraktar 1989; van de Weerd et al. 2018). The age of the different localities (late Eocene vs. early Oligocene) is consequently either based on the occurrence of other taxa presenting a significant evolution through the Eocene–Oligocene transition (e.g., theridomorph rodents), on chronostratigraphical data, on absolute dating when available, or relatively to the “Oi-1 Glaciation” event when paleoclimatic data were available.
Turkey.—The Süngülü locality (de Bruijn et al. 2003) yielded a rodent fauna displaying ambivalent characteristics, suggestive of a late Eocene or early Oligocene age. However, de Bruijn et al. (2003) also noted the occurrence of a glyptosaur, which is more consistent with a late Eocene age. More recently, Métais et al. (2023) confirmed a likely late Eocene age for Süngülü, although an early Oligocene age cannot be entirely excluded. In this study, we adopt this late Eocene interpretation. In contrast, the rodent assemblages from the Lignite-Sandstone Formation of the Ergene Basin were dated using fission track dating of apatite particles in volcanic deposits (analysis by Paul A.M. Andriessen in Ünay-Bayraktar 1989: 14), which indicated a late early Oligocene age for Kavakdere and Kocayarma (Rupelian, Ünay-Bayraktar 1989).
Romania.—The ages of the different Paleogene localities with small vertebrates from Transylvania are based on the regional chronostratigraphical data (see Geological setting above). Bociu and Treznea are the latest Eocene records of mammals whereas Mera, Suceag and Cetățuie are the first Oligocene records. The associations of cricetid rodents presented below demonstrate similarities with the late Eocene and early Oligocene-aged records of Serbia and Turkey (Ünay-Bayraktar 1989; de Bruijn et al. 2003, 2018, 2019; van de Weerd et al. 2018). These similarities support the chronological correlations of the Romanian localities.
Serbia.—The age of the Serbian localities is interpreted in accordance with the findings of de Bruijn et al. (2018). The Buštranje locality, situated within the basal coarse clastics part of the Buštranje Formation in the Pčinja Basin, is predominantly considered to be of late Eocene (Priabonian) age. In contrast, the Strelac-1, Strelac-3, and Valniš localities from the Koritnica-Babušnica Basin are rather interpreted as early Oligocene based on the association of fossil rodents (Heosminthus, Heterocricetodon, Pseudocricetodon montalbanensis, Paracricetodon dehmi, and a diatomyid). In addition to the cricetid rodents, the diatomyid rodents found in the Strelac area (de Bruijn et al. 2018) also support an early Oligocene age by comparison with the record of Balochistan (Flynn et al. 1986).
Czech Republic.—Fejfar (1987) published a new locality, Dětañ, from a section in northwest of the Bohemia Massif in Czech Republic. The bottom of the section yielded an assemblage of fossil mammals together with an absolute age based on K/Ar dating of biotite/smectite crystals of the tuffs in the fossiliferous layer, providing an age of 37.7 ± 1.5 Ma (Priabonian, late Eocene). Later on, Fejfar and Kaiser (2005) considered the age of this locality as 37.5 Ma (still Priabonian). However, in accordance with the prevailing view that cricetids (along with other immigrant taxa) arrived only at the beginning of the Oligocene in Europe, Fejfar (1987) and Fejfar and Kaiser (2005) disregarded the datation (arguing a possible alteration of the biotite in Mikuláš et al. 2003) and concluded that the locality must be early Oligocene. In a subsequent analysis, Mikuláš et al. (2003) conducted a second K/Ar study on the basaltic lava flow that overlies the tuffs and tuffites at the top of the same section. This analysis yielded an age of 32.6 ± 1.7 Ma, which correlates with the very base of the Oligocene. However, the error margin does not exclude a late Eocene age. A logical conclusion from both datations would be that the majority of the section (if not all) is late Eocene in age. However, Mikuláš et al. (2003: 91) still concluded that “according to relative paleontological dating, the locality belongs to mammalian Zone MP21”. In the present study, we propose to disregard the biochronological value of the immigrant taxa, rather than disregarding both absolute datations. Consequently, we consider the mammalian assemblage of Dětañ to be Priabonian, late Eocene in age, implying that cricetids arrive earlier than the Oligocene in this region (as in Eastern Europe).
South Germany.—A series of fissure fillings in Southern Germany records the evolution of mammalian communities from the late Eocene to the early Oligocene, including the Eocene–Oligocene transition (Heissig 1987; EOT). However, the precise dating of the EOT within this series is subject to debate (Schmidt-Kittler and Vianey-Liaud 1975). In contrast to Stehlin’s (1909) initial description of the “Grande Coupure”, the succession of fissure fillings from Southern Germany demonstrates asynchronous first appearances of new taxa (at Möhren 19) and extinctions of endemic European taxa (mainly at Bernloch 1). Heissig (1987) proposed that the arrival of immigrant taxa (including Cricetidae) at Möhren 19 correlates with the EOT. In contrast, Legendre (1987a, b) suggested that extinctions caused by global climatic change are more likely to be synchronous at a large geographic scale than migrations, thus correlating Bernloch 1 with the EOT, due to the disappearance of endemic Europeans taxa in this site. Furthermore, Legendre (1987c) provided additional support for his interpretation by analysing the structure of fossil mammal assemblages, utilising cenograms (Valverde 1964; Legendre 1986). These cenograms indicated a significantly colder and drier climate, from Bernloch 1 onwards. Subsequently, Héran et al. (2010) conducted a geochemical analysis of the δ18O of tooth phosphate from rodents at the same South German localities, confirming a significant temperature drop at Bernloch 1. A quantitative analysis of the rodent diversity in these localities, in conjunction with the δ18O values (Fig. 7), demonstrates that the climatic change occurred concurrently with a pronounced diversification of cricetids and the local extinction of several Theridomyidae, Gliridae, Sciuridae and Aplodontidae lineages (Suevosciurus demi, Pseudosciurus suevicus, Gliravus majori, Gliravus minor, Oligodyromys balhoi, Oligopetes lophulus, and Oligopetes obtusus; Heissig 1987). This occurred subsequent to the arrival of the first immigrant taxa (Cricetidae, Eomyidae, Sciuridae, Aplodontidae) at Möhren 19. In the present study, we follow the interpretation of Legendre (1987b, c) and correlate the climatic change recorded in South Germany with the global “Oi-1 event” and the end of the EOT (ca. 33.9 Ma; Zachos et al. 2001; Jovane et al. 2006; Ladant et al. 2014; Hutchinson et al. 2021). Consequently, all localities older than Bernloch 1 are considered to be late Eocene (Table 1), which implies that cricetids arrived in this region earlier than the Oligocene, in a similar manner to that observed in Eastern Europe.
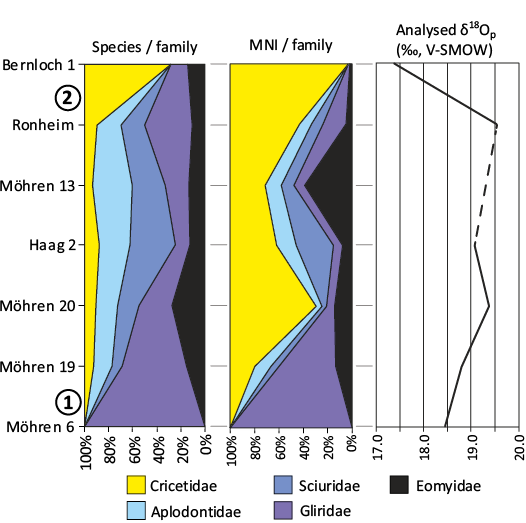
Fig. 7. Quantitative composition of rodent families (Theridomyidae excluded, because over represented in the assemblages): raw number of species per family and minimal number of individual (MNI) per family. The right column shows the analysed δ18Op (‰, V-SMOW) from the teeth apatite of the fossil rodents (values extracted from Héran et al. 2010). The analysed δ18Op serves as a proxy to paleoclimate indicating a significant drop of the annual temperature between the localities Ronheim and Bernloch 1. (1): Arrival of Cricetidae, Aplodontidae, Sciuridae and Eomyidae, (2): Cricetidae diversification event and drop of the δ18Op (‰, V-SMOW) values, marking the EOT.
Western Europe (Belgium, England, France, Spain, Switzerland).—Among the westernmost European localities, the Theridomyidae are frequently employed to provide a relative chronology of the mammal assemblages, due to their rich diversity and rapid evolution through the Eocene–Oligocene transition (e.g., Schmidt-Kittler 1990; Biochrom’97; Hugueney 1997; Vianey-Liaud and Marivaux 2016). First, some genera are indeed restricted to the uppermost Eocene (Priabonian) such as: Ectropomys Bosma & Schmidt-Kittler, 1972 (e.g., Ectropomys exigus Bosma & Schmidt-Kittler, 1972; Ectropomys monacensis Vianey-Liaud, Schmidt-Kittler, & Peláez-Campomanes, 1994; Ectropomys gliriformis [de Bruijn, Sondaar, & Sanders, 1979]), Thalerimys Tobien, 1972 (e.g., Thalerimys fordi [Bosma & Insole, 1972]), Oltinomys Stehlin & Schaub, 1951 (Oltinomys platyceps [Filhol, 1877]), and Patriotheridomys Vianey-Liaud, 1975 (e.g., Patriotheridomys altus Vianey-Liaud, 1975). Even more significantly, other genera occur in both the latest Eocene and earliest Oligocene but present a noticeable evolution (size, crown height, occlusal pattern) that leads to a turnover at the species level across the Eocene–Oligocene transition: Issiodoromys Croizet in Gervais, 1848 (Issiodoromys hartenbergeri [Vianey-Liaud & Ringeade, 1993] at the end of the Eocene vs. Issiodoromys nanus [Thaler, 1969], Issiodoromys medius [Vianey-Liaud, 1976], and Issiodoromys minor Schlosser, 1884, in the early Oligocene), Pseudoltinomys Lavocat, 1951 (Pseudoltinomys cuvieri [Pomel, 1852–1953] for the latest Eocene vs. Pseudoltinomys gaillardi Lavocat, 1951, for the early Oligocene), Blainvillimys Stehlin & Schaub, 1951, although it might be polyphyletic (Vianey-Liaud and Marivaux 2016) also shows a noticeable evolution (Blainvillimys civracensis Vianey-Liaud & Ringeade, 1993, and Blainvillimys rotudidens Schlosser, 1884, for the late Eocene vs. Blainvillimys langei Vianey-Liaud, 1972, Blainvillimys gregarius [Schlosser, 1884] and Blainvillimys gemellus Vianey-Liaud, 1989, for the early Oligocene), and Theridomys Jourdan, 1837, which is also likely polyphyletic (Vianey-Liaud and Marivaux 2016) shows significant changes (Theridomys bonduelli [Lartet, 1869] and Theridomys golpei Hartenberger, 1973, for the late Eocene vs. Theridomys calafensis Anadon, Vianey-Liaud, Cabrera, & Hartenberger, 1987, Theridomys aquatilis Aymard, 1849, and Theridomys major Depéret, 1906, for the early Oligocene).
Table 1. Succession of fissure fillings from Southern Germany (Heissig 1987) and proposed revision of the age (in bold characters) based on the fossil record (Heissig 1978, 1987) and paleoclimatic data (Legendre 1987c; Héran et al. 2010).
|
South German |
Age according to Heissig (1987) and Biochrom’97 |
New age proposed in this study |
|
Burgmagerbein 2 |
Oligocene (MP23) |
Oligocene |
|
Ehingen 1 |
Oligocene (MP23) |
Oligocene |
|
Bernloch 1 |
Oligocene (MP23) |
Oligocene |
|
Schelklingen 1 |
Oligocene (MP23) |
Eocene |
|
Ronheim 1 |
Oligocene (MP22) |
Eocene |
|
Herrlingen 1 |
Oligocene (MP22) |
Eocene |
|
Möhren 13 |
Oligocene (MP22) |
Eocene |
|
Grafenmühle 10 |
Oligocene (MP22) |
Eocene |
|
Grafenmühle 6 (b) |
Oligocene (MP22) |
Eocene |
|
Grafenmühle 7 |
Oligocene (MP22) |
Eocene |
|
Burgmagerbein 3/5/8 |
Oligocene (MP21) |
Eocene |
|
Haag 2 |
Oligocene (MP21) |
Eocene |
|
Möhren 4 |
Oligocene (MP21) |
Eocene |
|
Möhren 20 |
Oligocene (MP21) |
Eocene |
|
Möhren 7/16 |
Oligocene (MP21) |
Eocene |
|
Möhren 31 |
Oligocene (MP21) |
Eocene |
|
Möhren 19 |
Oligocene (MP21) |
Eocene |
|
Frohnstetten |
Eocene (MP20) |
Eocene |
|
Weissenburg 2/9 |
Eocene |
Eocene |
|
Weissenburg 3 |
Eocene |
Eocene |
|
Möhren 2 |
Eocene |
Eocene |
|
Möhren 23 |
Eocene |
Eocene |
|
Möhren 6 |
Eocene (MP19) |
Eocene |
|
Oppertshofen 2 |
Eocene |
Eocene |
|
Weissenburg 8 |
Eocene |
Eocene |
|
Grafenmühle 6 (a) |
Eocene |
Eocene |
|
Mähringen |
Eocene |
Eocene |
The family Theridomyidae consequently allows to secure the age of the many localities, independently from immigrant taxa, including the following that are referred to in the discussion herein: for the uppermost Eocene, Bembridge Limestone (Hooker et al. 1995), Escamps (Biochrom’97), Saint-Capraise-d’Eymet (Biochrom’97), San Cugat de Gavadons (Antunes et al. 1997), Villarrosano 1, 12 (Peláez-Campomanes 2000), Mormont-Entreroches (Biochrom’97), Obergösgen (Biochrom’97); for the lowermost Oligocene: Balm (Engesser and Mödden 1997), Ollala 4A (Freudenthal 1997), Paquera 1 (Hugueney and Adrover 1982), Villebramar (Biochrom’97), Baraval (Sigé et al. 1998), Mazan (Maridet et al. 2013), Lower Hamstead beds (Hooker 2010), Hoogbutsel (Smith 2003; Hooker 2010), Ronzon (Lavocat 1952; Hugueney 1997), Aubrelong 1 (Schmidt-Kittler 1987; Vianey-Liaud and Schmid 2009) Ravet (Schmidt-Kittler 1987).
Paleogeographic and biogeographical frameworks
The paleogeographic reconstruction used herein for the late Eocene and early Oligocene of Europe are a compilation of maps proposed by different authors (Meulenkamp et al. 2000 and Popov et al. 2004 for the late Eocene, and Popov et al. 2004 and Barrier and Vrielynck 2008 for the early Oligocene). The studied localities are part of the Moesian land sensu Popov et al. (2004), one of the emerged lowland platforms within the late Eocene and early Oligocene Paratethys sea. From a palaeobiogeographical point of view, the Transylvanian localities are part of the Balkanatolia (Licht et al. 2022) together with several other previously published localities which also provided an assemblage of cricetid taxa around the EOT: Zvonce and Buštranje from the upper Eocene of Serbia (de Bruijn et al. 2018, 2019; van de Weerd et al. 2018), Süngülü probably also upper Eocene from Turkey (de Bruijn et al. 2003), Strelac, Valniš and Raljin from the lower Oligocene of Serbia (de Bruijn et al. 2018, 2019; van de Weerd et al. 2018), and Kavakdere and Kocayarma from the lower Oligocene of Turkey (Ünay-Bayraktar 1989).
Material and methods
The studied material is composed of isolated cheek teeth obtained by screen-washing sediments with various screen down to a 0.5 mm mesh. The terminology used to describe the molars follows Maridet and Ni (2013). No mandible or maxilla have been found allowing to associate incisor and cheek teeth, so the microstructure of incisors enamel cannot be analysed as a complementary systematic approach. The definition of subfamilies is based on the phylogenetic results of Maridet and Ni (2013). Specimens are deposited in the collections of the Museum of Paleontology-Stratigraphy of the Babeş-Bolyai University in Cluj-Napoca (MPSUBB) and are catalogued with the numbers: MPSUBB v172/1, MPSUBB v171/1+2 and MPSUBB v1084 to v1136. All measurements are in millimeters.
Systematic palaeontology
Order Rodentia Bowdich, 1821
Family Cricetidae Fischer von Waldheim, 1817
Subfamily incertae sedis
Genus Witenia de Bruijn, Ünay, Saraç, & Yïlmaz, 2003
Type species: Witenia flava de Bruijn, Ünay, Saraç, & Yïlmaz, 2003; Süngülü A, upper Eocene?, Turkey.
Witenia sp.
Fig. 8A, B.
Material.—Two lower molars from Treznea, Priabonian, upper Eocene, Turbuța Formation, Meseș sedimentary area, Transylvanian Basin, Romania.
Measurements.—See Table 2.
Table 2. Measurements [in mm] of molars of Witenia sp. from the late Eocene of Treznea.
|
Tooth |
Specimen number |
L |
W |
|
Left m1 |
MPSUBB v1084 |
1.97 |
1.37 |
|
Right m3 |
MPSUBB v1085 |
1.80 |
1.51 |
Description.—Both molars have bulk cuspids with a relatively high crown compared to other late Eocene specimens studied herein.
The m1 has no anterolophulid nor anteroconid but has a metaconid spur connecting the anterolophid to the metaconid. The metaconid is much more mesially located than the protoconid, with its tip very close to the antero-lingual border of the tooth. The metaconid and the protoconid are connected by a long V-shaped protoconid hind arm. The metaconid ridge is thick and ends with a strongly developed mesostylid, but does not totally close the mesosinusid, whereas a low and weakly developed cingulid closes the sinusid labially. The entoconid is transversally elongated and shows a shot distal spur. The posterosinusid is large and surrounded by a long and strongly curved posterolophid. A short labial posterolophulid delimits a small posterosinusid. The roots are not preserved.
The m3 has thick anterolophids, but not reaching the metaconid and the protoconid so the metasinusid and protosinusid remain open, respectively. The protoconid hind arm is large, oriented backward and reaches the middle of the mesoflexid, whereas the mesolophid is short and is oriented forward. The metaconid ridge is long, without mesostylid, but does not totally close the mesoflexid, whereas a low and weakly developed cingulid closes the sinusid labially. The posterosinusid is nearly closed by a low, but incomplete cingulid. The roots are not preserved.
Remarks.—The two teeth from Treznea show characteristics that are diagnostic of the genus Witenia on lower check teeth (de Bruin et al. 2003): the anteroconid of the m1 developed as a narrow antero-lingually directed crest of the protoconid and the generally large sinusid and lingually closed by an antero-labially postero-lingually directed oblique crest. However, the statistically larger m3 compared to m1, another diagnostic feature of the genus, cannot be observed here based on two teeth only. In addition, the teeth from Treznea are characterized by large and elongated crests (lophodont trend) and large sinusid that differentiate Witenia from most other cricetid genera.
There are so far four species referred to Witenia: Witenia yolua Gomes Rodrigues, Marivaux, & Vianey-Liaud, 2012; W. flava de Bruijn, Ünay, Saraç, & Yïlmaz, 2003; Witenia fusca de Bruijn, Ünay, Saraç, & Yïlmaz, 2003, and Witenia europea de Bruijn, Marković, Wessels, & van de Weerd, 2019. De Bruijn et al. (2018) also tentatively referred a few teeth from Strelac-1, Strelac-3, and Valniš to the the species W. fusca as W. cf. fusca. The m1 from Treznea shows several morphological similarities with W. fusca: the lingually directed spur instead of an anteroconid; the protoconid and metaconid near one another (so the mesial part of the tooth is noticeably narrower than the distal part) and connected by the distal arm of the protoconid; the oblique ectolophid making the shape of a cross with the mesolophid/ectomesolophid; the hypolophid inserted on the ectolophid just in front of the hypoconid; and the long posterolophid with a bulge at its labial extremity. Likewise, the m3 from Treznea shows more similarity with W. fusca: the parallel metalophid and hypolophid directed somewhat mesially and inserting respectively on the protoconid and hypoconid mesial arms; a large sinusid with the long distal arm of the protoconid but a much shorter mesolophid; and a wide strong posterolophid constricted just distally to the entoconid.
The two teeth are otherwise noticeably smaller than in all species of Witenia, including W. cf. fusca from Strelac-1, Strelac-3, and Valniš (the smallest population referred to Witenia so far: de Bruin et al. 2018). We tentatively refer them to Witenia, they might consequently belong to a new and smaller species, but the material is unfortunately insufficient to secure the generic identification and formally define a new species. These two teeth are identified as Witenia sp. until more material is found.
Genus Eocricetodon Wang, 2007
Type species: Eucricetodon meridionalis Wang & Meng, 1986, Caijiachong, upper Eocene of Yunnan, China
Eocricetodon cf. Eo. meridionalis (Wang & Meng, 1986)
Fig. 8C–K.
2001 Pseudocricetodon sp.; Baciu and Hartenberger 2001: 444.
Material.—Seven upper molars and four lower molars from Treznea, Priabonian, upper Eocene, Turbuța Formation, Meseș sedimentary area, Transylvanian Basin, Romania.
Measurements.—See Table 3.
Table 3. Measurements of molars (in mm) of Eocricetodon cf. Eo. meridionalis from the upper Eocene of Treznea.
|
Tooth |
Specimen number |
L |
W |
|
Right M1 Left M1 |
MPSUBB v172/1 MPSUBB v1086 |
1.62 1.75 |
– 1.19 |
|
Left M2 Left M2 Left M2 Right M2 Mean M2 StD M2 |
MPSUBB v1087 MPSUBB v1088 MPSUBB v1089 MPSUBB v1090 |
1.31 1.40 1.28 1.42 1.35 0.07 |
1.23 1.36 1.07 – 1.22 0.14 |
|
Right M3 |
MPSUBB v1091 |
1.13 |
1.02 |
|
Right m1 |
MPSUBB v1092 |
1.40 |
1.06 |
|
Right m2 Right m2 |
MPSUBB v1093 MPSUBB v171/1 |
1.42 1.39 |
1.16 1.15 |
|
Right m3 |
MPSUBB v171/2 |
1.34 |
1.09 |
Description.—In lateral view, the molars show a low crown with acute cusps and cuspids. On upper molars, especially M2s, when the teeth are not worn out, the paracone appears noticeably higher than the metacone.
M1s are characterized by an elongated and narrow mesial lobe. The anterocone is not divided but is transversally elongated; it is connected to the protocone by a long and well-developed anterolophule. The mesial protolophule is either incomplete or absent whereas the distal one is complete. The paracone spur is either weak or absent. The mesosinus is labially open despite the presence of a well-developed mesostyle. The mesoloph can be short or long, when long it merges with the mesostyle. The mesocone is small with an entomesoloph either very small or absent. One of the M1s (Fig. 8D) shows a very small protocone distal arm (sensu Maridet and Ni 2013), much lower and weaker than the entoloph. The metalophule is transverse and connects to the mesial half of the hypocone. The posterosinus is wide and closed labially long posteroloph. The roots are not preserved.
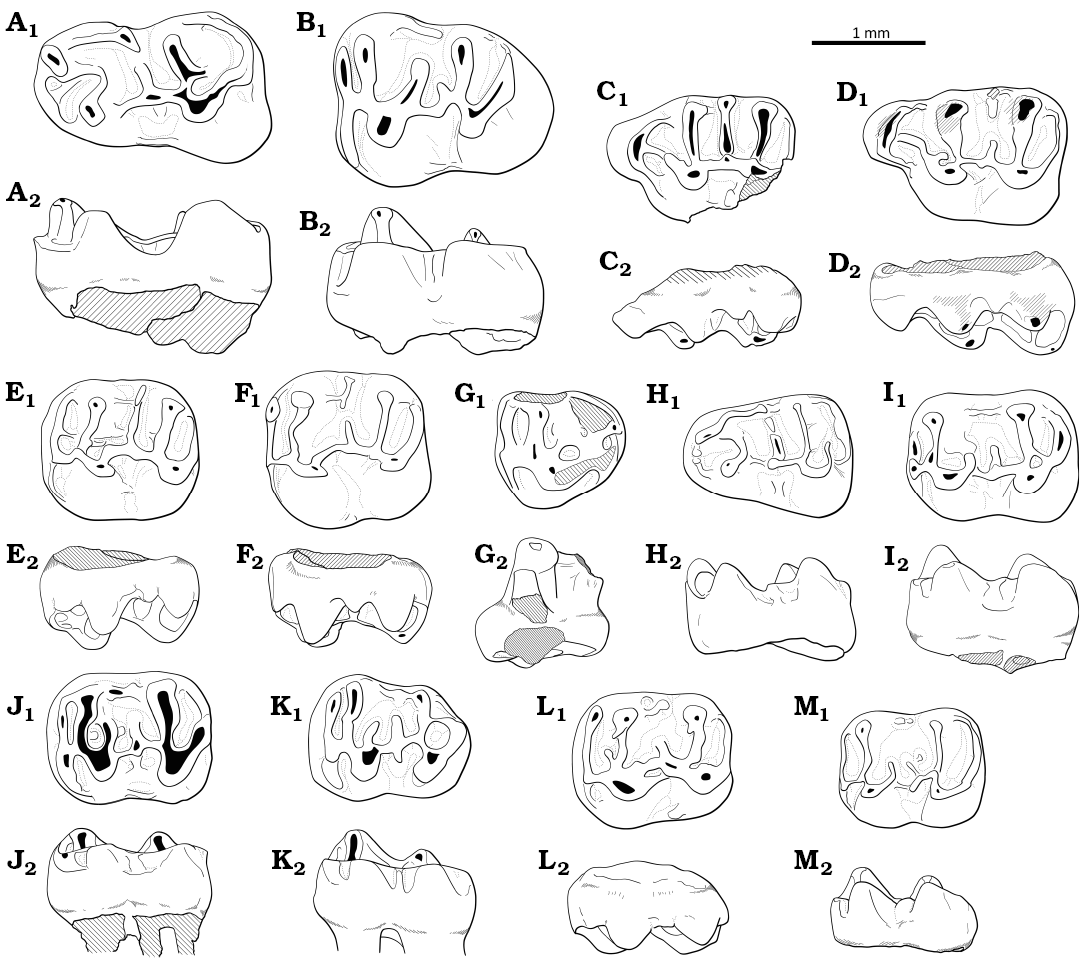
Fig. 8. Drawings of upper and loyer molars. A, B. Witenia sp. from the Priabonian, upper Eocene of Treznea, Romania. A. Left m1 (MPSUBB v1084). B. Right m3 (MPSUBB v1085, reversed). C–K. Eocricetodon cf. Eo. meridionalis (Wang & Meng, 1986), from the late Eocene (Priabonian) of Treznea, Romania. C. Right M1 (MPSUBB v172/1, reversed). D. Left M1 (MPSUBB v1086). E. Left M2 (MPSUBB v1087). F. Left M2 (MPSUBB v1088). G. Right M3 (MPSUBB v1091, reversed). H. Left m1 (MPSUBB v1092, reversed). I. Right m2 (MPSUBB v1093). J. Right m2 (MPSUBB v171/1, reversed). K. Right m3 (MPSUBB v171/2, reversed). L–M. Bustrania cf. B. dissimile de Bruijn, Marković, Wessels, & van de Weerd, 2019, from the Priabonian, upper Eocene of Bociu, Romania. L. Right M2 (MPSUBB v1094, reversed). M. Left m2 (MPSUBB v1095). A1–M1, occlusal view; A2–M2, labial view.
M2s have a strong labial anteroloph whereas the lingual anteroloph is much less developed and lower. One M2 (Fig. 8E) has a second anterolophule connecting the middle of the labial anteroloph to the mesial protolophule. Another M2 (Fig. 8F) has a parastyle located labially on the labial anteroloph. Both the mesial protolophule and metalophule are oblique and connected mesially to the protocone and hypocone respectively. The distal protolophule is either weaker than the mesial one, or incomplete or absent. The mesostyle is well developed and linked to the paracone by a paracone distal spur (or postparacrista; Fig. 8E, F). The mesoloph is long and can merge with the mesostyle. One of the M2s shows a very small protocone distal arm, much lower and weaker than the entoloph. The posterosinus is wide and closed labially by the posteroloph. The roots are not preserved.
The only M3 is strongly worn out and partly broken; however, it is possible to observe that the tooth has rounded shape due to much reduced metacone and hypocone. The labial anteroloph bears a very small parastyle. A spur starting between the protolophule and the protocone seem to join the mesoloph to form a small pit in the middle of the tooth. The M3 has three roots.
The only m1 (Fig. 8H) has a very small anteroconid and a metastylid both next to each other, but no anterolophulid or metalophulid. The labial anterolophid connect directly the anteroconid to the protoconid mesial slop; the lingual anterolophid is very short as the metaconid is much more mesially located than the protoconid, hence close to the metastylid. The metaconid and the protoconid are connected by the curved protoconid hind arm. The metaconid ridge is long and follows the lingual border up to the entoconid so the mesosinusid is closed. The mesoconid is small but well developed. The mesolophid starts from the mesoconid, is large and long but does not reach the lingual border; it is also interrupted in its middle. In contrast, the ectomesolophid is very weakly developed, limited to a fold of the enamel in the middle of the sinusid, and does not seem to connect to the mesoconid or the ectolophid. The hypoconid hind arm is long and ends in the middle of the posterosinusid. There is a little depression between the distal side of the hypoconid and the base of the posterolophid. The roots are not preserved.
The m2s have long and thick lingual and labial anterolophids. The antero-lingual sinusid is closed, whereas the antero-labial one is closed only in one of the two m2s. Both the metalophulid and the protoconid hind arms are present and well developed; the protoconid hind arm bends and connects to the metaconid, but it is slightly lower than the metalophulid. One of the m2s (Fig. 8I) has short distal spurs starting from the metalophulid and joining the protoconid hind arm. The mesoconid is small but well developed; the mesolophid starts from the mesoconid, it is long but stops in the middle of the mesosinusid. The ectomesolophid also starts from the mesoconid and is short in one m2, but it is absent in the other one. The metaconid ridge is long and reaches the entoconid so the mesosinusid is closed ligually. A cingulid closes the sinusid labially in one m2 but not in the other one. The hypoconid hind arm connects to the hypolophulid in one m2 and is absent in the other. The posterolophid is large and long, it forms a bulge on the distal border and closes the postero-sinusid lingually. One of the m2s has two preserved roots.
The only m3 (Fig. 8K) has long and thick lingual and labial anterolophids like for m2s. Both the metalophulid and the protoconid hind arm are present and well developed but the protoconid hind arm does not connects to the metaconid. Mesoconid, mesolophid and ectomesolophid are all well developed; both the mesolophid and ectomesolophid start from the mesoconid; the mesolophid is long but does not reach the lingual border whereas the ectomesolophid is short. The metaconid ridge is long and reaches the entoconid so the mesosinusid is closed ligually whereas the sinusid remains open labially. The posterolophid forms a loop and connects to the hypolophulid delimiting a small rounded posterosinusid. An additional small depression exists between the posterolophid, the entoconid and a cingulum that follows the postero-lingual border. The tooth has two roots.
Remarks.—With a well-developed mesial lobe in M1, a small but also well-developed anteroconid in m1, the occurrence of a hypoconid hind arm in m1–2, and the reduced third molars, these teeth from Treznea show all the characteristics of a small eucricetodontine species. Among small size eucricetodontines, the teeth from Treznea noticeably differs from Lignitella suemengeni Ünay-Bayraktar, 1989, in having a hypoconid hind arm in m1–2 and being noticeably bigger, and from Oxynocricetodon erenensis Wang, 2007, in having a complete anterolophule and a long mesoloph in M1, and a much reduced M3.
Among Eocene and Oligocene eucricetodontines, many species have been referred to the genus Eucricetodon, and the genus or subgenus Atavocricetodon: Eu. (A.) atavoides Freudenthal, 1996; Eu. (A.) atavus Misonne, 1957; Eu. (A.) hugueneyae Freudenthal, 1996; Eu. (A.) minusculus Freudenthal, 1996; Eu. (A.) nanoides Freudenthal, 1996; Eu. (A.) nanus Peláez-Campomanes, 1995; Eu. (A.) paaliensis Marivaux, Vianey-Liaud, & Welcomme, 1999; Eu. caducus (Shevyreva, 1967); Eu. huberi (Schaub, 1925); Eu. huerzeleri Vianey-Liaud, 1972; Eu. leptaleos (Wang & Meng, 1986); Eu. murinus (Schlosser, 1884); Eu. asiaticus (Matthew & Granger, 1923); Eucricetodon occasionalis Lopatin, 1996; Eu. praecursor (Schaub, 1925). All these species differ from the above described specimens of Treznea in having more robust cusp(id)s, stouter loph(id)s, a larger mesial lobe in M1, in missing the long and complete anterolophule (this morphology is very rare in the Eucricetodon/Atavocricetodon group, and the anterolophule is stouter and better developed in the rare cases when it occurs, e.g., see Freudenthal 1996), often shorter mesolophs and mesolophids with less developed mesostyl(id)s, and better developed anteroconid in m1 with a longer mesial lobe. The teeth from Treznea show nevertheless some similarities with Eu. (A.) kurthi de Bruijn, Ünay, Saraç, & Yïlmaz, 2003 in having an elongated and narrow mesial lobe, a long mesoloph in M1 and M2 sometimes reaching the labial cingulum, a small and rounded M3 with very weakly developed hypocone and metacone in M3. Nevertheless, they still noticeably differ from it in being larger, in having a complete anterolophule on M1, both protolophules but with a less developed distal one in M2, and the mesial protolophule connecting directly to anteroloph in M3.
The long but narrow mesial lobe with a well-developed and complete anterolophule in M1 associated with a poorly-developed anterocone in M1 are in fact characteristic of another genus tentatively referred to Eucricetodontines but never described in Europe so far: Eocricetodon Wang, 2007. The diagnosis of this genus describes indeed many morphological characters also observed in Treznea such as the obtuse main cusps and slender lophs, the elongated and narrower anterior lobe, single anterocone, and complete thin anterolophule in M1, the well-developed mesostyle and mesoloph in upper molars, the ml and m2 of subequal length, the short mesial part (trigonid) and the weakly-developed anteroconid without metalophid I and anterolophid in m1, and metaconid and entoconid more mesially located than protoconid and hypoconid in m2. The only main difference is the presence of well-developed distal arm of hypoconid in the m1 and m2 of Treznea whereas the feature is considered absent in the diagnosis of Eocricetodon. It is, however, worth noticing that both the m1 figured in Wang (2007: fig. 4C) and the m2 figured in Wang and Meng (1986: pl. 1: 8) show a little bulge at the base of the posterolophid which could indicate that this feature is not really absent but rather weakly developed. There are so far two species referred to this genus: Eocricetodon meridionalis (Wang & Meng, 1986) and Eocricetodon borealis Wang, 2007. The two species are very similar in size and morphology, but Eo. meridionalis slightly differs by the absence of protoconule in m1, the larger and more mesially located metaconid and complete distal metalophid in m1, and the the transverse mesial metalophid connecting mesially to protoconid in m2. The teeth from Treznea show the same features thus displaying a size and morphology very close to Eo. meridionalis at the exception of the distal well-developed arm of hypoconid in m1 and m2. Therefore, we tentatively refer these specimens from Treznea to this species as Eo. cf. Eo. meridionalis, which indicates the first occurrence of this genus outside Asia.
Genus Bustrania de Bruijn, Marković, Wessels, & van de Weerd, 2019
Type species: Bustrania dissimile de Bruijn, Marković, Wessels, & van de Weerd, Buštranje, upper Eocene, Serbia.
Bustrania cf. B. dissimile de Bruijn, Marković, Wessels, & van de Weerd, 2019
Fig. 8L, M.
2001 Atavocricetodon cf. nanoides; Baciu and Hartenberger 2001: 444.
Material.—One upper molar and one lower molar from Bociu, Priabonian, upper Eocene, Jebucu Formation, Gilău sedimentary area, Transylvanian Basin, Romania.
Measurements.—See Table 4.
Table 4. Measurements of molars (in mm) of Bustrania cf. B. dissimile from the upper Eocene of Bociu.
|
Tooth |
Specimen number |
L |
W |
|
Right M2 |
MPSUBB v1094 |
1.27 |
1.21 |
|
Left m2 |
MPSUBB v1095 |
1.22 |
1.08 |
Description.—In lateral view, the molars are characterized by a low crown, slender and less acute cusp(id)s compared to Eo. cf. Eo. meridionalis from Treznea; also, as opposed to Eo. cf. Eo. meridionalis from Treznea, the paracone of the M2 is not noticeably higher than the metacone.
The M2 (Fig. 8L) has a long labial anteroloph ending by a little parastyle whereas the lingual anteroloph is lower and much shorter so the protosinus is limited to a small pit mesial to the protocone. The protocone spur is present but is short and a second small spur projects into the anterosinus from the paracone. The paracone also has a well-developed distal spur ending by a mesostyle. A small cingulum is present on the labial border just distal to the paracone; it merges with the paracone distal spur to form a small pit. The mesostyle and the metacone are not connected so the mesosinus remains labially open. A cingulum is also present lingually; it is connected to the hypocone, thick but short, so the sinus remains open lingually. Both the entoloph and the protocone distal arm are well developed; they connect the protocone to a large mesocone. The mesoloph is short and starts from the mesial part of the mesocone whereas the metalophule connects to the distal part of the mesocone (and not to the hypocone). The posteroloph is long, delimiting a wide posterosinus. The roots are not preserved.
The m2 (Fig. 8M) has a long lingual anterolophid but a short labial anterolophid so the protosinusid remains open labially. There is no metaconid ridge but a small mesostyle; nevertheless, the mesosinusid remains open lingually. Likewise, the sinusid is opened labially. The protoconid hind arm is long but ends in the mesosinusid. The mesolophid starts from the mesoconid and is short; it ends in the mesosinusid. Additionally, a little spur starts from the mesolophid extremity and is oriented towards the hypolophulid. The ectolophid is lower and weaker mesially to the mesoconid (between the protoconid hind arm and the mesoconid) than distally (between the mesoconid and the hypolophulid). The entolophulid is straight and connects to the ectolophid, mesially to the hypoconid. The tooth has no hypoconid hind arm, no labial posterolophulid or labial posterosinusid, but the posterolophid is long and delimits a wide posterosinusid. The roots are not preserved.
Remarks.—These teeth represent the smallest cricetid found so far in the upper Eocene of Transylvania. They are characterized by a low crown, thin crests and gracile cusp(id)s, the sinus(id) oriented backward, and a mesosinus much larger than the sinus in M2 and a mesosinusid much larger than the sinusid in m2 (due to the lingual position of the entoloph and the labial position of the entolophid respectively). Additionnaly the protocone distal arm is long, almost longitudinal and connects directly to the mesial arm of the hypocone, which is a characteristic only seen in taxa referred to Paracricetodontinae or Pappocricetodontinae (Maridet and Ni 2013; de Bruijn et al. 2018). The above-described morphological features ressemble that of Bustrania dissimile de Bruijn, Marković, Wessels, & van de Weerd, 2019, from the Eocene of Serbia (Buštranje). One of the diagnostic features if B. dissimile is the high morphological variability composed by an “irregular array of low ridges and cuspules” and “the complex unstable pattern within the main basins” (de Bruijn et al. 2019: 522). With only two teeth, such a variabililty can not be observed here. However, the M2 from Bociu shows a complexe pattern with several spurs starting from the protocone, the paracone and the mesostyle. Likewise, the m2 also shows a very irregular entolophid and a small isolated spur starting from the mesolophid. The teeth from Bociu only differ from B. dissimile from Buštranje in being slightly larger and missing the hypoconid hind arm in m2, although this character is very variable and is not always present in B. dissimile. We consequently tentatively refer the teeth from Bociu to this species as B. cf. B. dissimile
Subfamily Eucricetodontinae Mein & Freudenthal, 1971
Genus Eucricetodon Thaler, 1966
Type species: Cricetodon collatus Schaub, 1925, Küttigen, upper Oligocene, Switzerland.
Eucricetodon aff. Eu. huerzeleri Vianey-Liaud, 1972
Fig. 9A–D.
Material.—Two upper molars and three lower molars from Cetățuie, Rupelian, lower Oligocene, Dâncu Formation, Gilău sedimentary area, Transylvanian Basin, Romania.
Measurements.—See Table 5.
Table 5. Measurements of molars (in mm) of Eucricetodon aff. Eu. huerzeleri from the lower Oligocene of Cetățuie.
|
Tooth |
Specimen number |
L |
W |
|
Right M2 |
MPSUBB v1096 |
1.83 |
1.78 |
|
Right M3 |
MPSUBB v1097 |
1.50 |
1.63 |
|
Right m2 Left m2 Left m2 Mean m2 StD m2 |
MPSUBB v1098 MPSUBB v1099 MPSUBB v1100 |
2.01 1.94 1.96 1.85 0.20 |
1.69 1.71 – – – |
Description.—The molars are characterized by large cusp(id)s with a rounded shape in lateral view due to the wear.
For the M2 (Fig. 9A), the labial and lingual anterolophs are both long and respectively closing the anterosinus and the protosinus; however, the lingual anteroloph is noticeably lower and thinner than the labial one. A small paracone spur, close to the labial border, merges with the mesostyle, itself connected to the base of the metacone, so the mesosinus is labially closed. Both the mesial protolophule and the metalophule are slightly transverse, connected mesially to respectively the protocone and the hypocone. The distal protolophule is incomplete and ends in the middle of the mesosinus, like the mesoloph. Additionally, a small spur starts from the extremity of the mesoloph, oriented toward the metalophule. The posterosinus is narrow whereas the posteroloph is thick and connectes to the metacone so the posterosinus is closed labially. The M2 has three roots.
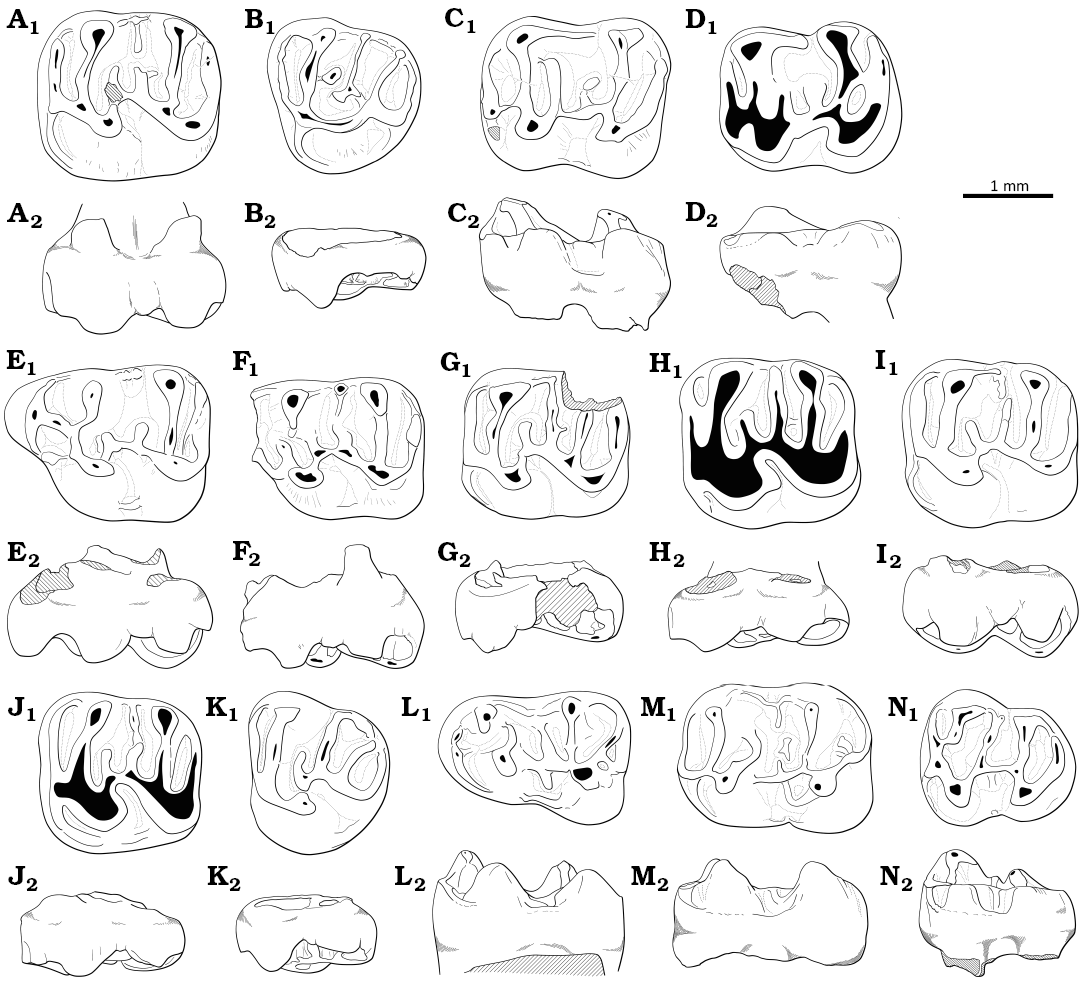
Fig. 9. Drawings of upper and lower molars. A–D. Eucricetodon aff. Eu. huerzeleri Vianey-Liaud, 1972, from Rupelian, lower Oligocene of Cetățuie, Romania. A. Right M2 (MPSUBB v1096, reversed). B. Right M3 (MPSUBB v1097, reversed). C. Right m2 (MPSUBB v1098, reversed). D. Left m2 (MPSUBB v1099). E–N. Tenuicricetodon arcemis gen. et sp. nov. from Rupelian, lower Oligocene of Cetățuie, Romania. E. Right M1 (MPSUBB v1116, reversed). F. Left M1 (MPSUBB v1117). G. Left M2 (MPSUBB v1118). H. Left M2 (MPSUBB v1120). I. Right M2 (MPSUBB v1121, reversed). J. Right M2 (MPSUBB v1122, reversed). K. Left M3 (MPSUBB v1123). L. Right m1 (MPSUBB v1124, reversed). M. Left m2 (MPSUBB v1125). N. Right m3 (MPSUBB v1126, reversed). A1–N1, occlusal view; A2–N2, labial view.
The M3 (Fig. 9B) displays a rounded shape due to the reduced hypocone and metacone. It has long labial and lingual anterolophs respectively closing the anterosinus and the protosinus. The protolophule, the mesoloph and the metalophule are slightly oblique. The mesolophe starts from a point between the protocone and the metalophule and reaches the mesostyle. A well-developed paraconule is present in the mesosinus between the protolophule and the mesoloph, it is connected to the protolophule. The mesosinus is closed labially by a long cingulum connecting the paracone to the metacone. The metacone is strongly reduced so it is not larger than the mesostyle. The posteroloph is long and closes the posterosinus labially. The roots are not preserved.
The m2s have a long and well-developed lingual anterolophid reaching the base of the metaconid whereas the labial anterolophid is short and poorly developed; as a result, the protosinusid remains opened labially. The protoconid hind arm is long and reaches the metaconid. The mesolophid is either short and ends in the middle of mesosinusid (2/3; with a little stylid at its extremity for one m2, Fig. 9C), or long. The metaconid ridge is well developed and long, it reaches the base of the entoconid lingually and closes the mesosinusid. An entoconid ridge is also present, merging with the posterolophid. The sinusid is also closed labially by a low cingulid. There are no hypoconid hind arm or ectomesolophid. The posterosinusid is large, closed lingually, and delimited by a thick posterolophid. The m2s have two roots
Remarks.—The five teeth described above from Cetățuie differ by their large size, slightly higher crown and more robust cusp(id)s when compared to Tenuicricetodon arcemis gen. et sp. nov. (see below). The robust but lower cusp(id)s, the absence of hypoconid hind arm in m2s and the reduced distal part of M3 (but more developed than in Tenuicricetodon arcemis gen. et sp. nov.) indicate that they belong to the Eucricetodontinae subfamily. They are much larger than most species referred to Eucricetodon (or Atavocricetodon): Eu. (A.) atavoides Freudenthal, 1996; Eu. (A.) atavus Misonne, 1957; Eu. (A.) minusculus Freudenthal, 1996; Eu. (A.) nanoides Freudenthal, 1996; Eu. (A.) nanus Peláez-Campomanes, 1995; Eu. (A.) hugueneyae Freudenthal, 1996; Eu. caducus (Shevyreva, 1967); Eu. huberi (Schaub, 1925); Eu. asiaticus (Matthew & Granger, 1923); Eu. occasionalis Lopatin, 1996; Eu. (A.) paaliensis Marivaux, Vianey-Liaud, & Welcomme, 1999; Eu. murinus (Schlosser, 1884); Eu. praecursor (Schaub, 1925); Eu. leptaleos (Wang & Meng, 1986) (see Freudenthal 1996; Lopatin 1996; Maridet et al. 2009, 2013; Vianey-Liaud 1972; Wang and Meng 1986 for size comparisons). However, these teeth from Cetățuie fit in the size range of Eu. huerzeleri Vianey-Liaud, 1972, with the exception of the M3 which is noticeably wider. Their morphology also mostly fit the description of Eu. gergovianum (Gervais, 1848–1852) by Schaub (1925) later emended into a diagnosis for Eu. huerzeleri by Vianey-Liaud (1972): the generally thick and short mesoloph(id)s and the low cingulums and cingulids compared to cusp(id)s, the rounded M3 without complete entoloph and with poorly-developed hypocone and metacone, and the elongated m2 with an oblique ectolophid. The m2s also possess a well-developed mesoconid as described by Schaub (1925) whereas the only M2 from Cetățuie does not show a distinct mesocone as opposed to the emended diagnosis from Vianey-Liaud (1972). There are also some significant differences in the teeth from Cetățuie such as the well-developed distal protolophule in M2 and protoconid hind arm in m2s, and the better developed lingual anteroloph in M2. This material is consequently referred to an affine form of Eu. huerzeleri, waiting for more material to confirm the occurrence of a new species in the lower Oligocene of Transylvania.
Genus Tenuicricetodon nov.
Zoobank LSID: urn:lsid:zoobank.org:act:A0A9A6A2-DF05-492A-AF EE-B695083E2877.
Etymology: From Latin tenuis, thin or fine, and the genus Cricetodon Lartet, 1851.
Type species: Tenuicricetodon arcemis gen. et sp. nov.
Diagnosis.—Medium-sized cricetid with thin and narrow cusp(id)s and loph(ids). M1 with a long anterolophule reaching the anterocone or not. Thick posteroloph forming a bulge in M1, with possibility of a posterostyle extending from the posteroloph lingually in the posterosinus. M1 and M2 with long and straight mesoloph often reaching the labial mesostyle. A continuous lingual cingulid extends from the metaconid to the entoconid in both m1 and m2. Both m1 and m2 have two hypoconid hind arms, one being smaller than the other is.
Differential diagnosis.—Differs from:
– Raricricetodon Tong, 1997, Palasiomys Tong, 1997, Pappocricetodon Tong, 1992, in having a well-developed mesial lobe in upper and lower first molars, and missing the protocone distal arm (sensu Maridet and Ni 2013) in upper molars.
– Eucricetodon Thaler, 1966 (including Atavocricetodon Freudenthal, 1996) in having thinner and narrower cusp(id)s and loph(ids) giving a more lophodont aspect. Also differs in having a long mesial arm of the protocone (more developed than the distal one) which can reach the anterocone (possible but very rare in Eucricetodon see Freudenthal 1996) and a concave labial border in M1, and a divided anteroconid in m1.
– Eocricetodon Wang, 2007, in being larger, having a shorter mesial lobe in M1; also, in having a more elongated m1 with longer mesolophid and ectomesolophid, well-developed anterolophids and a divided anteroconid.
– Oxynocricetodon Wang, 2007, in having a smaller mesial lobe in M1, having mesolophs and distal protolophule in upper molars, also in having long mesolophids and a continuous lingual cingulid extends from the metaconid to the entoconid in both m1 and m2.
– Pseudocricetodon Thaler, 1969 (including Allocricetodon Freudenthal, 1994) in having a short and narrow mesial lobe and a mesial protolophule more developed than the distal one in M1. It also differs in missing the anterolophulid but having a long and oblique mesolophid in m1. Another striking difference is the presence of one, or even two, hypoconid hind arms in m1 and m2.
– Witenia de Bruijn, Ünay, Saraç, & Yïlmaz, 2003, in being smaller with more gracile cusp(id)s and loph(id)s (more lophodont occlusal pattern), in having also much less developed distal part of 3rd molars (upper and lower), and a broader mesial lobe of M1.
– Heterocricetodon Schaub, 1925, in having a lower crown and less elongated molars, especially much-reduced third molars, and in having hypoconid hind arms in m1 and m2.
– Adelomyarion Hugueney, 1969, in having longitudinal and complete entoloph(id)s (as opposed to the often strongly oblique and interrupted entoloph(id)s in Adelomyarion) and having hypoconid hind arms in m1 and m2.
– Bustrania de Bruijn, Marković, Wessels, & van de Weerd, 2019, in being much larger, missing the irregular array of low ridges and cuspules present on all molars of Bustrania, in having a sinus of M1 and M2 directed mesially (as opposed to the distally for Bustrania) and in having a well-developed anteroconid on m1.
– Kerosinia Ünay-Bayraktar, 1989, in missing the direct connection between the anterocone and paracone in M1, in having a more developed distal part of the M3 including a long mesoloph, also in missing the complete anterolophulid linking the anteroconid to the protoconid in m1 and in missing the distal arm of the hypoconid in m1 and m2.
– Ulaancricetodon Daxner-Höck, 2000, in being much larger, in missing the trapezoidal shape and the weakly developed mesial lobe and anterocone in M1, also in having the entoconid on m1 located mesially to the protoconid (the opposite for Ulaancricetodon).
Stratigraphic and geographic range.—Dâncu Formation (Rupelian), Transylvania (Romania).
Tenuicricetodon arcemis sp. nov.
Figs. 9E–N, 10.
Zoobank LSID: urn:lsid:zoobank.org:act:FD1C5030-1D79-42F2-B07 B-3E8C478F5D72.
Etymology: From Arcem the latin name of the locality Cetățuie, in Latin arcemis.
Holotype: Right M1 (MPSUBB v1116).
Type locality: Cetățuie, Transylvanian Basin, Romania.
Type horizon: Rupelian, lower Oligocene, Dâncu Formation, Gilău sedimentary area,
Species diagnosis.—Same as the generic diagnosis.
Material.—Eight upper molars and three lower molars, all from the type locality and horizon.
Measurements.—See Table 6.
Table 6. Measurements of molars of Tenuicricetodon arcemis gen. et sp. nov. from the lower Oligocene of Cetățuie.
|
Tooth |
Specimen number |
L |
W |
|
Right M1 Left M1 |
MPSUBB v1116 MPSUBB v1117 |
2.30 – |
1.75 1.58 |
|
Left M2 Right M2 Left M2 Right M2 Right M2 Mean M2 StD M2 |
MPSUBB v1118 MPSUBB v1119 MPSUBB v1120 MPSUBB v1121 MPSUBB v1122 |
1.84 1.93 1.95 1.91 1.83 1.89 0.05 |
1.74 1.83 1.91 1.83 1.80 1.82 0.06 |
|
Left M3 |
MPSUBB v1123 |
1.55 |
1.78 |
|
Right m1 |
MPSUBB v1124 |
2.09 |
1.48 |
|
Left m2 |
MPSUBB v1125 |
2.15 |
1.73 |
|
Right m3 |
MPSUBB v1126 |
1.71 |
1.54 |
Description.—The molars are characterized by a low crown and small narrow cusp(id)s and loph(ids) providing a slightly lophodont aspect; in lateral view the cusp(id)s have either an angular shape when the wear is weak, or a low rounded shape when the wear is strong.
The M1s display a short and narrow mesial lobe. The anterocone is small, crescent-like and transversally elongated; the lingual anteroloph ends with a protostyle connected to the protocone. The protocone spur (sensu Maridet and Ni 2013) is either short (ending in the anterosinus), or long and prolonged by an anterolophule reaching the anterocone. Both the mesosinus and the sinus are closed respectively labially and lingually by a cingulum; additionally, there is a mesostyle on the labial cingulum, with or without a spur. The mesial protoloph is complete (connected to the anterolophule) in one M1 (Fig 10A) and interrupted in the other (Fig. 10B). The distal protoloph is short and it connects to the paracone in one M1. The entoloph and the distal protoloph form a continuous oblique crest between the hypocone and the distal protolophule whereas the connection between the protocone and the entoloph is less developed. The protocone and its mesial spur being oblique, the sinus is also oblique. The mesoloph starts from a small mesocone and it reaches the mesostyle spur in one M1. The metalophule is straight and connects the hypocone. The posteroloph is inflated. In one M1 a posterostyle extends from the posteroloph lingually in the posterosinus (Fig. 10A). The M1s have three roots.
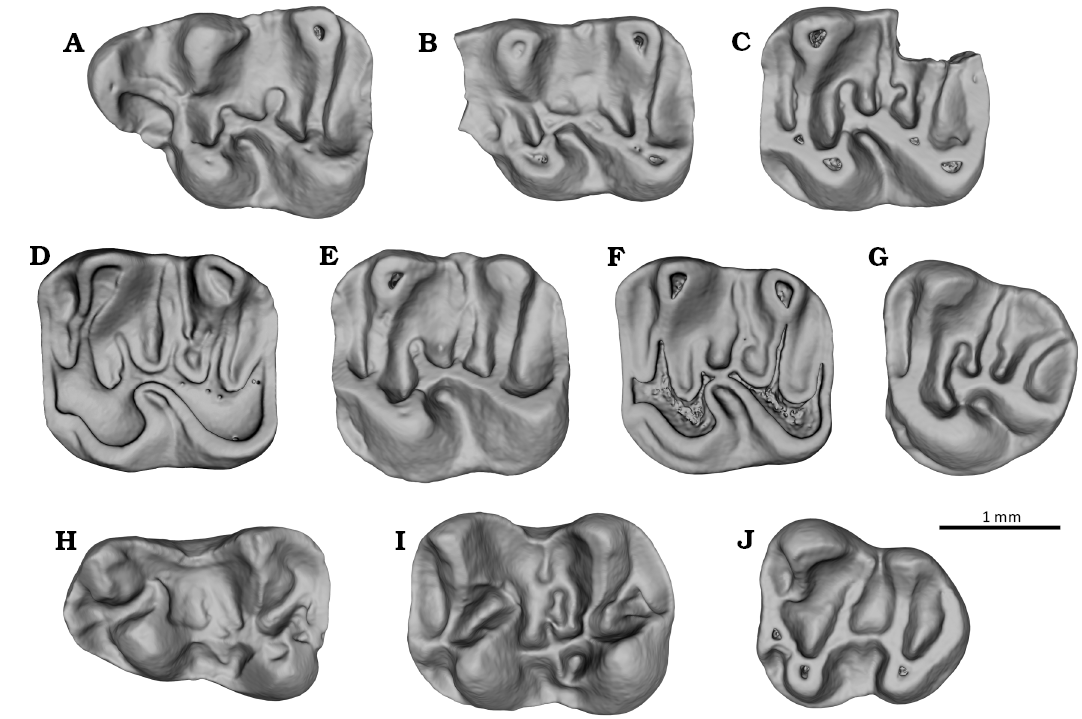
Fig. 10. Tenuicricetodon arcemis gen. et sp. nov. from Rupelian, lower Oligocene of Cetățuie, Romania. A. Right M1 (MPSUBB v1116, reversed). B. Left M1 (MPSUBB v1117); C. Left M2 (MPSUBB v1118). D. Left M2 (MPSUBB v1120). E. Right M2 (MPSUBB v1121, reversed). F. Right M2 (MPSUBB v1122, reversed). G. Left M3 (MPSUBB v1123). H. Right m1 (MPSUBB v1124, reversed). I. Left m2 (MPSUBB v1125). J. Right m3 (MPSUBB v1126, reversed). Occlusal view.
In M2s, the lingual anteroloph is much shorter and lower than the lingual one, it can reach the protocone and close the protosinus (3/5). The mesial protolophule is complete and better developed than the distal one; the distal protolophule is long but does not reach the paracone. Like in M1s, the entoloph and the distal protoloph form a continuous oblique crest between the hypocone and the paracone whereas the connection between the protocone and the entoloph is shorter; also, the protocone is elongated distally and the sinus is strongly curved. The paracone spur is located on the labial border, it is long and closes the mesosinus in all but one M2 (Fig. 10E). The mesoloph is long, it either reach directly the labial cingulum (3/5) or merges with a somewhat lower and thinner mesostyle spur. The sinus either is closed by a low and weakly developed cingulum (2/5) or labially open (3/5). The metalophule is slightly oblique and connects mesially to the hypocone. The posteroloph is long and generally closes the posterosinus (3/5), but the posterosinus can also remain open labially (1/5), and the postero-labial corner of one M2 is broken so the labial end of the posterosinus is not discernable (1/5). The M2s have three roots.
The M3 (Fig. 10G) has a well-developed labial anteroloph, the lingual one being absent. The mesial protolophule is strong, connects the anterolophule whereas the distal protolophule is short, and does not reach the paracone. Like in M1s and M2s, the entoloph forms a continuous oblique crest between the hypocone and the distal protolophule, and the connection between the protocone and the entoloph is interrupted. The mesoloph is long and reaches the labial cingulum; the metacone is reduced to a postero-labial bulge on the cingulum. The metalophule connects distally the metacone and mesially the hypocone. The roots are not preserved.
The m1 (Fig. 10H) has both equally developed anteroconid and metastylid. The lingual anterolophid, between the metastylid and the metaconid, is very short whereas the labial one is long and closes the protosinusid labially. A small spur starts distally to the anteroconid but there is no anterolophulid connecting the anteroconid to the protoconid. The protoconid hind arm is well developed and long, and connects to the metaconid. Both the metaconid ridge and the entoconid spur are long; they join each other lingually and close the mesosinusid. Likewise, a strong ridge starting from the hypoconid is prolonged by a cingulid reaching the protoconid so the sinusid is closed labially. The entolophid, mesolophid and ectomesolophid are all thick and form a cross in the middle of the molar. The mesolophid is long and reaches the lingual cingulid whereas the ectomesolophid ends in the middle of the sinusid. The hypolophulid is straight, transverse, and connects mesially to the hypoconid. The hypoconid hind arm is long and connects to the posterolophid, delimiting a small pit distally to the hypoconid. This small pit is itself divided by an additional spur (second hypoconid hind arm) between the hypoconid hind arm and the distal border (Fig. 10H). The roots are not preserved.
The m2 (Fig. 10I) has both strong lingual and labial anterolophid closing the anterosinusid and protosinusid respectively. The metalophulid is oblique and connects on the anterolophulid; the protoconid hind arm is long, bends mesially and connects to the metaconid distal slope. The metaconid ridge and the entoconid spur are long and join each other along the lingual border, forming a cingulid that closes the mesosinusid. A spur extends in the mesosinusid from this lingual cingulid, but does not reach the mesolophid; additionally, a crest is present in the middle of the mesosinusid, isolated between this spur and the mesolophid. A strong cingulid also closes the sinusid labially. The ectomesolophid is long and merges with a ridge connected to the hypoconid. The hypolophulid is oblique, parallel to the metalophulid, and connect on the ectolophid, mesially to the hypoconid. The posterosinusid is very large, and closed lingually by a long posterolophid; the hypoconid hind arm is short but well developed and ends in the middle of the posterosinusid. A second minute hypoconid hind arm is present next to the first one. The roots are not preserved.
The m3 (Fig. 10J) displays clearly separated metaconid and protoconid, indeed the metalophid connects on the lingual anterolophid whereas the anterolophulid connects on the labial anterolophid, and the protoconid hind arm does not connect to the metaconid, but on the metaconid distal ridge next to minute mesostylid. There is no mesolophid, and the ectomesolophid is very weak, limited to a fold of the enamel labially to the entolophid. The mesosinusid and the sinusid are open respectively lingually and labially. The posterolophid is long and merges with the entoconid distal ridge that closes the posterosinusid lingually. The m3 has two roots.
Remarks.—Hugueney (1980) and later Comte (1985) discussed in detail the differences between Eucricetodon huberi (Schaub, 1925) and Pseudocricetodon incertus (Schlosser, 1884). Indeed, the two species are large and present similar sizes, but some morphological differences can be observed that, in turn, can also secure the identification at generic level. These differences led Engesser (1987) to refer these genera to two different subfamilies, Eucricetodontinae and Pseudocricetodontinae respectively.
The teeth described above differ from Eucricetodon aff. Eu. huerzeleri from the same locality by being slightly smaller, having a M3 with a less reduced distal part, and having a lower crown with thinner and narrower cusp(id)s and loph(ids) which gives it a more lophodont aspect. These characteristics are usually associated with Pseudocricetodon (Engesser 1987; Hugueney 1999). They also display a long mesoloph in M2 and M3 which is characterictic of Ps. incertus and absent in Eu. huberi (Comte 1985), a short and narrow anteroloph usually characteristic of Pseudocricetodon (Engesser 1987), a long mesial arm of the protocone in M1 more frequent in Pseudocricetodon (Engesser 1987; Hugueney 1999), and a concave labial border whereas it is rather convexe in M1 of Eu. huberi (Comte 1985; and more generally in Eucricetodontinae, Engesser 1987), and a divided anteroconid in m1 which is rare in Eucricetodon (Hugueney 1999). Among Pseudocricetodontinae this population from Cetățuie otherwise strongly differs from Heterocricetodon Schaub, 1925, in having a much lower crown and less elongated molars, especially the third molars; it also strongly differs from Adelomyarion Hugueney, 1969, in being less lophodont (thicker crests and larger cusps and cuspids), and missing the often strongly oblique or interrupted entoloph(id).
In contrast these teeth from Cetățuie also display characters that are usually associated with Eucricetodon: The mesial lobe is short and narrow, and the mesial protolophule more developed than the distal one in M1 (Engesser 1987; Hugueney 1999); the anterolophulid absent in m1 whereas it is usually long and complete in Ps. incertus (Hugueney 1980); the mesolophid of m1 is long and oblique in Eu. huberi whereas it is usually small or even absent in Ps. incertus (Hugueney 1980); and the hypoconid hind arm is always absent in Pseudocricetodon and more generally in all Pseudocricetodontinae (Engesser 1987; Hugueney 1999).
The ambiguous association of morphological characters on molars led to refering this population from Cetățuie to a new genus: Tenuicricetodon gen. nov. However, the question of the subfamily it belongs to remains open. Most of the characters mentioned above present a noticeable variability when taking into account large populations (e.g., Freudenthal 1994; Freudenthal et al. 1994; Maridet et al. 2009), but not all of them. The short and narrow mesial lobe in M1, the absence of anterolophulid in m1 and the presence of a hypoconid hind arm in m1 seem to be consistent features differentiating Eucricetodontinae from Pseudocricetodontinae (Freudenthal et al. 1994; Hugueney 1999). Despite the morphological similarities with Pseudocricetodon, we refer this new genus to the subfamily Eucricetodontinae.
Stratigraphic and geographic range.—Dâncu Formation (Rupelian), Transylvania (Romania).
Subfamily Pseudocricetodontinae Engesser, 1987
Genus Pseudocricetodon Thaler, 1969
Type species: Pseudocricetodon montalbanensis Thaler, 1969, Montalbán, lower Oligocene, Spain.
Pseudocricetodon cf. Ps. montalbanensis Thaler, 1969
Fig. 11A–C.
Material.—One upper molar from Suceag and two lower molars from Cetățuie, Rupelian, lower Oligocene, Dâncu Formation, Gilău sedimentary area, Transylvanian Basin, Romania.
Measurements.—See Table 7.
Table 7. Measurements of molars (in mm) of Pseudocricetodon cf. Ps. montalbanensis from the lower Oligocene of Cetățuie and Suceag.
|
Tooth |
Locality |
Specimen number |
L |
W |
|
Left M1 |
Suceag |
MPSUBB v1127 |
– |
1.03 |
|
Left m1 |
Cetățuie |
MPSUBB v1128 |
1.21 |
0.92 |
|
Left m3 |
Cetățuie |
MPSUBB v1129 |
1.08 |
0.96 |
Description.—The cheek teeth are small, with a very low crown, and with thin and acute cusp(id)s providing a slightly more lophodont aspect compared to other cricetids described herein.
The mesial part of the M1 is lost so the mesial lobe is not observable (Fig. 11A). The protocone spur is long but ends free in the anterosinus, and the anterolophule seems absent. There is no mesial protolophule whereas the distal one is well developed, oblique, and connects distally to the protocone on the entoloph. The large mesostyle closes the mesosinus whereas the sinus remains open lingually. The mesoloph is long and located rather distally in the mesosinus (noticeably closer to the metalophule than the protolophule), and a longitudinal spur connects the mesoloph to the metalophule. The metalophule is slightly oblique and connects mesially to the hypocone. A short mesiodistal crestule connects the mesolophe and the metalophule. The posterosinus is elongated and surrounded by a long posteroloph that closes it labially. The roots are not preserved.
The m1 (Fig. 11B) has a small but well-developed anteroconid, surrounded by both equally developed anterolophids, and connected to the protoconid mesial slope by a long anterolophulid. There is no metalophulid but a strong protoconid hind arm connecting directly the protoconid to the metaconid. The metaconid ridge is long but does not reach the entoconid so the mesosinusid remains partly open; labially a small and low cingulid is present but so weakly developed that the sinusid appears open in lateral view. The mesolophid is short and, as in M1 for the mesoloph, distally located (closer to the hypolophulid than to the protoconid hind arm). The tooth is highly worn so the hypoconid hind arm is not clearly visible; however, a small bulge starting between the hypoconid and the posterolophid, and extending into the posterorsinusis suggests that it is present. Both small labial posterolophulid and labial posterosinusid are present. The m1 has two roots.
The m3 (Fig. 11C) displays a well-developed lingual anterolophid whereas the labial one is shorter and lower; as a result the anterosinusid is closed lingually whereas the protosinusid remains open. Both the metalophulid (projecting distally) and the protoconid hind arm are long but none of them reaches the metaconid, which is consequently isolated from the protoconid. The metaconid ridge is long and follows the lingual border, reaching the entoconid spur, thus closing the mesosinusid. Labially, the sinusid remains open. The ectomesolophid is low and weakly developed, but much mesially located compared to the mesolophid, as in m1, the mesolophid is indeed closer to the hypolophulid than the protoconid hind arm. The posterosinusid is long and elongated, closed lingually, and surrounded by a long posterolophid bearing a bulge on the distal border. The tooth has two roots.
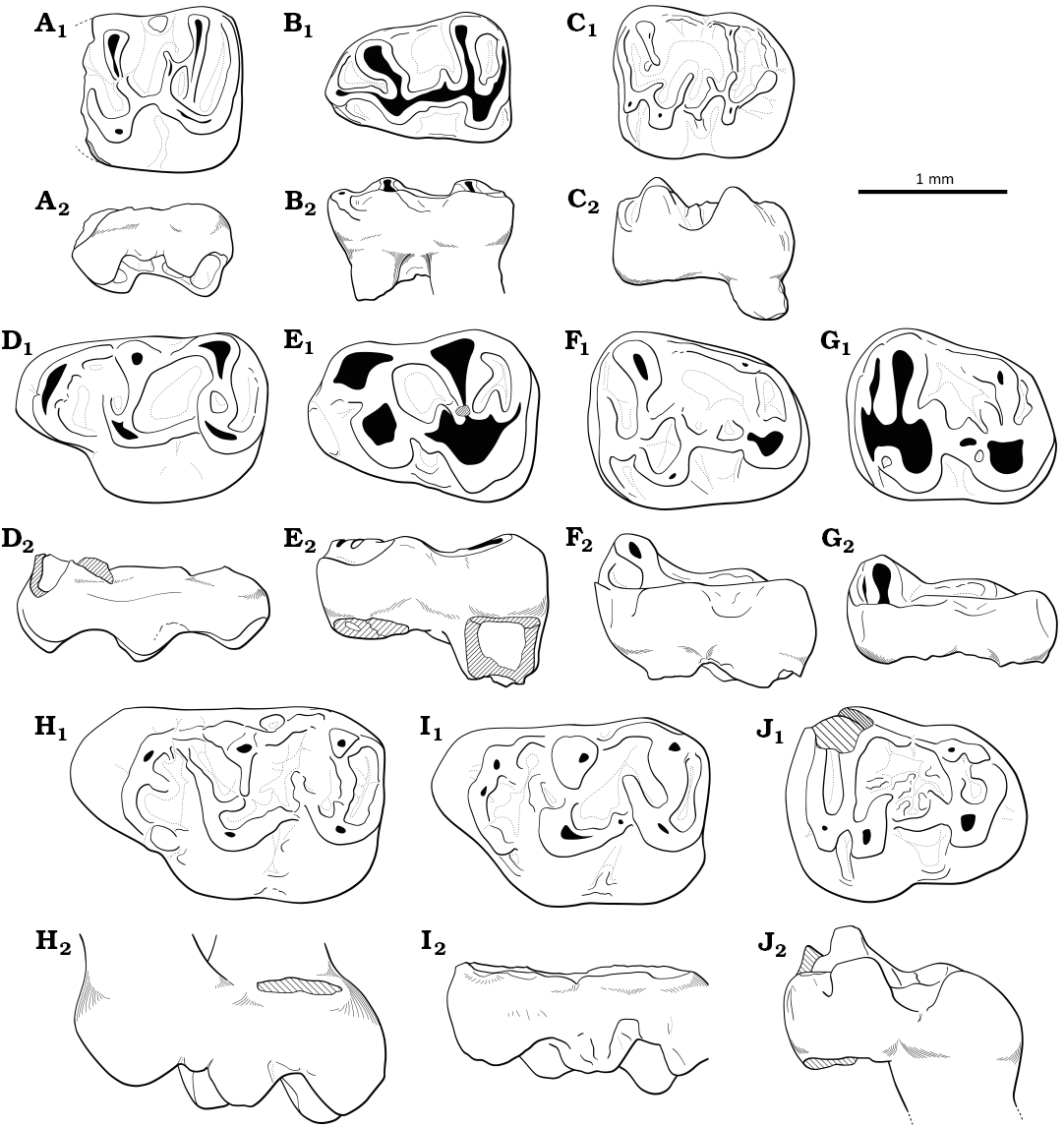
Fig. 11. Drawings of upper and lower molars. A–C. Pseudocricetodon cf. Ps. montalbanensis Thaler, 1969, from the Rupelian, lower Oligocene of Cetățuie and Suceag, Romania. A. Left M1 (MPSUBB v1127). B. Left m1 (MPSUBB v1128). C. Left m3 (MPSUBB v1129). D–G. Paracricetodon aff. Pa. stojonovici van de Weerd, de Bruijn, Marković, & Wessels, 2018, from the Rupelian, lower Oligocene of Cetățuie and Suceag, Romania. D. Left M1 (MPSUBB v1130). E. Left m1 (MPSUBB v1131). F. Right m3 (MPSUBB v1132, reversed). G. Right m3 (MPSUBB v1133, reversed). H–J. Paracricetodon wentgesi de Bruijn, Ünay, Saraç, & Yïlmaz, 2003, from the Rupelian, lower Oligocene of Mera and Cetățuie, Romania. H. Right M1 (MPSUBB v1134, reversed). I. Right M1 (MPSUBB v1135, reversed). J. Right m3 (MPSUBB v1136, reversed). A1–J1, occlusal view; A2–J2, labial view.
Remarks.—Several characters fit the emended diagnosis of Pseudocricetodon as provided by Freudenthal et al. (1994): the ectolophid laying labially and presence of the hypoconid hind arm in m1; the distal part of the m3 not much reduced with well-developed hypoconid and entoconid; the protoconid hind arm long and connected to the metaconid in lower molars; the long mesoloph in upper molars; and the lingual border of M1 forming an angle of c. 90° with the distal border. Additionally, all teeth fit in the size range of Ps. montalbanensis Thaler, 1969 (see Freudenthal et al. 1994) but the morphology slightly differ in displaying a generally simpler occlusal pattern with a single short mesolophid and no ectomesolophid in m1, and an incomplete metalophulid in m3. The teeth are otherwise slightly smaller than Ps. moguntiacus (Bahlo, 1975) and larger than Ps. philippi Hugueney, 1971, and Ps. heissigi Marković, Wessels, van de Weerd, & de Bruijn, 2020 (for size comparisons see: Hugueney 1971; Comte 1985; Freudenthal et al. 1994; Marković et al. 2020); for these reasons and because of the scarcity of the material they are referred to Ps. cf. Ps. montalbanensis.
Subfamily Paracricetodontinae Mein & Freudenthal, 1971
Genus Paracricetodon Schaub, 1925
Type species: Cricetodon spectabilis Schlosser, 1884, Quercy (precise locality unknown), Oligocene, France.
Paracricetodon aff. Pa. stojonovici van de Weerd, de Bruijn, Marković, & Wessels, 2018
Fig. 11D–G.
Material.—One upper molar from Suceag and three lower molars from Cetățuie, Rupelian, lower Oligocene, Dâncu Formation, Gilău sedimentary area, Transylvanian Basin, Romania.
Measurements.—See Table 8.
Table 8. Measurements of molars (in mm) of Paracricetodon aff. Pa. stojonovici from the lower Oligocene of Cetățuie and Suceag.
|
Tooth |
Locality |
Specimen number |
L |
W |
|
Left M1 |
Suceag |
MPSUBB v1130 |
1.54 |
1.08 |
|
Left m1 |
Cetățuie |
MPSUBB v1131 |
1.51 |
1.15 |
|
Right m3 Right m3 |
Cetățuie Cetățuie |
MPSUBB v1132 MPSUBB v1133 |
1.46 1.38 |
1.21 1.16 |
Description.—The M1 (Fig. 11D) shows a long mesial lobe with strong anterolophs. The anterocone is transversally elontaged with a small notch on its mesial border, and with both well-developed labial and lingual anterolophs respectively reaching the paracone and the metacone. Two weakly developed spurs start distally from the anterocone, but they do not connect to any loph or cusp, so there are no anterolophule or mesial protolophule. The protocone spur is short and ends free in the anterosinus. The distal protolophule is transverse and almost straight, connecting directly the protocone to the paracone. A strong spur starts from the paracone and reaches the labial border where it merges with the metacone. The mesosinus is consequently closed whereas the sinus remains open lingually. There is no entoloph but instead the protocone is elongated and prolonged by distal arm connected directly to the hypocone. In addition to the metalophule, a spur starts from the mesial part of the hypocone and connects to the metacone, so both this spur and the metalophule surround a small pit located between the hypocone and the metacone. The posterolophid is low but long and closes the posterosinus labially. The roots are not preserved.
The m1 (Fig. 11E) is strongly worn out but some morphological features can be observed. The anteroconid is transversally elongated and seems noticeably lower than the other cuspids. The mesosinusid is closed lingually by a large metaconid hind arm reaching the entoconid; labially the sinusid is also closed by a low but thick cingulid. There is a spur extending lingually from the entolophid into the mesosinusid; however, due to the wear it is not clear if it is a mesolophid or an mesial spur starting from the hypoconid. Despite the wear the hypoconid hind arm is clearly visible, extending into the posterosinusid. The tooth has two roots.
The two m3s have a well-developed lingual anterolophid closing the anterosinusid whereas the labial anterolophid is less developed and the protosinusid remains open. The anterolophids are connected to the protoconid by a large anterolophulid; the metalophulid is straight and connects on the anterolophulid. On one m3, the protoconid hind arm is very long and forms a loop to connect on the metalophulid delimiting a large but shallow pit between the metaconid and the protoconid (Fig. 11F). On the other m3, the advanced stage of the wear makes it difficult to confirm similar connections between the metaconid and the protoconid (Fig. 11G). The m3s have a short mesolophid and the spur starting mesially from the hypoconid, both ending free in the mesosinusid. The hypoconid is noticeably larger than the entoconid and they are directly connected to each other by a straight hypolophulid. The roots are preserved for one of the m3s and it has two roots.
Remarks.—This Paracricetodon is characterised by a small size and a relatively simple morphology. It is indeed the smallest Paracricetodon found in the lower Oligocene of Transylvania and among the smallest compared to all known species of Paracricetodon (see van de Weerd et al. 2018 for an exhaustive size comparison of all species of Paracricetodon). The teeth described herein are often close to the size of Pa. stojonovici, Pa. gracilis, and Pa. wentgesi, but otherwise much smaller than all other species of Paracricetodon (Fig. 12).
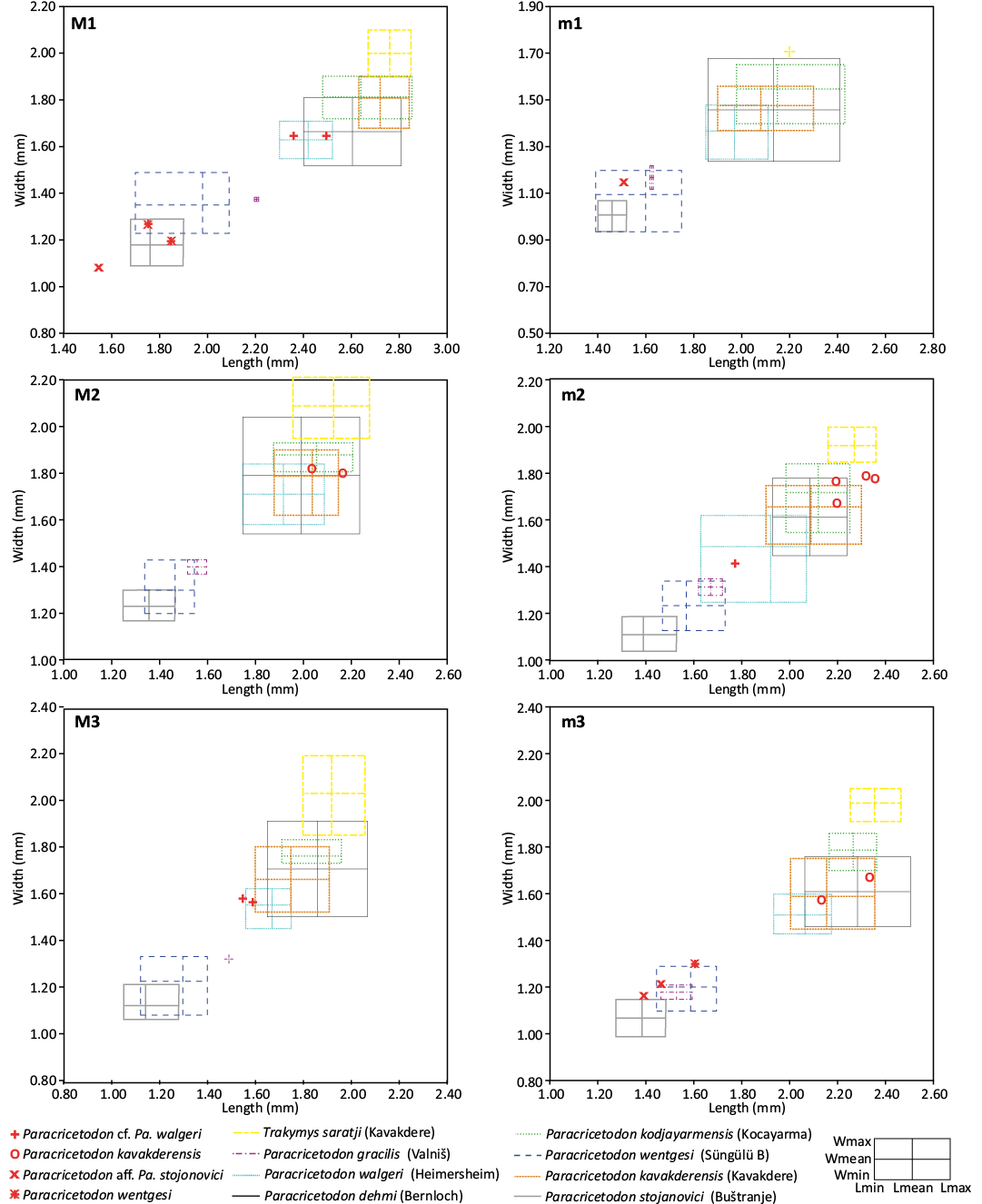
Fig. 12. Scatter diagram of sizes (length vs. width) for the molars of Paracricetodon from the lower Oligocene of Transylvania. Comparative data are taken from van de Weerd et al. (2018a) for Buštranje, Walniš, Kavakdere and Kocayama; de Bruijn et al. (2003) for Süngülü B; Bahlo (1975) for Heimersheim, and Hrubesh (1957) for Bernloch.
The M1 (Fig. 11D) that was found in Suceag is characterized by a very short protocone spur without anterolophule. The short protocone spur and the absence of anterolophule can be observed in Pa. stojonovici but is absent in Pa. gracilis and in Pa. wentgesi. The M1 also has a crescent-like anterocone and no protostyle, similar to Pa. stojonovici, but as opposed to Pa. gracilis and Pa. wentgesi. The m1 from Cetățuie is similar to those of Pa. stojonovici, Pa. gracilis and Pa. wentgesi with the metaconid and entoconid situated mesially to the protoconid and hypoconid, the strong hind arm of the hypoconid and the short mesolophid. The m1 also differs from these species in having a very weakly-developed anteroconid and no ectomesolophid. The two m3s from Cetățuie have a relatively weakly-developed entoconid, similar to Pa. stojonovici, but they differ in having both short mesolophid and mesial arm of the hypoconid, and missing the ectomesolophid.
A few morphological similarities suggest an affinity with Pa. stojonovici, but the noticeable differences with all small species rather support the identification of the above-described teeth as a new species. However, the four teeth found at Suceag and Cetățuie constitute an insufficient sample to create a new species. These specimens are consequently referred to an affine form of Pa. stojonovici, waiting for more material to confirm the occurrence of a new species in the lower Oligocene of Transylvania.
Paracricetodon wentgesi de Bruijn, Ünay, Saraç, & Yïlmaz, 2003
Fig. 11H–J.
Material.—One upper molar from Mera and 2 molars from Cetățuie, Rupelian, lower Oligocene, Dâncu Formation, Gilău sedimentary area, Transylvanian Basin, Romania.
Measurements.—See Table 9.
Table 9. Measurements of molars (in mm) of Paracricetodon wentgesi from the lower Oligocene of Mera and Cetățuie.
|
Tooth |
Locality |
Specimen number |
L |
W |
|
Right M1 Right M1 |
Mera Cetățuie |
MPSUBB v1134 MPSUBB v1135 |
1.85 1.75 |
1.19 1.27 |
|
Right m3 |
Cetățuie |
MPSUBB v1136 |
1.60 |
1.30 |
Description.—The two M1s show a long mesial lobe with an anterocone transversally elontaged and two well-developed labial and lingual anterolophs. The protostyle is present and well-developed, it has two protostyle spurs connecting to the protocone. One or two weakly developed spurs start distally from the anterocone, but they do not connect to any loph or cusp. The mesial protolophule is oblique or bent and connects to either the protocone mesial spur (Fig. 11H) or directly to the protocone (Fig. 11I). A strong ridge connects the paracone and the metacone on the labial border, closing the mesosinus. A short and low cingulum can be present on the border next to the paracone delimiting a little pit labially to the paracone. The sinus is also closed lingually by a low cingulum; one of the M1s also shows a low and short spur starting from this cigulum. There is no entoloph but instead the protocone is elongated and prolonged by its distal arm connected directly to the hypocone. The occurence of a mesocone suggests the possibility of a short entoloph in the continuity of the postprotocrista. In addition to the metalophule, a spur starts from the mesial part of the hypocone and end free in the mesosinus. The posteroloph is long and closes the posterosinus lingually. The roots are preserved for one of the M1s (Fig. 11H), it has three roots.
The m3 (Fig. 11J) has a well-developed lingual anterolophid whereas the labial anterolophid is less developed. The protosinusid is open labially, but the antero-lingual corner of the tooth is broken so the lingual extremity of the anterosinusid is not observable. The anterolophids are connected to the protoconid by a large anterolophulid; the metalophulid is straight and connects on the anterolophulid. The protoconid hind arm is strong but short and ends free in the mesosinusid; likewise, a spur starts from the junction of the distal end of the entolophid with the mesial side of the hypoconid which interpreted as a very weakly developed mesolophid. There are otherwise several very low folds of the enamel in the mesosinusid, which could be interpreted as a complex association of weakly developed spurs staring from the metalophulid, the entolophid and the hypolophulid. The hypoconid is noticeably larger than the entoconid and they are directly connected to each other by a straight hypolophulid. The posterolophid is short and thick delimiting a small and rounded posterosinusid. The tooth has two roots.
Remarks.—The teeth are slightly larger than Pa. aff. Pa. stojonovici from Transylvania and with a more complex occlusal pattern. The size of the teeth falls in the range of Pa. stojonovici or Pa. wentgesi (Fig. 12), however, the morphology of the M1s (from Mera and Cetățuie) is very similar to Pa. wentgesi with: a short anterolophule and a well-developed anterocone, the occurrence of a protostyle connected to the protocone by spurs, the long hypocone mesial arm parallel to the anterolophule, and the so called “burgee-shaped” distal spur of the paracone (de Bruijn et al. 2003: 58) forming a continuous ectoloph connecting to the metacone (de Bruijn et al. 2003). Likewise, the m3 from Cetățuie shows a morphology close to Pa. wentgesi with: a long protocone distal arm projecting backward, a long mesial arm of the hypocone projecting forward, and a well-developed entoconid on m3. The three teeth from Mera and Cetățuie are consequently referred to Pa. wentgesi.
Stratigraphic and geographic range.—Priabonian (late Eocene) to Rupelian (early Oligocene) respecticvely of Lesser Caucasus (Turkey) and Transylvania (Romania).
Paracricetodon cf. Pa. walgeri Bahlo, 1975
Fig. 13A–E.
Material.—Two upper molars from Suceag and 4 molars from Cetățuie, Rupelian, lower Oligocene, Dâncu Formation, Gilău sedimentary area, Transylvanian Basin, Romania.
Measurements.—See Table 10.
Table 10. Measurements of molars (in mm) of Paracricetodon cf. Pa. walgeri from the lower Oligocene of Cetățuie and Suceag (upper level).
|
Tooth |
Locality |
Specimen number |
L |
W |
|
Left M1 Right M1 Right M1 Mean M1 StD M1 |
Suceag Suceag Cetățuie |
MPSUBB v1101 MPSUBB v1102 MPSUBB v1103 |
2.49 2.36 – – – |
1.64 1.64 1.58 1.62 0.03 |
|
Left M3 Right M3 |
Cetățuie Cetățuie |
MPSUBB v1104 MPSUBB v1105 |
1.54 1.59 |
1.58 1.55 |
|
Right m2 |
Cetățuie |
MPSUBB v1106 |
1.77 |
1.41 |
Description.—The molars are characterized by strong loph(id)s and rounded cusp(id)s in lateral view. The molars are slightly smaller than that of Eucricetodon aff. Eu. huerzeleri and Tenuicricetodon arcemis gen. et sp. nov., whereas the crown is proportionally slightly higher.
The M1s have a wide mesial lobe with a strong anterocone bearing two anterostyles associated to a well-developed protostyle. The anterocone is connected to the protocone by a long and thick anterolophule. Additionally, in one tooth a distal spur is starting from the protostyle (Fig. 13A), but not reaching the protocone; another tooth has a minute parastyle located on the labial border just mesially to the paracone. The teeth have one protolophule connected either on the protocone or on its distal part. The mesostyle is well developed, either connected to the paracone and metacone by a cingulum closing the mesosinus, or isolated on the labial border. A short and low cingulum can be present on the border next to the paracone delimiting a little pit labially to the paracone. The mesoloph is long but does not always reach the mesostyle. The sinus is deep and closed by a low but strong lingual cingulum; one tooth also has a well-developed ectostyle located on this cingulum. The metalophule is straight, transverse, and connects on the hypocone. The posterosinus is small, closed labially and delimited by a short but very thick posteroloph. One M1 shows three roots (Fig. 13A).
M3s are characterized by a long and strong labial anteroloph whereas the lingual anteroloph is thin and much lower. The labial anteroloph does not reach the paracone but a small cingulum links the anteroloph to the paracone so the anterosinus remains closed labially. The mesial protolophule connects on the anterolophule. A cingulum closes the mesosinus and and connects to the thick posteroloph. Three crests are present in the middle of M3s, connected by the entoloph: the distal protolophule (short), the mesoloph (long and reaching the labial cingulum) and the metalophule (joining the posteroloph labially). The connections between the protocone and the entoloph are either weak or discontinuous, forming a longitudinal valley between a mesosinus and the sinus. The metacone is either absent or too small to be observable. One of the M3s has three preserved roots (Fig. 13D).
The m2 (Fig. 13E) has both the lingual and labial anterolophulid equally long and well developed; the anterosinusid and protosinusid are closed respectively lingually and labially. The metalophulid can be present or absent whereas the protoconid hind arm is always well developed and almost reaches the metaconid. The mesolophid can be short or long; it starts from a small entomesolophid. The hypolophulid is slightly oblique and connected on the ectolophid, at a point between the mesoconid and the hypoconid. The mesosinusid is closed lingually by a low cingulid whereas the labial cingulid is discontinuous so the sinusid remains labially open. The posterosinusid is wide, closed lingually, delimited by a long posterolophid. The roots are not preserved.
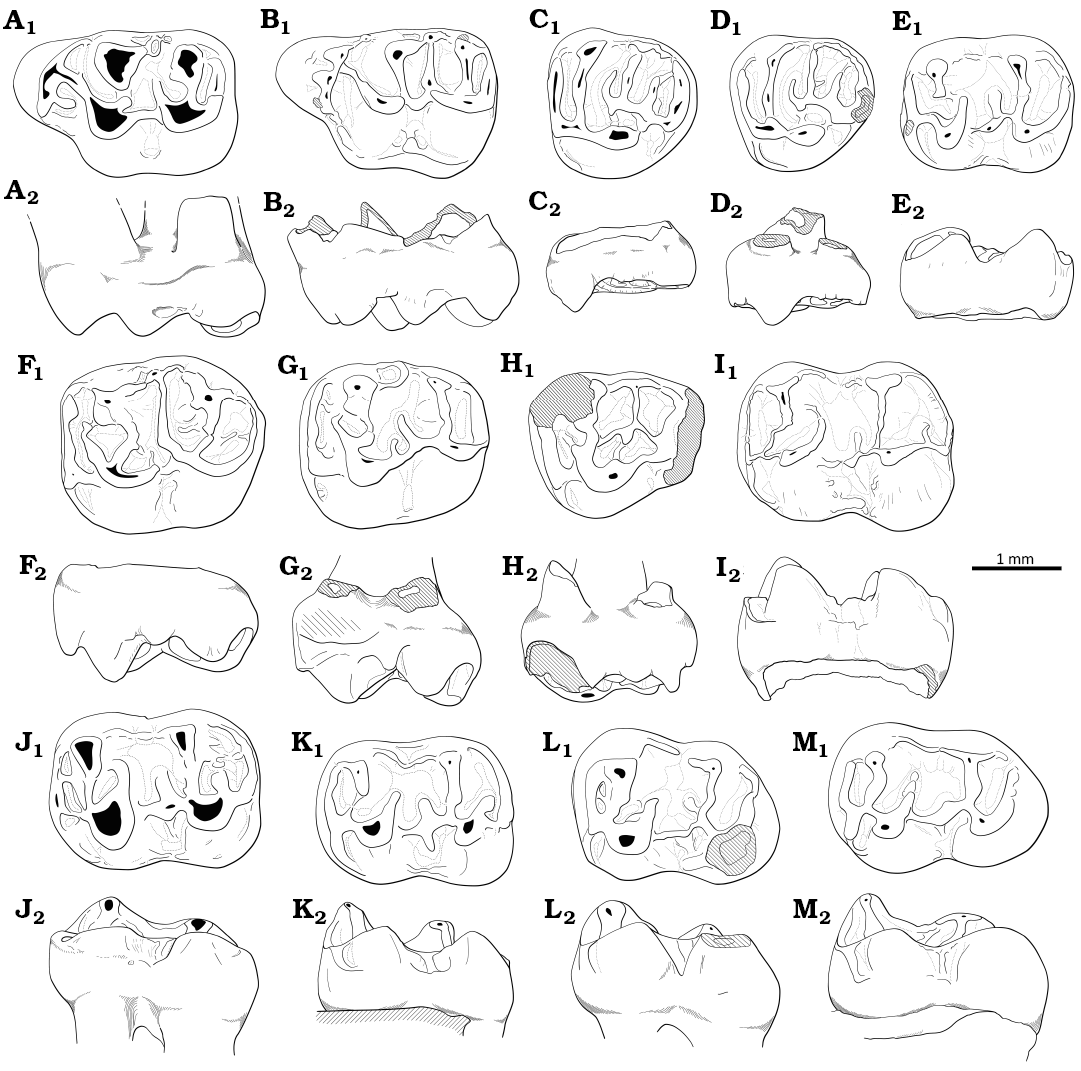
Fig. 13. Drawings of upper and lower molars. A–E. Paracricetodon cf. Pa. walgeri Bahlo, 1975, from the Rupelian, lower Oligocene of Cetățuie and Suceag, Romania. A. Left M1 (MPSUBB v1101). B. Right M1 (MPSUBB v1102, reversed). C. Left M3 (MPSUBB v1104). D. Right M3 (MPSUBB v1105, reversed). E. Right m2 (MPSUBB v1106, reversed). F–M. Paracricetodon kavakderensis Ünay-Bayraktar, 1989, from the Rupelian, lower Oligocene of Mera, Cetățuie and Suceag, Romania. F. Left M2 (MPSUBB v1107). G. Right M2 (MPSUBB v1108, reversed). H. Right M3 (MPSUBB v1109, reversed). I. Right m2 (MPSUBB v1110, reversed). J. Left m2 (MPSUBB v1111). K. Right m2 (MPSUBB v1113, reversed). L. Right m3 (MPSUBB v1115, reversed). M. Right m3 (MPSUBB v1114, reversed). A1–M1, occlusal view; A2–M2, labial view.
Remarks.—The size of these teeth from Cetățuie and Suceag (Fig. 12) fall within the range of Paracricetodon walgeri from its type locality of Heimersheim (Bahlo 1975) except for one M2, which is only slightly larger. The teeth are otherwise often smaller than Paracricetodon kodjayarmensis from Kocayarma (van de Weerd et al. 2018), than Paracricetodon kavakderensis from Kavakdere (van de Weerd et al. 2018) and than Paracricetodon dehmi from Bernloch (Hrubesch 1957). They are also noticeably smaller than Trakymys saratji, Paracricetodon spectabilis, Paracricetodon cadurcensis and Paracricetodon confluens (see van de Weerd et al. 2018 for an exhaustive size comparison of all species of Paracricetodon). Finally, they are constantly larger than Paracricetodon stojanovici from Buštranje (van de Weerd et al. 2018), Paracricetodon gracilis from Valniš (van de Weerd et al. 2018a) and Paracricetodon wentgesi from Süngülü B (de Bruijn et al. 2003).
Among the species of closest sizes, the specimens from Transylvania differ from Paracricetodon kodjayarmensis and Paracricetodon kavakderensis in having M1s with rather distally oriented protolophule and straight metalophule. They also differ in missing the hypoconid hind arm and in having a longer mesolophid in m2, and in having more complex M3s with both the protocone hind arm and entoloph joining the hypocone and two mesolophs. In contrast, some features observed on the above-described specimens fit well the diagnosis of Paracricetodon walgeri: The weak or interrupted metalophid as opposed to the well-developed entolophid and protoconid hind arm in m2. The thin or interrupted entoloph in m2. The protoloph oriented backward and connected, either to the distal part of the protocone, or to its hind arm whereas the hypoconid is straight and connects directly to the hypocone in M1. They otherwise differ in missing the protoconid hind arm in m2 and having shorter M3s.
Therefore, considering the few differences compared to Paracricetodon walgeri, and keeping in mind that we do not know much about the variability of this population from Transylvania, we tentatively refer these specimens from Suceag (upper level) and Cetățuie to this species as Paracricetodon cf. Paracricetodon walgeri.
Paracricetodon kavakderensis Ünay-Bayraktar, 1989
Fig. 13F–M.
Material.—One lower molar from Mera, seven molars from Cetățuie and one upper molar from Suceag, Rupelian, lower Oligocene, Dâncu Formation, Gilău sedimentary area, Transylvanian Basin, Romania.
Measurements.—See Table 11.
Table 11. Measurements of molars (in mm) of Paracricetodon kavakderensis from the lower Oligocene of Mera (upper level), Cetățuie and Suceag (lower level).
|
Tooth |
Locality |
Specimen number |
L |
W |
|
Left M2 Right M2 |
Suceag Cetățuie |
MPSUBB v1107 MPSUBB v1108 |
2.17 2.04 |
1.80 1.82 |
|
Right M3 |
Cetățuie |
MPSUBB v1109 |
- |
1.67 |
|
Right m2 Left m2 Right m2 Right m2 Mean m2 StD m2 |
Cetățuie Cetățuie Cetățuie Cetățuie |
MPSUBB v1110 MPSUBB v1111 MPSUBB v1112 MPSUBB v1113 |
2.19 2.32 2.36 2.19 2.27 0.09 |
1.77 1.79 1.78 1.67 1.75 0.06 |
|
Right m3 Right m3 |
Cetățuie Mera |
MPSUBB v1114 MPSUBB v1115 |
2.33 2.13 |
1.67 1.57 |
Description.—The molars are characterized by a high crown (at least higher than the other early Oligocene taxa described herein); in lateral view the cusp(id)s are bulk and high separated by deep valleys.
The two M2s have a very long labial anteroloph starting from the protostyle, itself connected directly to the protocone; in contrast, the lingual anteroloph is weak and low. The protocone spur does not reach the anteroloph but instead connect to the paracone forming an mesial protolophule. The distal protolophule is also complete; it starts from protocone (Fig. 13F) or from the protocone mesial spur (Fig. 13G). The entoloph interrupts between the mesoloph and the protocone, instead the protocone distal arm connects directly to the mesoloph or distally to the mesoloph. In one M2 (Fig. 13G), the paracone spur forms a loop delimiting a small pit labially to the paracone, in the other M2 it connects to the mesostyle and a low cigulum delimits the same small labial pit. Additionally, a cingulum between the paracone spur and the metacone closes the mesosinus labially. Lingually the sinus can be open or closed by a small cingulum. The metalophule connects to the entoloph at a point between the hypocone and the mesoloph. One M2 displays additional weakly-developed spurs mesially and distally to the metalophule (Fig. 13F). The posterosinus is large and the posteroloph is long. One of the M2s has three preserved roots (Fig. 13G).
The M3 is partly broken (the antero-labial corner and distal part, Fig. 13H). Like the M2s, it has a very long labial anteroloph and a weak and low lingual anteroloph. The protocone spur does not reach the anteroloph or the paracone but ends in the anterosinus. Both the entoloph and the protocone distal arm are present; the entoloph connects the distal protoloph to the mesoloph whereas the protocone distal arm directly connects the protocone to the hypocone. The mesoloph is long, with a very small mesostyle at its extremity. The mesostyle is merged into a cingulum that closes the mesosinus labially. Additionally, a small transversal spur starts opposite to the mesoloph and links the entoloph to the protocone. The M3s has three roots.
In m2s, both the lingual and labial anterolophids are well developed; they are connected to the protoconid by a long anterolophulid. The metalophulid connects on the anterolophulid; additionally, one m2 has a metaconid spur that connects to the lingual anterolophid (Fig. 13J). The protoconid hind arm is long; it can end in the mesosinusid (Fig. 13K) or form a loop and connect to the metaconid (Fig. 13J). The mesoconid is always well developed and is the starting point of both a short mesolophid and a short ectomesolophid. The metaconid ridge is long and reaches the entoconid so the mesosinusid is always closed lingually. Likewise, a cingulum always closes the sinusid labially. The hypolophulid is straight and connects on the ectolophid, mesially to the hypoconid. The hypoconid hind arm is always well developed; it can end in the posterosinusid (2/4) or connect to the entoconid distal slope (2/4). Both the posterosinusid and the posterolophid are well developed too, but in contrast labial posterolophulid and labial posterosinusid are either very weakly (2/4) developed or absent (2/4). The m2s have two roots.
The two m3s display a mesial part similar to that of the m2s: both the lingual and labial anterolophids are well developed and connected to the protoconid by an anterolophulid; the metalophulid also connects on the anterolophulid and one m3 has a metaconid spur that connects to the lingual anteroloph (Fig. 13L); the protoconid hind arm is long and ends in the mesosinusid. Otherwise, as opposed to m2s, the mesolophid and ectomesolophid are absent, the labial cingulum either is interrupted or absent, so the sinusid remains open. The distal part of the tooth is rounded due to very large posterosinusid and posterolophid, but the hypoconid hind arm, the labial posterolophulid and the labial posterosinusid are absent. The m3s have two roots
Remarks.—Except for two m2s slightly larger, the above specimens fit quite well in the size range of both Paracricetodon dehmi from Bernloch and Paracricetodon kavakderensis from Kavakdere (Fig. 12). They are otherwise slightly smaller than Pa. kodjayarmensis from Kocayarma and much smaller than Trakymys saratji from Kavakdere. They are also larger than Paracricetodon stojanovici from Buštranje (van de Weerd et al. 2018), Paracricetodon gracilis from Valniš (van de Weerd et al. 2018) and Paracricetodon wentgesi from Süngülü B (de Bruijn et al. 2003). Paracricetodon spectabilis, Paracricetodon cadurcensis, and Paracricetodon confluens are also much larger; see van de Weerd et al. (2018) for an exhaustive size comparison of all species of Paracricetodon.
It is worth noticing that one M2 from Suceag (Fig. 13F) and one m2 from Cetățuie (Fig. 13I) show more complex occlusal patterns due to the presence of low and weakly developed spurs, which remind the morphology of Trakymys saratji, but in a lesser extant. However, the size and the morphology of the other teeth seem to exclude a close affinity with this species.
Ünay-Bayraktar (1989) noticed the similar size between Pa. kavakderensis and Pa. dehmi but emphasized that they clearly differ in Pa. kavakderensis “having a smaller m3, a weaker hypocone and shallower sinus in the M3”. The size of m3s from Transylvania falls in the range of both species, but the only M3 yielded by the layer of Cetățuie shows a weak hypocone and shallow sinus that better fit the description of Pa. kavakderensis (Ünay-Bayraktar 1989). Paracricetodon kavakderensis and Pa. kodjayarmensis are also of similar sizes, but Ünay-Bayraktar (1989) stated that the lower molars of Pa. kavakderensis differs from Pa. kodjayarmensis by being narrower, especially m3s. The width of m3s from Transylvania (Fig. 12) is noticeably less than those of Pa. kodjayarmensis which also suggest a similarity with Pa. kavakderensis.
The above specimens and Pa. kavakderensis also share several morphological features that also differentiate them from Pa. kodjayarmensis and Pa. dehmi: generally, longer distal arms of the labial cusps in lower molars and longer mesial arms of the lingual cusps in upper molars. In M2, a well-developed protocone spur connects to the paracone to form a mesial protolophule. Both the mesial and distal protolophules delimiting a large pit between the protocone and paracone; the paracone distal spur forms a loop delimiting a small pit labially to the paracone. In M3 the metalophule is well developed and connects the hypocone to the distal cingulum.
We consequently refer these specimens from Suceag, Cetățuie and Mera to Pa. kavakderensis.
Stratigraphic and geographic range.—Rupelian (Early Oligocene) of Lesser Caucasus (Turkey) and Transylvania (Romania)
Discussion
New data on the distribution of cricetid rodents in the upper Eocene and lower Oligocene of Eastern Europe.—The Cricetidae described in the present study constitute the first update of their Transylvanian record (Table 12) since Baciu and Hartenberger (2001). The Eocene record consists of the three genera Witenia, Bustrania, and Eocricetodon. Witenia and Bustrania were referred to the subfamily Pappocricetodontinae by de Bruijn et al. (2003, 2018). However, Maridet and Ni (2013) have shown that this subfamily is polyphyletic and therefore cannot be considered valid. Witenia and Bustrania are referred here as incertae sedis. Eocricetodon has been referred to the subfamily Eucricetodontinae since Wang (2007). Maridet and Ni (2013) were unable to confirm this referal to this subfamily, or of any other subfamily, which is why the genus is also referred here as incertae sedis. In fact, none of the subfamilies Eucricetodontinae, Pseudocricetodontinae, and Paracricetodontinae seems to be present in the upper Eocene of Transylvania. In contrast, the lower Oligocene localities show a slightly greater diversity with four genera referred to these three subfamilies, and none of the genera present in the upper Eocene. The Eocene–Oligocene transition is thus characterised by a complete turnover at the specific, generic and probably even subfamilial levels (Table 12). This turnover illustrates a second phase of migration after the late Eocene one, but also a local disappearance, at the beginning of the Oligocene, of some Asian taxa that arrived earlier in the late Eocene.
Table 12. Summary of the fossil record of Cricetidae from upper Eocene (Treznea and Bociu) and lower Oligocene (Mera, Cetățuie, and Suceag) localities in Transylvania, Romania. The values correspond to the number of molars found for each species in each locality (the sum per subfamily and the total per locality are also given).
| |
Upper Eocene |
Lower Oligocene |
|||
|
Treznea |
Bociu |
Mera |
Cetățuie |
Suceag |
|
|
Incertae sedis |
13 |
2 |
0 |
0 |
0 |
|
Witenia sp. |
2 |
|
|
|
|
|
Bustrania cf. B. dissimile |
|
2 |
|
|
|
|
Eocricetodon cf. Eo. meridionalis |
11 |
|
|
|
|
|
Eucricetodontinae |
0 |
0 |
0 |
16 |
0 |
|
Eucricetodon aff. Eu. huerzeleri |
|
|
|
5 |
|
|
Tenuicricetodon arcemis gen. et sp. nov. |
|
|
|
11 |
|
|
Pseudocricetodontinae |
0 |
0 |
0 |
2 |
1 |
|
Pseudocricetodon cf. Ps. montalbanensis |
|
|
|
2 |
1 |
|
Paracricetodontinae |
0 |
0 |
2 |
16 |
4 |
|
Paracricetodon cf. Pa. walgeri |
|
|
|
4 |
2 |
|
Paracricetodon kavakderensis |
|
|
1 |
7 |
1 |
|
Paracricetodon aff. Pa. stojonovici |
|
|
|
3 |
1 |
|
Paracricetodon wentgesi |
|
|
1 |
2 |
|
|
Total |
13 |
2 |
2 |
34 |
5 |
Over the last 20 years, much progress has been made on the context of the transition between the Eocene and the Oligocene in Europe, both in terms of understanding palaeogeography and the faunas of Central and Eastern Europe (e.g., Baciu and Hartenberger 2001; de Bruijn et al. 2003, 2018, 2019; Delfino et al. 2003; Codrea et al. 2011; Grandi and Bona 2017; Mennecart et al. 2018; Tissier et al. 2018; van de Weerd et al. 2018; Licht et al. 2022; Lihoreau et al. 2023). As discussed above, the age of several fossil localities may be called into question, leading to a new interpretation of the distribution of mammals across Europe. These new biochronological interpretations will no doubt be discussed in the years to come. However, together with the new data on cricetid diversity, they serve here as a basis for establishing a new working hypothesis on the processes of change in rodent communities on a European scale during the Eocene–Oligocene transition. More generally, this working hypothesis makes it possible to discuss and re-evaluate the concept of “Grande Coupure” set out by Hans Georg Stehlin more than 100 years ago.
Paleogeography and paleobiogeography of the latest Eocene.—The geographical configuration of Europe (Fig. 14) at the end of the Eocene is characterised by highly fragmented landmasses from the Middle East to the western edge of Europe (Popov et al. 2004). Nevertheless, the different landmasses are close together and exchanges of fauna cannot be ruled out depending on occasional variations in sea level. This is the case, for example, of the lands in the north of Europe, which are separated by shallow seas (between the Russian Land and the Bohemian–Vohynian Highs). In the same way, the various strips of land that make up southern Europe (Tisza, Dinarian High, Moesian Land, Balkanian High, and Anatolian Land) are separated by narrow inlets, most of which are shallow. However, the separation between the northern and southern regions is underlined by the presence of several deep basins such as the Peri-Alpine Sea and the Great Causasus Basin, the only exception being the narrow inlet that separates the Volhynian High and the Moesian Land. Finally, the western edge of Europe is a large landmass, separated from the rest of Europe by the western part of the Alpine Sea and the Rhine Sea, both of which are shallow basins (Fig. 14).
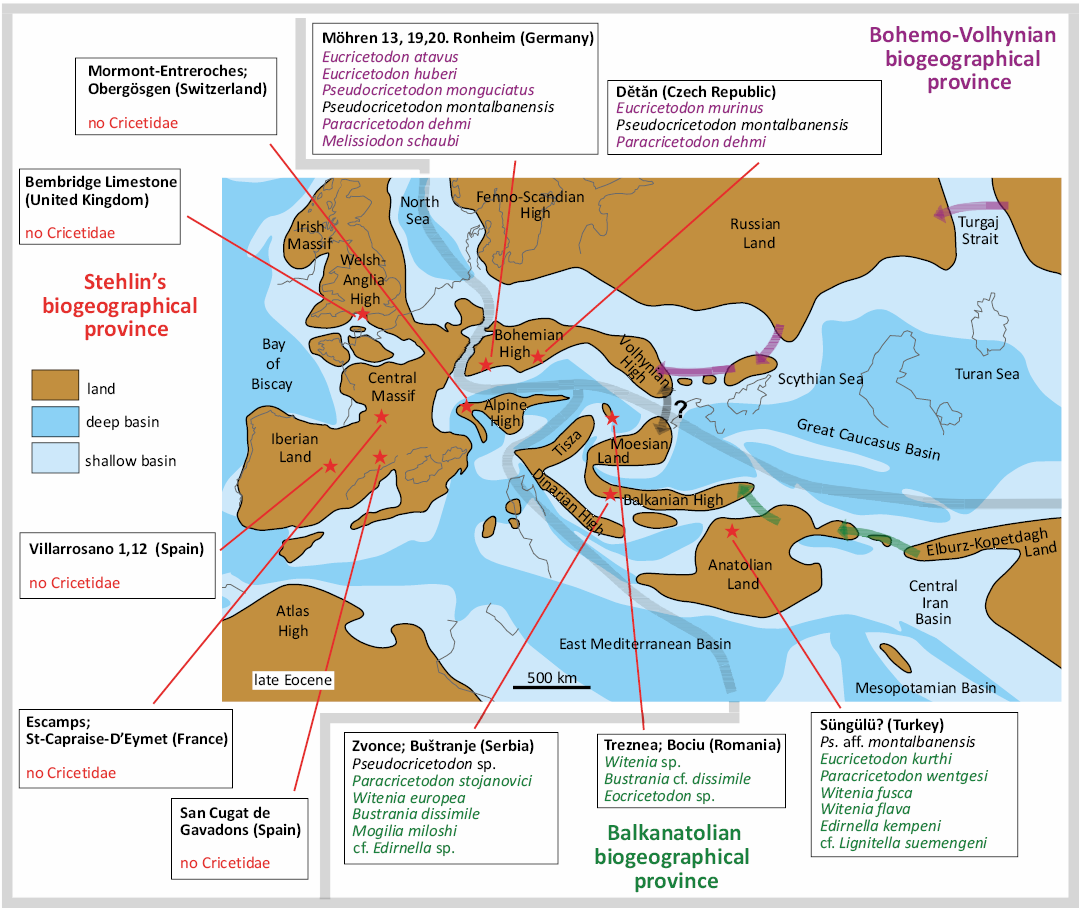
Fig. 14. Priabonian, latest Eocene paleogeographic maps (modified from Popov et al. 2004 and Meulenkamp et al. 2000) and distribution of cricetid taxa. Taxa that are endemic to the Bohemian and Volhynian land are in purple, taxa that are endemic to the Moesian, Balkanian and Anatolian lands (Balkanatolia sensu Licht et al. 2022) are in green, whereas black taxa can be found in both paleobiogeographic regions. Arrows show the possible migration passes for taxa of the same colors as the arrow.
While the geographical context already suggests that there may be barriers restricting the migration of fauna, the distribution of Cricetidae further confirms the existence of real biogeographical barriers, or at least of different provinces. The first biogeographical zone includes the Anatolian Land, the Balkanian High, Tisza, and the Moesian Land, all characterised by endemic species of cricetids (Fig. 14). This province is not new, as it corresponds well to the Balkanatolia, already identified as a distinct biogeographic province based on the occurrence of endemic large mammals during the middle Eocene (Licht et al. 2022). Another biogeographic region stands out on the basis of cricetid assemblages in northern Europe. The Bohemian and Volhynian landmasses provide a species record that is markedly different from that of the Balkanatolian province, which we propose to call the Bohemo-Volhynian province. The only species present in the two provinces is Pseudocricetodon montalbanensis (even though the species is identified as an affine form in the Anatolian region). The common presence of this species may indicate limited exchanges of fauna between the two provinces, perhaps at the level of the arm of the sea between the Volhynian High and the Moesian Land.
Finally, the last geographical province corresponds to the landmasses of the western edge of Europe, including the Alpine High Land. The fossil record of this province is known from a very large number of localities (see Biochrom’97 for a summary). This fossil record is characterized by the absence of cricetids prior to the Oligocene, and therefore suggests a total isolation from the rest of Europe, at least during the late Eocene. In contrast to the rest of Europe, the single faunal turnover at the beginning of the Oligocene in this province corresponds to the Eocene–Oligocene transition as described by Stehlin (1909). For this reason, we propose to name this western edge, including the Alpine High Land, the Stehlin’s biogeographical province.
On the basis of the cricetids, the Stehlin’s province appears to be totally isolated biogeographically from the other two provinces, although the distribution of some other mammalian taxa indicates affinities between the Bohemo-Volhynian province and the Stehlin province. Indeed, the same species of Palaeotherium are recorded at the same time in the upper Eocene of Germany (Frohnstetten, Möhren 13/19/31; Heissig 1987) and in many localities of the upper Eocene of France (Rémy 1992; Biochrom’97), whereas Plagiolophus fraasi is only known from the late Eocene of the Bohemo-Volhynian province and the uppermost Eocene of the Bembridge Marls of England (Remy 2004), dated from MP20. Plagiolophus minor, however, was recovered from many upper Eocene localities in Stehlin’s province and Bohemo-Volhynian province, but is also found in a few lower Oligocene localities (from Stehlin’s province only), such as Ronzon and Soumailles (Remy 2004). Similarly, the genus Suevosciurus occurs in the upper Eocene of Germany (e.g., Möhren 13/19/20/31, Haag 2, Herrlingen 1, Ronheim 1; Heissig 1987), England (e.g., Headon Hill, Creechbarrow; Hooker 1986, 1994) and France (Chéry-Chartreuve, Escamps; Comte et al. 2012; Vianey-Liaud and Marivaux 2016). Finally, Elomeryx occurs in the upper Eocene of Germany (Möhren 13; Heissig 1987), the upper Eocene of Italy (Grancona; Grandi and Bona 2017), and the upper Eocene of France (La Débruge; Biochrom’97). Additionally, it is also worth mentioning that Lebküchner (1974) published and illustrated several remains of Elomeryx woodi from Oligocene deposits of Turkish Thrace, although a late Eocene age cannot be excluded awaiting for both a chronologic and systematic revision of these deposits and their fossil content. These affinities could indicate either occasional and limited faunal passages between the two provinces during the late Eocene (but excluding the passage of the Cricetidae), or an earlier Eocene connection that predates the arrival of the Cricetidae in Bohemo-Volhynian province. Moreover, many taxa present in the late Eocene of the Bohemo-Volhynian province also indicate an isolation of this province during the late Eocene, and are only found in other provinces after the EOT, notably rhinocerotoids such as Eggysodon, Epiaceratherium and Ronzotherium (Becker 2009) and some artiodactyls such as Plesiomeryx cadurcensis (Weppe et al. 2024) or Entelodon antiquus (Brunet 1979).
Paleogeography and paleobiogeography of the early Oligocene.—The geography of Europe (Fig. 15) at the beginning of the Oligocene was less fragmented and had more extensive landmasses, due to the sea-level drop caused by the Oi-1 glaciation. This new configuration is therefore more favorable to faunal exchanges between the three provinces identified at the end of the Eocene, although several significant inlets remain, notably between the Bohemian High and the western edge, and between the Volhynian High and the Moesian Land. The new distribution of Cricetidae confirms that faunal exchanges were possible in the early Oligocene, based on the arrival of the first species in Stehlin’s province. In this province, at the base of the Oligocene, the Cricetidae remain relatively poorly diversified (compared with the Bohemo-Volhynian province) and are only represented by the genus Eucricetodon. The species Eu. atavus, which was already present in the Bohemo-Volhynian province in the late Eocene, suggests that the genus Eucricetodon arrived in the western margin via a northern migration route. Although faunal exchanges are possible, the greater diversification of Cricetidae in the Bohemo-Volhynian province indicates the persistence of a biogeographic differentiation with the Stehlin’s province, at least at the beginning of the Oligocene.
Species that were endemic to the Bohemo-Volhynian province in the late Eocene (purple in Figs. 14, 15) are also found in the Balkanatolian province in the early Oligocene (Eucricetodon atavus, Paracricetodon dehmi, and Pseudocricetodon moguntiacus). Conversely, species that were endemic to the Balkanatolian province in the late Eocene (green in Fig. 14, 15,) remain endemic to this province, despite exchanges between the different landmasses that make up this province. The arrival of Pa. kavakderensis, Pa. aff. stojanovici, and Pa. wentgesi in Transylvania (Moesian Land) bears witness to these faunal exchanges within the province. The maintenance of several endemic species and the much greater diversity of Cricetidae in the Balkanatolian province confirms the persistence of its biogeographic differentiation at the earliest Oligocene.
Finally, several Cricetidae that appeared for the first time in this province (Pa. walgeri, H. schlosseri, Ps. philippi), are present later in other localities in central and western Europe during the late early and early late Oligocene (e.g., Aubenas-les-Alpes, Dürrenberg, LaBlache, Offenbach, Saint Martin de Castillon; Hugueney et al. 1971; Kälin 2013; Maridet et al. 2010, 2019; Ziegler and Storch 2008), suggesting the establishment of a new migration route from the south.
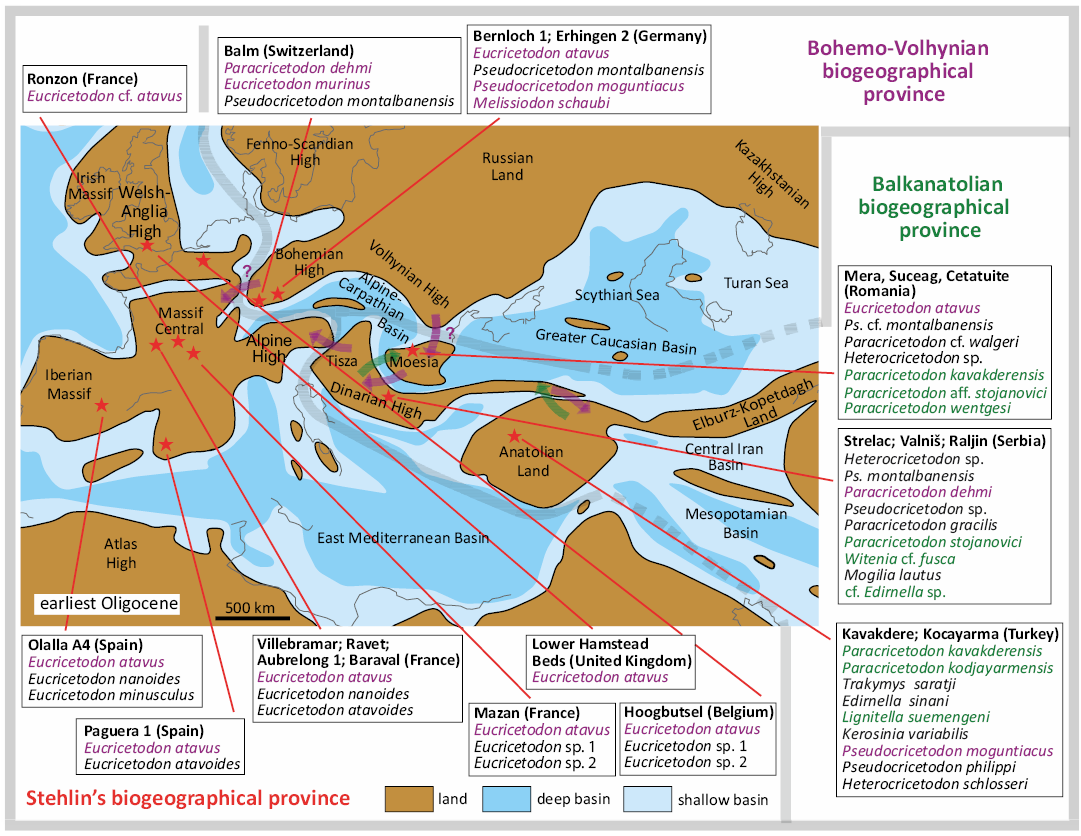
Fig. 15. Rupelian, earliest Oligocene paleogeographic maps (modified from Popov et al. 2004 and Barrier and Vrielynck 2008) and distribution of cricetid taxa. Taxa that were endemic to the Bohemian and Volhynian lands during the late Eocene are in purple, taxa that were endemic to the Moesian, Balkanian and Anatolian lands (Balkanatolia sensu Licht et al. 2022) during the late Eocene are in green, whereas black taxa are either new or can be found in all paleobiogeographic regions. Arrows show the possible migration pass for taxa of the same colors as the arrow.
Toward a new scenario for the “Grande Coupure” in Europe.—In light of the Asian origin of the Cricetidae (Vianey-Liaud 1979: 222–226; Wang and Dawson 1994; Maridet and Ni 2013), the biogeographical patterns described above also tell a story of successive migrations between Asia and Europe around the Eocene–Oligocene transition. Based on current palaeogeographic knowledge and the distribution of Cricetidae, two independent migration passes are possible: a northern passway and a southern passway. These two routes contributed to two distinct biogeographical provinces towards the end of the Eocene (the Balkanatolian province and the Bohemo-Volhynian province). However, neither of these routes altered the faunal assemblages in the western edge of Europe during the late Eocene (Stehlin’s province). At the base of the Oligocene, the taxa that arrived by the northern passway seem to have been the first to migrate in the other provinces. Taxa that arrived via the southern passway were initially restricted to the Balkanatolian province at the beginning of the Oligocene, but point to the establishment of another migration route that could potentially reach the western edge of Europe slightly later in the Oligocene.
This scenario of two distinct migration passways and several successive migrations from the late Eocene and then during the Oligocene is not novel. Indeed, several publications have already described a similar biogeographical pattern based on other groups of mammals (e.g., Becker 2009; Grandi and Bona 2017; Mennecart et al. 2018, 2021; Scherler et al. 2018; Tissier et al. 2018; Licht et al. 2022; Lihoreau et al. 2023). The biogeographic differentiation of an Anatolian-Balkans zone on the basis of Eocene–Oligocene rodent assemblages (van de Weerd et al. 2023) has already been formalised as a distinct biogeographic province and named Balkanatolia by Licht et al. (2022). This attests to a southern migration route for taxa arriving from Asia, which did not generally reach the western edge or the north of Europe. Although recent studies on the Cricetidae of Serbia, Turkey, and Romania represent a significant advancement in our understanding of the evolutionary history of mammals in Europe around the Eocene/Oligocene boundary, the fossil record of rodents in the Balkanatolian province remains limited. The new species of Cricetidae that arrived in this province at the beginning of the Oligocene are represented by a mixture of species of Bohemo-Volhynian origin (Eucricetodon atavus, Paracricetodon dehmi, Pseudocricetodon mogunciatus) and of very probably Asian origin (in any case unknown at that time in the rest of Europe: Mogilia lautus, Trakymys saratji, Edirnella sinani, Kerosinia variabilis). The discovery of Diatomyidae in the lower Oligocene of southeastern Serbia (de Bruijn et al. 2018) confirms the existence of new migrations from Asia (Mein and Ginsburg 1997), but limited to the Baklkanatolian province. One exception, however, shows that rare but more complex faunal exchanges also exist between the different provinces: the probable occurrence of the Asian family Ctenodactylidae in the lower Oligocene locality of Paguera 1 (Majorca; Hugueney and Adrover 1982) indicates that some taxa of Asian origin may still have reached Western Europe during the early Oligocene. In addition, the presence of the rare genus Moissenetia Hugueney & Adrover, 1995 (originally described at Paguera 1; Hugueney and Adrover 1995) in the upper Eocene of Baden-Württemberg and Bavaria (Möhren, Ehrenstein, Grafenmühle, Burgmagerbein, Ehingen; Berger 2008) also suggests a complex network of passes, potentially changing with variations in relative sea level, between the different biogeographical provinces.
Further observations can be made on the rodent assemblages of the Bohemo-Volhynian province. The succession of localities in Southern Germany here considered to be upper Eocene (from Möhren 19 to Schelklingen 1; Heissig 1987) is well documented and already demonstrates a high diversity of Sciuridae, Dipodiae, Gliridae, Eomyidae and Castoridae (Heissig 1987; Berger 2008; Maridet et al. 2010). These include many taxa that are usually considered to be new immigrants and markers of the “Grande Coupure” sensu Stehlin (1909). This similarity between the late Eocene assemblages of Southern Germany and those known later in the Oligocene of Western Europe provides further evidence in favour of a northern arrival route for the majority of the taxa that subsequently dispersed in Western Europe from the beginning of the Oligocene onward.
In addition, several other recent studies have refined a migration history around the Eocene/Oligocene boundary in Europe based on large mammals. Grandi and Bona (2017) compared the fossil record of Anthracotheriidae at the end of the Eocene in different regions of Europe. They confirmed the presence of Elomeryx as early as the late Eocene (Italy, Grancona), but found that Anthracotheriidae differed significantly in regions further east (equivalent regions of the Balkanatolian province sensu Licht et al. 2022). They concluded from this that there were two distinct migration routes (a northern one and a southern one) and that the arrival of Elomeryx, which was not present in the Balkanatolian province (assuming that the Oligocene age of Elomeryx published by Lebküchner [1974] from Turkish Thrace is correct), must have been via a northern route, without finding any evidence for this. A revision of the dating of the Möhren 13 locality as suggested herein (see the European chronological frameworks above) confirms the presence of Elomeryx in the Bohemo-Volhynian province as early as the late Eocene and supports the conclusions of Grandi and Bona (2017). Still on the Anthracotheriidae, Scherler et al. (2019) noted the occurrence of the sole genus Prominatherium in the late Eocene of the Balkanatolian province. This genus disappeared at the beginning of the Oligocene with the successive arrival of Anthracotherium and, slightly later, Paenanthracotherium. The presence of these two genera in Pakistan led Scherler et al. (2019) to suggest an arrival by a southern migration route through the Balkanatolian province. In fact, Anthracotherium was also present in the Bohemo-Volhynian province during the late Eocene (Burgmagerbein 8, Grafenmuhle 7, Möhren 13, Herrlingen 1; Heissig 1987). As a result, while the later arrival of Paenanthracotherium via a southern route is very likely, the arrival of the genus Anthracotherium was not necessarily via a southern route. Concerning the fossil record of other Artiodactyla, Mennecart et al. (2018, 2021) also suggested the existence of two distinct migration routes, the northern route by which the first taxa immigrated during the “Grande Coupure” and a southern route whose taxa were initially restricted to the Balkanatolian province and arrived in the western part of Europe later, towards the end of the Early Oligocene (Bachitherium dispersal event sensu Mennecart et al. 2021). Still among large mammals, the Perissodactyla record tells a similar story. The Amynodontidae are present in Eastern Europe from the late Eocene (Kretzoi 1940; von Nikolov and Heissig 1985; Tissier et al. 2018), but represented by taxa (Amynodontopsis, Sellamynodon, Amynodon, and Cadurcodon) different from the only taxon which arrived in Western Europe at the beginning of the Oligocene (Cadurcotherium; Ménouret 2018), suggesting an arrival by another route, from the north of Europe. With regard to other Rhinocerotoidea, Becker (2009) also discussed the two possible migration routes for the arrival of the genera Epiaceratherium, Eggysodon, and Ronzotherium in Western Europe. He confirmed the strong similarities between the rhinocerotoid assemblages of several lower Oligocene Western European localities such as Villebramar, Bournoncle, Barlières, Soumailles, Lagny-Torigny (France; Brunet 1979; Hugueney 1997; Uhlig 1999), Monteviale (Italy; Uhlig 1999), Kleinblauen and Bressaucourt (Switzerland; Becker 2009), but the similarities observed also include taxa from several localities considered herein to be upper Eocene, such as Detăn (Czech Republic; Fejfar 1987), Ronheim 1, Grafenmühle sensu lato, and Möhren sensu lato (Germany; Heissig 1987; Uhlig 1999). Thus, the occurence of the genera Epiaceratherium, Eggysodon, and Ronzotherium in the Bohemo-Volhynian province in the late Eocene and the absence of Epiaceratherium and Eggysodon from the fossil record of the Balkanatolian province also suggest an arrival of Rhinocerotoidea in Western Europe (Stehlin’s province) by a northern route via the Bohemo-Volhynian province.
Conclusions
The distribution of Cricetidae in Europe underwent a significant shift around the Eocene/Oligocene boundary. This was documented by a surge in knowledge about European mammals, particularly in Eastern Europe, and a more precise understanding of the biogeographical context. These developments have led to a scenario of the “Grande Coupure” that is markedly different from the one put forward by Stehlin over a hundred years ago. Indeed, the renewal of faunas on a European scale involved geographical barriers and several successive migrations. The Stehlin’s (1909) “Grande Coupure” sensu stricto (a single drastic faunal turnover at the beginning of the Oligocene) now appears to be only partially true for the western edge of Europe, where the extinction of endemic fauna and the arrival of new taxa from Asia are coeval. For the rest of Europe, if a significant turnover and a redistribution of diversity does occur at the Eocene/Oligocene boundary, it is as part of a stepwise faunal change that started in the late Eocene with the arrival of the first Asian immigrant. In Eastern Europe, the disappearance of taxa at the Eocene/Oligocene boundary doesn’t involve endemic taxa but mostly late Eocene Asian immigrants that are progressively replaced during the early Oligocene by other Asian immigrants arrived by a northern migration passway.
Since Stehlin (1909), several studies tried to find a “Grande Coupure” sensu lato outside of Europe. Osborn (1910; for North America) and Matthew and Granger (1923a, b, 1924, 1925; for Mongolia) were the first to describe noticeable terrestrial faunal turnovers around the Eocene/Oligocene boundary in other regions of the northern hemisphere. However, it is only since the 1990’s (e.g., Pascual et al. 1985; Marshall and Cifelli 1989; Rasmussen et al. 1992; Prothero and Heaton 1996; Hartenberger 1998; Meng and McKenna 1998) and the development of modern datation methods, with more precise calibration of the geological sections and better correlations with the marine record that researchers tried to truly refer the faunal changes observed across the Eocene–Oligocene transition to the “Grande Coupure” and the global climatic change (Ladant et al. 2014; Hutchinson et al. 2021). Most studies failed to find a drastic event comparable to the “Grande Coupure” sensu stricto as described by Stehlin (1909). Studies have shown either a different pattern of faunal renewal compared with the European transition (e.g., Rasmussen et al. 1992; Meng and McKenna 1998), or faunal renewal that is not synchronous with climate change or the Eocene/Oligocene boundary (e.g., Prothero and Heaton 1996; Kraatz and Geisler 2010; Woodburne et al. 2014; Antoine et al. 2021), or simply an insufficient fossil record (e.g., Pascual et al. 1985; Marshall and Cifelli 1989). Consequently, the scenario proposed here for the Eocene–Oligocene transition in Europe represents a significant advance in our understanding of this period. It challenges the traditional view of a “Grande Coupure” sensu stricto, as described by Stehlin (1909), involving a single faunal turnover at the Eocene/Oligocene boundary, and offers a more nuanced approach to studying this faunal transition on different continents as a “Grande Coupure” sensu lato, i.e. any significant faunal event occurring during the EOT.
A summary of the Eocene–Oligocene transition for European mammals is as follows. By the late Eocene, two migration routes were in place, one in the north and one in the south, and new taxa from Asia were arriving. These routes appear to have been largely isolated from each other and from Western Europe, with the arrivals of different taxa leading to notably different assemblages of taxa and thus to two distinct biogeographical provinces (the Balkanatolian province in the south and the Bohemo-Volhynian province in the north). The separation of these two migration routes is likely to have resulted from a latitudinal biogeographical differentiation that was already well established in Asia during the late Eocene (Tsubamoto et al. 2004). This produced two distinct northern and southern faunas with Asian origin for each of these migration routes. The new taxa arriving in Europe during the late Eocene did not reach the western edge of Europe (herein Stehlin’s province), where an endemic fauna remained due to its geographic isolation. At the beginning of the Oligocene, the drop in sea level and the geographical evolution of Europe led to a change in the configuration of the landmasses, facilitating new exchanges between the three provinces. Bohemo-Volhynian taxa subsequently migrated into the two other provinces. The Stehlin province is the sole European region where the extinction of numerous endemic taxa is coeval with a single migration of taxa of eastern origin (the “Grande Coupure” sensu stricto as defined by Stehlin in 1909).
In Eastern Europe, the beginning of the Oligocene was characterised by a redistribution of biodiversity. This process occurred gradually, with the further arrival of Asian taxa through several successive migrations during the early Oligocene (Scherler et al. 2018; Mennecart et al. 2021). However, Bohemo-Volhynian taxa dominated this redistribution, spreading to almost all of Europe. In contrast, the taxa of the other two provinces remained largely confined to those provinces. Despite the faunal exchanges and the redistribution of biodiversity that took place at the beginning of the Oligocene, biogeographic differences remained, as did the three geographic provinces of the late Eocene. In this scenario, it is likely that global climate change played a significant role at the European scale (Legendre 1989; Hooker et al. 2004; Costa et al. 2011; Sheldon et al. 2016). First, the progressive climate change that took place after the Middle Eocene Climatic Optimum (MECO, around 40 Ma) and especially rapid toward the end of the Eocene (Zachos et al. 2001; Wade and Pearson 2008; Inglis et al. 2015; Tramoy et al. 2016; Carter et al. 2017; Śliwińska et al. 2019) lead to noticeable environmental alterations (Legendre 1989; Escarguel et al. 2008; Gebhardt et al. 2013; Kocsis et al. 2014) and variations in sea levels (Houben et al. 2012; Kocsis et al. 2014; Sheldon et al. 2016) prior to the Oligocene. These changes were likely the initial stages of the arrival of immigrant taxa in the late Eocene. Subsequently, the Oi-1 glaciation (Coxall and Pearson 2007) resulted in a further drop of sea level (Houben et al. 2012; De Lira Mota et al. 2023), facilitating the expansion of landmasses and the formation of new land bridges (Rögl 1998; Popov et al. 2004), but also a drastic change towards more seasonal climate and more open landscapes (Hartenberger 1973; Legendre 1989; Legendre and Hartenberger 1992; Bozukov et al. 2009; Kvaček et al. 2014; Pound and Salzmann 2017; Toumoulin et al. 2022) in a colder climatic context (Liu et al. 2009; Hren et al. 2013). The disappearance of numerous endemic taxa can likely be attributed directly to environmental evolutions linked to climate change, rather than to competition with newcomers, as demonstrated by Weppe et al. (2023) with the fossil record of Quercy. The taxa from the Bohemo-Volhynian province, which arrived in Western Europe by a northern route, likely underwent an evolution constrained by regional environmental characteristics in higher latitudes, which would have resulted in the development of ecological characteristics better adapted to colder climatic condition. Such pre-adaptation to open, colder environments found in high latitudes, which became established throughout Europe at the beginning of the Oligocene, may explain their success and their subsequent redistribution throughout Europe, in contrast to the taxa of the other two provinces.
As previously stated, this new scenario is currently a working hypothesis. To confirm, refute, or refine this hypothesis, two key steps must be taken. Firstly, a revision of the biochronological framework at the European scale is necessary. Indeed, the arrival in Eastern Europe during the late Eocene of taxa considered as markers of the Oligocene in the mammalian biochronological scale of the Palaeogene (MP biochronologic units; Biochrom’97) has been demonstrated since Baciu and Hartenberger (2001). In general, the accuracy of the biochronological units around the Eocene–Oligocene transition and the problem of the diachrony of the first appearances of taxa throughout Europe are not new and have already been the subject of several studies (e.g., Schmidt-Kittler and Vianey-Liaud 1975; Rage 1984; Legendre 1987c, 1989; Legendre and Lévêque 1997; Hooker et al. 2004, 2007, 2009; Costa et al. 2011). Secondly, with a more comprehensive understanding of the fossil record in Central and Eastern Europe, it will eventually be possible to all the paleobiogeographic data (not only on mammals) in order to test the faunal affinities between the different regions (or landmasses) that make up Europe around the Eocene/Oligocene boundary.
Acknowledgments
We thank Loïc Costeur (Naturhistorishes Museum Basel, Switzerland) for access to the collections during the course of this study. We also thank Monique Vianey-Liaud (Institut des Sciences de l’Évolution de Montpellier, Montpellier, France) and Sevket Sen (Muséum national d’Histoire naturelle, Paris, France) for helpful comments on a previous version of this publication. OM’s research is supported by two Swiss National Science Foundation (SNF) grants (CKSP_190584 and 200021_162359). For the Romanian authors, this work was supported by a grant of the Romanian Ministry of Research, Innovation and Digitization, CNCS-UEFISCDI, project number PN-III-P4-PCE-2021-0351, within PNCDI II and the National Geographic Foundation Grant 8535-08.
Editor: Eli Amson.
References
Antoine, P.-O., Yans, J., Castillo, A.A., Stutz, N., Abello, M.A., Adnet, S., Custódio, M.A., Benites-Palomino, A., Billet, G., Boivin, M., Herrera, F., Jaramillo, C., Mártinez, C., Moreno, F., Navarrete, R.E., Negri, F.R., Parra, F., Pujos, F., Rage, J.-C., Ribeiro, A.M., Robinet, C., Roddaz, M., Tejada-Lara, J.V., Varas-Malca, R., Ventura Santos, R., Salas-Gismondi, R., and Marivaux, L. 2021. Biotic community and landscape changes around the Eocene–Oligocene transition at Shapaja, Peruvian Amazonia: Regional or global drivers? Global and Planetary Change 202: 103512. Crossref
Antunes, M.T., Casanovas, M.L., Cuesta, M.A., Checa, L., Santafé, J.V., and Agustí, J. 1997. Eocene mammals from Iberian Peninsula. Mémoires et Travaux de E.P.H.E. de l’Institut de Montpellier 21: 337–352.
Baciu, C. and Hartenberger, J.-L. 2001. Un exemple de correlation marin-continental dans le Priabonien de Roumanie. Remarques sur la Grande Coupure. Compte Rendu de l’Académie des Sciences serie 2 333: 441–446. Crossref
Bahlo, E. 1975. Die Nagetierfauna von Heimersheim bei Alzey (Rheinhessen, Westdeutschland) aus dem Grenzbereich Mittel-/Oberoligozän und ihre stratigrafische Stellung. Abhandlungen des Hessischen Landesamtes für Bodenforschung 71: 1–182.
Balintoni, I., Mészáros, N., and Györfi, I. 1998. La Transylvanie, dépression et bassins. Studia Universitatis Babeș-Bolyai, Geologia 43 (1): 43–58.
Barrier, E. and Vrielynck, B. 2008. Palaeotectonic Maps of the Middle East: Tectono-Sedimentary-Palinspastic Maps from Late Norian to Pliocene, 14 maps. CCGM/CGMW, Paris.
Becker, D. 2009. Earliest record of rhinocerotoids (Mammalia: Perissodactyla) from Switzerland: systematics and biostratigraphy. Swiss Journal of Geosciences 102: 489–504. Crossref
Berger, G. 2008. Die fossilen Schlafmäuse (Gliridae, Rodentia, Mammalia) aus süddeutschen Spaltenfüllungen des Obereozäns und Unteroligozäns. Münchner Geowissenschaftliche Abhandlungen Reihe A: Geologie und Paläontologie 41: 1–128.
Biochrom’97. 1997. Synthèse et tableaux de corrélations. In: J.-P. Aguilar, S. Legendre, and J. Michaux (eds.), Actes de Congrès BiochroM’97. Memoires et travaux de l’Institut de Montpellier de l’Ecole Pratique des Hautes Etudes, 769–805. Institut de Montpellier de l’Ecole Pratique des Hautes Etudes, Montpellier.
Blondel, C. 2001. The Eocene–Oligocene ungulates from Western Europe and their environment. Palaeogeography Palaeoclimatology Palaeoecology 168: 125–139. Crossref
Bozukov, V., Utescher, T., and Ivanov, D. 2009. Late Eocene to early Miocene climate and vegetation of Bulgaria. Review of Palaeobotany and Palynology 153: 360–374. Crossref
Brunet, M. 1979. Les Grands Mammifères Chefs de File de l’Immigration Oligocène et le Problème de la Limite Eocène–Oligocène en Europe. 281 pp. Fondation Singer-Polignac édition, Paris.
Carter, A., Riley, T.R., Hillenbrand, C.-D., and Rittner, M. 2017. Widespread Antarctic glaciation during the late Eocene. Earth and Planetary Science Letters 458: 49–57. Crossref
Codrea, V.A. and Dica, P. 2005. Upper Cretaceous–lowermost Miocene lithostratigraphic units exposed in Alba Iulia-Sebeş-Vinţu de Jos area (SW Transylvanian basin). Studia Universitatis Babeş-Bolyai, Geologia 50 (1–2): 19–26. Crossref
Codrea, V.A. and Fărcaş, C. 2002: Principalele asociaţii de tetrapode continentale paleogene din Transilvania: distribuţie stratigrafică şi semnificaţii paleoambientale. Muzeul Județean Arad, Armonii Naturale 4: 80–90.
Codrea, V.A. and Godefroit, P. 2008: New Late Cretaceous dinosaur findings from northwestern Transylvania (Romania). Comptes Rendus Palevol 7: 289–295. Crossref
Codrea, V.A. and Şuraru, N. 1989. Uber einen Amynodontiden: “Cadurcodon” zimborensis sp. nov. in den Zimborer-Schichten von Zimbor, kreis Sălaj im Nord-Western des Transsylvanischen Beckens. In: I. Petrescu (ed.), The Oligocene from the Transylvanian Basin, 319–338. University of Cluj-Napoca, Cluj-Napoca.
Codrea, V.A. and Venczel, A. 2020. The fossil record of Palaeogene crocodilians in Romania: preliminary data. Nymphaea, Folia naturae Bihariae 46–47: 67–82.
Codrea, V.A., Venczel, M., Solomon, A.A., Bordeianu, M., Fărcaș, C., and Veress, L. 2022. Paleogene terrestrial vertebrates of Transylvania—key for a better understanding of the ‘Grande Coupure’ Event. 100th Anniversary of the Carpathian-Balkan Geological Association. XXII International Congress of the Carpathian-Balkan Geological Association (CBGA), Abstracts, 63. Bulgarian Academy of Sciences, Plovdiv.
Codrea, V.A., Maridet, O., Venczel, M., Fărcaş, C., and Solomon, A.A. 2011. New data on the terrestrial Eocene/Oligocene boundary in Transylvania (Romania). In: A. van de Geer and A. Athanassiou (eds.), 9th Annual Meeting of the European Association of Vertebrate Palaeontologists, Abstract volume, 19. European Association of Vertebrate Palaeontologists, Heraklion.
Comte, B. 1985. Eléments nouveaux sur l’évolution des genres Eucricetodon et Pseudocricetodon (Eucricetodontinae, Rodentia, Mammalia) de l’Oligocéne d’Europe occidentale. Palaeovertebrata 15 (1): 1–69.
Comte, B., Sabatier, M., Marandat, B., and Vianey-Liaud, M. 2012. Les rongeurs de Chéry-Chartreuve et Rocourt-Saint-Martin (est du bassin de Paris; Aisne, France). Leur place parmi les faunes de l’Eocène Moyen d’Europe. Palaeovertebrata 37 (4–5): 167–271. Crossref
Costa, E., Garcés, M., Sáez, A., Cabrera, L., and López-Blanco, M. 2011. The age of the “Grande Coupure” mammal turnover: New constraints from the Eocene–Oligocene record of the Eastern Ebro Basin (NE Spain). Palaeogeography Palaeoclimatology Palaeoecology 301: 97–107. Crossref
Coxall, H.K. and Pearson, P.N. 2007. The Eocene–Oligocene transition. In: M. Williams, A.M. Haywood, F.J. Gregory, and D.N. Schmidt (eds.), Deep-Time Perspectives on Climate Change: Marrying the Signal from Computer Models and Biological Proxies, 351–387. The Micropalaeontological Society, Special Publications, London. Crossref
De Bruijn, H., Marković, Z., Wessels, W., Milivojević, M., and Van De Weerd, A.A., 2018. Rodent faunas from the Paleogene of south-east Serbia. Palaeobiodiversity and Palaeoenvironments 98: 441–458. Crossref
De Bruijn, H., Marković, Z., Wessels, W., and van de Weerd, A.A. 2019. Pappocricetodontinae (Rodentia, Muridae) from the Paleogene of south-east Serbia. Palaeobiodiversity and Palaeoenvironments 99: 511–526. Crossref
de Bruijn, H., Ünay, E., Saraç, G., and Yïlmaz, A. 2003. A rodent assemblage from the Eo/Oligocene boundary interval near Süngülü, Lesser Caucasus, Turkey. Coloquios de Paleontología Vol. Ext. 1: 47–76.
De Lira Mota, M.A., Dunkley Jones, T., Sulaiman, N., Edgar, K.M., Yamaguchi, T., Leng, M.J., Adloff, M., Greene, S.E., Norris, R., Warren, B., Duffy, G., Farrant, J., Murayama, M., Hall, J., and Bendle, J. 2023. Multi-proxy evidence for sea level fall at the onset of the Eocene–Oligocene transition. Nature Communication 14: 4748. Crossref
Delfino, M., Rage, J.C., and Rook, L. 2003. Tertiary mammal turnover phenomena: what happened to the herpetofauna? Deinsea 10: 153–161.
Engesser, B. 1987. New Eomyidae, Dipodidae and Cricetidae (Rodentia, Mammalia) of the lower freshwater molasse of Switzerland and Savoy. Eclogae Geologicae Helvetiae 80: 943–994.
Engesser, B. and Mödden, C. 1997. A new version of the biozonation of the lower freshwater molasse (Oligocene and Agenian) of Swizerland and Savoy on the basis of fossil mammals. In: J.-P. Aguilar, S. Legendre, and J. Michaux (eds.), Actes du Congrès BiochroM’97. Mémoires et Travaux de l’E.P.H.E., Institut de Montpellier 21: 475–499.
Escarguel, G., Legendre, S., and Sigé, B. 2008. Unearthing deep-time biodiversity changes: The Palaeogene mammalian metacommunity of the Quercy and Limagne area (Massif Central, France). Comptes Rendus Geoscience 340: 602–614. Crossref
Fabiani, R. 1905. Studio geo-paleontologico dei Colli Berici. Atti del Regio Istituto Veneto di Scienze, Lettere ed Arti 64: 1797–1839
Fărcaş, C. and Codrea, V.A. 2004. Evolution of Knowledge on Paleogene Land Formations from the NW border of the Transylvanian Basin. Complexul Muzeal Judeţean Bistriţa-Năsăud, Studii şi cercetări, Geologie-Geografie 9: 13–46.
Fărcaș C. and Codrea V.A. 2005. “La Grande Coupure”, main Cenozoic bioevent. Complexul muzeal Bistrița-Năsăud, Studii şi cercetări, Geologie-Geografie 10: 33–37.
Fejfar, O. 1987. A lower Oligocene mammalian fauna from Dětaň and Dvérce NW Bohemia, Czechoslovakia. Münchner Geowissenschaftliche Abhandlungen Reihe A: Geologie und Paläontologie 10: 253–264.
Fejfar, O. and Kaiser, T.M. 2005. Insect bone-modification and paleoecology of Oligocene mammal-bearing sites in the Doupov Mountains, northwestern Bohemia. Palaeontologia Electronica 8 (1): 8A.
Flynn, L.J., Jacobs, L.L., and Cheema, I.U. 1986. Baluchimyinae, a new ctenodactyloid rodent subfamily from the Miocene of Baluchistan. American Museum Novitates 2841: 1–60.
Freudenthal, M. 1994. Cricetidae (Rodentia, Mammalia) from the upper Oligocene of Mirambueno and Vivel del Río (prov. Teruel, Spain). Scripta Geologica 104: 1–55.
Freudenthal, M. 1996. The early Oligocene rodent fauna from Olalla 4A (Teniel, Spain). Scripta Geologica 112: 1–67.
Freudenthal M. 1997. Paleogene rodents faunas from the province of Teruel, Spain. In: J.-P. Aguilar, S. Legendre, and J. Michaux (eds.), Actes de Congrès BiochroM’97. Mémoires Travaux E.P.H.E., Institut Montpellier 21: 397–415.
Freudenthal, M., Hugueney, M., and Moissenet, E. 1994. The genus Pseudocricetodon (Cricetidae, Mammalia) in the upper Oligocene of the province of Teruel (Spain). Scripta Geologica 104: 57–114.
Gebhardt, H., Ćorić, S., Darga, R., Briguglio, A., Schenk, B., Werner, W., Andersen, N., and Sames, B. 2013. Middle to late Eocene paleoenvironmental changes in a marine transgressive sequence from the northern Tethyan margin (Adelholzen, Germany). Austrian Journal of Earth Sciences 106 (2): 45–72.
Grandi, F. and Bona, F. 2017. Prominatherium dalmatinum from the late Eocene of Grancona (Vicenza, NE Italy). The oldest terrestrial mammal of the Italian peninsula. Comptes Rendus Palevol 16 (7): 738–745. Crossref
Hartenberger, J.L. 1973. Les rongeurs de l’Eocène d’Europe. Leur évolution dans leur cadre biogéographique. Mémoires du Muséum National d’Historie Naturelle, Sciences de la Terre 24 (3): 49–70.
Hartenberger, J.-L. 1998. An Asian «Grande Coupure». Nature 394: 321. Crossref
Heissig, K. 1987. Changes in the rodent and ungulate fauna in the Oligocene fissure fillings of Germany. Münchner Geowissenschaftliche Abhandlungen Reihe A: Geologie und Paläontologie 10: 101–108.
Héran, M.-A., Lécuyer, C., and Legendre, S. 2010. Cenozoic long-term terrestrial climatic evolution in Germany tracked by δ18O of rodent tooth phosphate. Palaeogeography Palaeoclimatology Palaeoecology 285: 331–342. Crossref
Hooker, J.J. 1986. Mammals from the Bartonian (middle/late Eocene) of the Hampshire Basin, southern England. Bulletin of the British Museum of Natural History, Geology series 39: 191–478.
Hooker, J.J. 1994. Mammalian taphonomy and palaeoecology of the Bembridge Limestone Formation (late Eocene, S. England). Historical Biology 8: 49–69. Crossref
Hooker, J.J. 2010. The ‘‘Grande Coupure’’ in the Hampshire Basin, UK: taxonomy and stratigraphy of the mammals on either side of this major Palaeogene faunal turnover. In: J.E. Whittaker and M.B. Hart (eds.), Micropalaeontology, Sedimentary Environments and Stratigraphy: A Tribute to Dennis Curry (1912–2001), 147–215. The Micropalaeontological Society, Special Publications, London. Crossref
Hooker, J.J., Collinson, M.E., Grimes, S.T., Sille, N.P., and Mattey, D.P. 2007. Discussion on the Eocene–Oligocene boundary in the UK Journal, Vol. 163, 2006, pp. 401–415. Journal of the Geological Society 164: 685–688. Crossref
Hooker, J.J., Collinson, M.E., and Sille, N.P. 2004. Eocene–Oligocene mammalian faunal turnover in the Hampshire Basin, UK: calibration to the global time scale and the major cooling event. Journal of the Geological Society 161: 161–172. Crossref
Hooker, J.J., Collinson, M.E., Van Bergen, P.F., Singer, R.L., De Leeuw, J.W., and Jones, T.P. 1995. Reconstruction of land and freshwater palaeoenvironments near the Eocene–Oligocene boundary, southern England. Journal of the Geological Society 152: 449–468. Crossref
Hooker, J.J., Grimes, S.T., Mattey, D.P., Collinson, M.E., and Sheldon, N.D. 2009. Refined correlation of the UK Late Eocene–Early Oligocene Solent Group and timing of its climate history. In: C. Koeberl and A. Montanari (eds.), The late Eocene Earth-Hothouse, Icehouse and Impacts. The Geological Society of America, Special Paper 452: 179–195. Crossref
Houben, A.J.P., Van Mourik, C.A., Montanari, A., Coccioni, R., and Brinkhuis, H. 2012. The Eocene–Oligocene transition: Changes in sea level, temperature or both? Palaeogeography Palaeoclimatology Palaeoecology 335–336: 75–83. Crossref
Hren, M.T., Sheldon, N.D., Grimes, S.T., Collinson, M.E., Hooker, J.J., Bugler, M., and Lohmann, K.C. 2013. Terrestrial cooling in Northern Europe during the Eocene–Oligocene transition. Proceedings of the National Academy of Sciences of the United States of America 110: 7562–7567. Crossref
Hrubesh, K. 1957. Zahnstudien an tertiären rodentia als beitrag zu deren stammesgeschichte. Über die Evolution der Melissiodontidae, eine revision des genus Melissiodon. Abhandlungen Bayerische Akademie der Wissenschaften, Mathematische-Naturwissenschaftliche Klasse 83: 1–100.
Hugueney, M. 1969. Les rongeurs (Mammalia) de l’Oligocène supérieur de Coderet-Bransat (Allier). Documents des Laboratoires de Géologie de la Faculté des Sciences de Lyon 34: 1–227.
Hugueney, M. 1971. Pseudocricetodon philippi, nouvelle espece de Cricetidé (Rodentia, Mammalia) de l’Oligocene moyen de Saint-Martin-de-Castillon (Vaucluse). Comptes Rendus de l’Académie des Sciences Series D 272 (20): 2533–2535.
Hugueney, M. 1980. La faune de Mammifères de l’Oligocène moyen de Saint-Menoux (Allier). 1ere partie: Rongeurs (Mammalia, Rodentia). Revue Scientifique Bourbonnais 17: 1–40.
Hugueney, M. 1997: Biochronologie mammalienne dans le Paléogène et le Miocène inférieur du Centre de la France: synthèse réactualisée. In: J.-P. Aguilar, S. Legendre, and J. Michaux (eds.), Actes du Congrès Bio- chroM’97. Mémoires et Travaux de l’Ecole pratique des Hautes Etudes, Institut de Montpellier 21: 417–430.
Hugueney, M. 1999. Genera Eucricetodon and Pseudocricetodon. In: G.E. Rössner and K. Heissig (eds.), The Miocene Land Mammals of Europe, 347–358. Verlag Dr. Friedrich Pfeil, München.
Hugueney, M. and Adrover, R. 1982. Le peuplement des Baléares (Espagne) au Paléogène. Geobios 15 (Suplement 1): 439–449. Crossref
Hugueney, M. and Adrover, R. 1995. Moissenetia paqurensis n. gen. n. sp. (Rodentia, Gliridae) de l’Oligocène des Baléares: première apparition du schéma dentaire Eliomys. Comptes Rendus de l’Académie des Sciences – Series IIA – Earth and Planetary Science 321: 917–922.
Hugueney, M., Truc, G., and Philippe, M. 1971. Nouveaux gisements à micromammiferes et mollusques continentaux dans l’Oligocene moyen du synclinal d’Apt (Vaucluse, sud-est de la France). Comptes Rendus Hebdomadaires des Seances de l’Academie des Sciences Paris, Serie D 272 (19): 2430–2433.
Hutchinson, D.K., Coxall, H.K., Lunt, D.J., Steinthorsdottir, M., de Boer, A.M., Baatsen, M., von der Heydt, A., Huber, M., Kennedy-Asser, A.T., Kunzmann, L., Ladant, J.-B., Lear, Caroline, H., Moraweck, K., Pearson, P.N., Piga, E., Pound, M.J., Salzmann, U., Scher, H.D., Sijp, W.P., Śliwińska, K.K., Wilson, P.A., and Zhang, Z.S. 2021. The Eocene–Oligocene transition: a review of marine and terrestrial proxy data, models and model-data comparisons. Climate of the Past 17 (1): 269–315. Crossref
Inglis, G.N., Farnsworth, A., Lunt, D., Foster, G.L., Hollis, C.J., Pagani, M., Jardine, P.E., Pearson, P.N., Markwick, P., Galsworthy, A.M.J., Raynham, L., Taylor, K.W.R., and Pancost, R.D. 2015: Descent toward the Icehouse: Eocene sea surface cooling inferred from GDGT distributions. Paleoceanography 30: 1000–1020. Crossref
Jovane, L., Florindo, F., Sprovieri, M., and Pälike, H. 2006. Astronomic calibration of the late Eocene/early Oligocene Massignano section (central Italy). Geochemistry Geophysics Geosystems 7 (7): 1–10. Crossref
Kälin, D. 2013. A new Oligocene (MP 24) mammal fauna (Dürrenberg, Canton Jura, NW Switzerland) from the eastern slope of the ‘‘Rauracian depression’’. Swiss Journal of Geosciences 106: 207–218. Crossref
Kessler, E., Codrea, V., and Vremir, M. 1998. A fossil bird from the lower Oligocene of Cluj-Napoca (Western Romania). Studia Universitatis Babeş-Bolyai, Geologia 43 (2): 7–12.
Kocsis, L., Ozsvárt, P., Becker, D., Ziegler, R., Scherler, L., and Codrea, V.A. 2014. Orogeny forced terrestrial climate variation during the late Eocene–early Oligocene in Europe. Geology 42: 727–730. Crossref
Kraatz, B.P. and Geisler, J.H. 2010. Eocene–Oligocene transition in Central Asia and its effects on mammalian evolution. Geology 38: 111–114. Crossref
Kretzoi, M. 1940. Alttertiäre Perissodactylen aus Ungarn. Annales Musei historico-naturalis hungarici 33: 87–99.
Krézsek, C. and Bally, A.W. 2006. The Transylvanian Basin (Romania) and its relation to the Carpathian fold and thrust belt: Insights in gravitational salt tectonics. Marine and Petroleum Geology 23: 405–442. Crossref
Kvaček, Z., Teodoridis, V., Mach, K., Přikryl, T., and Dvořák, Z. 2014. Tracing the Eocene–Oligocene transition: A case study from North Bohemia. Bulletin of Geosciences 89: 21–66. Crossref
Ladant, J.-B., Donnadieu, Y., Lefebvre, V., and Dumas, C. 2014. The respective role of atmospheric carbon dioxide and orbital parameters on ice sheet evolution at the Eocene–Oligocene transition. Paleoceanography and Paleoclimatology 29: 810–823. Crossref
Lavocat, R. 1952. Révision de la faune des Mammifères oligocènes d’Auvergne et du Velay. 153 pp. Sciences et Avenir, Paris.
Lebküchner, R.F. 1974. Beitrag zur Kenntnis der Geologie des Oligozäns von Mittelthrakien (Turkei). Bulletin of the Mineral Research and Exploration Institute of Turkey 83: 1–30.
Legendre, S. 1986. Analyse of mammalian communities from the late Eocene and Oligocene of southern France. Palaeovertebrata 16: 191–212.
Legendre, S. 1987a. Concordance entre la paléontologie continentale et les évènements paléo-océaniques: exemple des faunes de mammifères du Paléogène du Quercy. Compte Rendu de l’Académie des Sciences, Paris 304 (3): 45–50.
Legendre, S. 1987b. Les immigrations de la “Grande Coupure” sont-elles contemporaines en Europe occidentale. In: N. Schmidt-Kittler (ed.), Münchner Geowissenschaftliche Abhandlungen Reihe A: Geologie und Paläontologie 10: 141–148.
Legendre, S. 1987c. Les communautés de mammifères d’Europe occidentale de l’Eocène supérieur et Oligocène: structures et milieux. Münchner Geowissenschaftliche Abhandlungen Reihe A: Geologie und Paläontologie 10: 301–312.
Legendre, S. 1989. Les communautés de mammifères du paléogène (eocène supérieur et Oligocène) d’Europe occidentale: structures, milieux et évolution. Münchner Geowissenschaftliche Abhanlungen 16: 1–110.
Legendre, S. and Hartenberger, J.L. 1992. Evolution of mammalian faunas in Europe during the Eocene and Oligocene. In: D.R. Prothero and W.A. Berggren (eds.), Eocene–Oligocene Climatic and Biotic Evolution, 516–528. Princeton University Press, Princeton. Crossref
Legendre, S. and Lévêque, F. 1997. Etalonnage de l’échelle biochronologiaue mammalienne du Paléogène d’Europe occidentale: vers une intégration à l’échelle globale. In: J.-P. Aguilar, S. Legendre, and J. Michaux (eds.), Actes du Congrès Bio-chroM’97. Mémoires et Travaux de l’Ecole pratique des Hautes Etudes, Institut de Montpellier 21: 461–473.
Licht, A., Métais, G., Coster, P., İbilioğlu, D., Ocakoğlu, F., Westerweel, J., Mueller, M., Campbell, C., Mattingly, S., Wood, M.C., and Beard, K.C. 2022. Balkanatolia: The insular mammalian biogeographic province that partly paved the way to the Grande Coupure. Earth-Science Reviews 226: 103929. Crossref
Lihoreau, F., Marjanac, L., Marjanac, T., Erdal, O., and Antoine, P.-O. 2023. A late Eocene palaeoamasiine embrithopod (Mammalia, Afrotheria) from the Adriatic realm (Island of Rab, Croatia). Palaeovertebrata 47: 47.1.e1. Crossref
Liu, Z., Pagani, M., Zinniker, D., DeConto, R., Huber, M., Brinkhuis, H., Shah, S.R., Leckie, R.M., and Pearson, A. 2009. Global cooling during the Eocene–Oligocene climate transition. Science 323: 1187–1190. Crossref
Lopatin, A.V. 1996. Stratigraphy and small mammals from the Aral Formation of the Altynshokysu locality (North Aral Region). Stratigraphy and Geological Correlation 4 (2): 166–180.
Mantea, G., Ștefan, A., Rusu, A., and Dimitrescu, R. 1987. Harta geologică 1 : 50000 29c, folio Răchițele, L-34-46-D. Insitutul de Geologie și Geofizică, București.
Maridet, O. and Ni, X. 2013. A new cricetid rodent from the early Oligocene of Yunnan, China, and its evolutionary implications for early Eurasian cricetids. Journal of Vertebrate Paleontology 33: 185–194. Crossref
Maridet, O., Hugueney, M., and Costeur, L. 2013. The mammalian assemblage of Mazan (Vaucluse, France) and its position in the early Oligocene European palaeobiogeography. Swiss Journal of Geosciences 106: 231–252. Crossref
Maridet, O., Hugueney, M., and Costeur, L. 2019. Aubenas-les-Alpes (S-E France). Part III—Last and final part of the mammalian assemblage with some comments on the palaeoenvironment and palaeobiogeography. Annales de Paléontologie 105: 139–153. Crossref
Maridet, O., Hugueney, M., and Heissig, K. 2010. New data about the diversity of early Oligocene eomyids (Mammalia, Rodentia) in Western Europe. Geodiversitas 32: 221–254. Crossref
Maridet, O., Wu, W., Ye, J., Bi, S., Ni, X., and Meng, J. 2009. Eucricetodon (Rodentia, Mammalia) from the late Oligocene of the Junggar basin, Northern Xinjiang, China. American Museum Novitates 3665: 1–21. Crossref
Marković, Z., Wessels, W., van de Weerd, A.A., and de Bruijn, H. 2020. Pseudocricetodontinae (Mammalia, Rodentia) from the Paleogene of south-east Serbia. Palaeobiodiversity and Palaeoenvironments 100: 251–267. Crossref
Marshall, L.G. and Cifelli. R.L. 1989. Analysis of changing diversity patterns in Cenozoic land lammal age faunas, South America. Palaeovertebrata 19 (4): 169–210.
Matthew, W.D. and Granger, W. 1923a. Nine new rodents from the Oligocene of Mongolia. American Museum Novitates 102: 1–10.
Matthew, W.D. and Granger, W. 1923b. The fauna of the Ardyn Obo Formation. American Museum Novitates 98: 1–5.
Matthew, W.D. and Granger, W. 1924. New insectivores and ruminants from the Tertiary of Mongolia, with remarks on the correlation. American Museum Novitates 105: 1–7.
Matthew, W.D. and Granger, W. 1925. New ungulates from the Ardyn Obo Formation of Mongolia, with faunal list and remarks on correlation. American Museum Novitates 195: 1–12.
Mein, P. and Ginsburg, L. 1997. Les mammifères du gisement miocène inférieur de Li Mae Long, Thaïlande: systématique, biostratigraphie et paléoenvironnement. Geodiversitas 19: 783–844.
Meng, J. and McKenna, M.C. 1998. Faunal turnovers of Palaeogene mammals from the Mongolian plateau. Nature 394: 364–367. Crossref
Mennecart, B., Aiglstorfer, M., Li, Y., Li, C., and Wang, S. 2021. Ruminants reveal Eocene Asiatic palaeobiogeographical provinces as the origin of diachronous mammalian Oligocene dispersals into Europe. Scientific Reports 11: 17710. Crossref
Mennecart, B., Geraads, D., Spassov, N., and Zagorchev, I. 2018. Discovery of the oldest European ruminant in the late Eocene of Bulgaria: Did tectonics influence the diachronic development of the Grande Coupure? Palaeogeography, Palaeoclimatology, Palaeoecology 498: 1–8. Crossref
Ménouret, B. 2018. Le genre Cadurcotherium (Rhinocerotoidea) en Europe ; synthèse des connaissances et révision systématique. Revue de Paléobiologie 37 (2): 495–517.
Métais, G., Coster, P., Licht, A., Ocakoğlu, F., and Beard, K.C. 2023. Additions to the late Eocene Süngülü mammal fauna in Easternmost Anatolia and the Eocene–Oligocene transition at the periphery of Balkanatolia. Comptes Rendus Palevol 22 (35): 711–727. Crossref
Meulenkamp, J.E., Sissingh, W., Londeix, L., Chahuzac, B., Calvo, J.P., Daams, R., Studencka, B., Kovac, M., Nagymarosy, A., Rusu, A., Badescu, D., Popov, S.V., Scherba, I.G., Roger, J., Platel, J.P., Hirsch, F., Sadek, A., Abdel-Gawad, G.I., Yaich, C., Ben Ismail-Lattrache, K., and Bouaziz, S. 2000. Late Rupelian (32–29 Ma). In: J. Dercourt, M. Gaetani, B. Vrielynck, E. Barrier, B. Biju-Duval, M.F. Brunet, J.P. Cadet, S. Crasquin, and M. Sandulescu (eds.), Atlas Peri-Tethys, Palaeogeographical Maps, 171–178. CCGM/CGMW, Paris.
Mikuláš, R., Fejfar, O., Ulrych, J., Žigová, A., Kadlecová, E., and Cajz, V. 2003. A study of the Dětaň locality (Oligocene, Doupovské hory Mts. Volcanic Complex, Czech Republic): collection of field data and starting points for interpretation. GeoLines 15: 91–97.
Miller, K.G., Wright, J.D., and Fairbanks, R.G. 1991: Unlocking the Ice House: Oligocene–Miocene oxygen isotopes, eustasy, and margin erosion. Journal of Geophysical Research: Solid Earth 96: 6829–6848. Crossref
Moisescu, V. 1963. Contribuţii la cunoaşterea faunei de moluşte oligocene din regiunea Ticu-Tămaşa (bazinul văii Almaşului, nord-vestul Transilvaniei). Studii şi Cercetări Geologice 8 (2): 201–214.
Moisescu, V. 1972. Mollusques et Echinides stampiens et egeriens de la région Cluj-Huedin-Românaşi (Nord-Ouest de la Transylnanie). Memoires Institute Géologique 16: 1–152.
Moisescu, V. 1975. Stratigrafia depozitelor paleogene şi miocen-inferioare din regiunea Cluj-Huedin-Românaşi (NW bazinului Transilvaniei). Anuarul Institutului de Geologie şi Geofizică 47: 5–212.
Moisescu, V. 1989. Biozonation des dépôts meriens-kiscelliens du Bassin de la Transylvanie sur la base des mollusques. In: I. Petrescu (ed.), The Oligocene from the Transylvanian Basin, 267–274. University of Cluj-Napoca, Cluj-Napoca.
von Nikolov, I. and Heissig, K. 1985. Fossile Säugetiere aus dem Obereozän und Unteroligozän Bulgariens und ihre Bedeutung für die Palaeogeographie. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Histor Geologie 25: 61–79.
Osborn, H.F. 1910. The Age of Mammals in Europe. Asia, and North America. 658 pp. Macmillan, New York. Crossref
Pascual, R., Vucetich, M.G., Scillato-Yané, G.J., and Bond, M. 1985. Main Passways of Mammalian Diversifi cation in South America. In: F. Stehli and S.D. Webb (eds.), The Great American Biotic Interchange, 219–247. Plenum, New York. Crossref
Peláez-Campomanes, P. 2000. Mammalian faunas from the Paleogene of the Sierra Palomera (Teruel, Spain). Journal of Paleontology 74: 336–348. Crossref
Petrescu, I. 2003. Palinologia Terțiarului cu aplicatii in biostratigrafie, paleoclimatologie, medii continentale. 249 pp. Carpatica, Cluj-Napoca.
Petrescu, I., Filipescu, S., Chira, C., Săsăran, E., Popa, M., and Bican-Brişan, N. 2002. Paleontologic and stratigraphic data on the Cenozoic formations in the well site Transgex H2, Cluj-Napoca (Transylvanian Depression). Studia Universitatis Babeș-Bolyai, Geologia Special Issue 1: 285–300.
Popescu, B. 1976. Lithostratigraphy of the cyclic continental to marine Eocene deposits in NW Transylvania. Romania. Archives Scientifiques de Genève 37 (1): 37–73.
Popescu, B., Bombia, G., Rusu, A., Iva, M., Gheta, N., Olteanu, R., Popescu, D., and Tautu, E. 1978. The Eocene of the Cluj-Huedin area. Dări de Seamă ale ședințelor Institutului de Geologie și Geofizică București 64 (4): 295–357.
Popov, S.V., Rögl, F., Rozanov, A.Y., Steininger, F.F., Shcherba, I.G., and Kovac, M. 2004. Lithological-paleogeographic maps of Paratethys. 10 maps late Eocene to Pliocene. Courier Forschungsinstitut Senckenberg 250: 1–46.
Pound, M.J. and Salzmann, U. 2017. Heterogeneity in global vegetation and terrestrial climate change during the late Eocene to early Oligocene transition. Scientific Reports 7: 43386. Crossref
Prothero, D.R. and Heaton, T.H. 1996. Faunal stability during the early Oligocene climatic crash. Palaeogeography, Palaeoclimatology, Palaeoecology 127: 257–283. Crossref
Rădulescu, C. and Samson, P. 1989. Oligocene mammals from Romania, In: I. Petrescu (ed.), The Oligocene from the Transylvanian Basin, 301–312. University of Cluj-Napoca, Cluj-Napoca.
Rage, J.-C. 1984. La “Grande Coupure” eocene/oligocene et les herpetofaunes (Amphibiens et Reptiles); problemes du synchronisme des evenements paleobiogeographiques. Bulletin de la Société Géologique de France S7-26 (6): 1251–1257. Crossref
Raileanu, G. and Saulea, E. 1956. Paleogenul din regiunea Cluj-Jibou (nord-vestul bazinului Transilvaniei). Anuarul Comitetului Geologic Bucuresti 29: 271–307.
Rasmussen, D.T., Bown, T.M., and Simons, E.L. 1992. 28. The Eocene–Oligocene transition in Continental Africa. In: D.R. Prothero and W.A. Berggren (eds.), Eocene–Oligocene Climatic and Biotic Evolution, 548–566. Princeton University Press, Princeton. Crossref
Reichenbacher, B. and Codrea, V.A. 1999: Fresh- to brackish water fish faunas from continental early Oligocene deposits in the Transylvanian Basin (Romania). Bulletin de l’Institut Royal de Sciences Naturelles Belgique, Sciences de le Terre 69: 197–207.
Remy, J.-A. 2004. Le genre Plagiolophus (Palaeotheriidae, Perissodactyla, Mammalia): révision systématique, morphologie et histologie dentaires, anatomie crânienne, essai d’interprétation fonctionnelle. Palaeovertebrata 33: 17–281.
Remy, J.A., Crochet, J.-Y., Sigé, B., Sudre, J., Bonis, M. de, Vianey-Liaud, M., Godinot, M., Hartenberger, J.-L., Lange-Badré, B., and Comte, B. 1987. Biochronologie des phosphorites du Quercy: mise à jour des listes fauniques et nouveaux gisements de mammifères fossiles. Münchner Geowissenschaftliche Abhandlungen Reihe A: Geologie und Paläontologie 10: 169–188.
Rögl, F. 1998. Palaeogeographic considerations for Mediterranean and Paratethys Seaways (Oligocene to Miocene). Annalen des Naturhistorischen Museums in Wien 99A: 279–310.
Rusu, A. 1970. Corelarea faciesurilor Oligocenului din regiunea Treznea – Bizușa (N-V bazinului Transilvaniei). Studii și cercetări Geologie, Geofizică, Geografie, seria Geologie 15 (2): 513–525.
Rusu, A. 1987. Ostreina biohorizons in the Eocene of the North-West Transilvania (Romania). In: I. Petrescu, L. Ghergari, N. Mészáros, and E. Nicorici (eds.), The Eocene from the Transylvanian Basin, Cluj-Napoca, Romania, 175–182. University of Cluj-Napoca, Cluj-Napoca.
Rusu, A. 1995. Eocene formations in the Calata region (NW Transylvania): a critical review. Romanian Journal of Tectonics and Regional Geology 76: 59–72.
Rusu, A., Popescu B., Moisescu V., Ignat V., Marinescu Fl., and Popescu A. 1977. Harta geologică 1 : 50000 29c, folio Meseș, L-34-35-C, Insitutul de Geologie și Geofizică, București.
Schaub, S. 1925. Die hamsterartigen Nagetiere des Tertiärs. Abhandlungen der Schweizerischen Palaeontologischen Gesellschaft 45: 1–114.
Scherler, L., Lihoreau, F., and Becker, D. 2018. To split or not to split Anthracotherium? A phylogeny of Anthracotheriinae (Cetartiodactyla: Hippopotamoidea) and its palaeobiogeographical implications. Zoological Journal of the Linnean Society 185: 487–510. Crossref
Schmidt-Kittler, N. (ed.) 1987. International Symposium on Mammalian Biostratigraphy and Paleoecology of the European Paleogene. Münchner Geowissenschaftliche Abhandlungen Reihe A: Geologie und Paläontologie 10: 1–311.
Schmidt-Kittler, N. 1990. A Biochronologic Subdivison of the European Paleogene Based on Mammals. Report on Results of the Paleogene Symposium Held in Mainz in February 1987. In: E.H. Lindsay, V. Fahlbusch, and P. Mein (eds.), European Neogene Mammal Chronology, 47–50. Springer US, Boston, MA. Crossref
Schmidt-Kittler, N. and Vianey-Liaud, M. 1975. Relations entre les faunes de rongeurs d’Allemagne du Sud et de France pendant l’Oligocène. Compte Rendu de l’Académie des Sciences 281: 511–514.
Sheldon, N.D., Grimes, S.T., Hooker, J.J., Collinson, M.E., Bugler, M.J., Hren, M., Price, G.D., and Sutton, P.A. 2016. Coupling of marine and continental oxygen isotope records during the Eocene–Oligocene transition. Geological Society of America Bulletin 128: 502–510. Crossref
Sigé, B., Hugueney, M., Crochet, J.-Y., Legendre, S., Mourer-Chauviré, C., Rage, J.-C., and Simon-Coinçon, R. 1998. Baraval, nouvelle faune de l’Oligocène inférieur (MP22) des phosphorites du Quercy : apport à la signification chronologique des remplissages karstiques. Bulletin de la Société d’Histoire Naturelle de Toulouse 134: 85–90.
Sheldon, N.D., Grimes, S.T., Hooker, J.J., Collinson, M.E., Bugler, M.J., Hren, M.T., Price, G.D., and Sutton, P.A. 2016. Coupling of marine and continental oxygen isotope records during the Eocene–Oligocene transition. Geological Society of America Bulletin 128: 502–510. Crossref
Śliwińska, K.K., Thomsen, E., Schouten, S., Schoon, P.L., and Heilmann-Clausen, C. 2019: Climate- and gateway-driven cooling of late Eocene to earliest Oligocene sea surface temperatures in the North Sea Basin. Scientific Reports 9: 4458. Crossref
Smith, R. 2003. Les vertébrés terrestres de l’Oligocène inférieur de Belgique (Formation de Borgloon, MP 21): inventaire et interpétation des données actuelles. Coloquios de Palaeontologia Volumen Extraordinario 1: 488–520.
Stehlin, H.G. 1909, Remarques sur les faunules de mammifères des couches Eocènes et Oligocènes du Bassin de Paris. Bulletin de la Société Géologique de France 9 (4): 488–520.
Stucky, R.K. 1992. Mammalian faunas in North America of Bridgerian to early Arikareen “ages” (Eocene and Oligocene). In: D.R. Prothero and W.A. Berggren (eds.), Eocene–Oligocene Climatic and Biotic Evolution, 464-493. Princeton University Press, Princeton. Crossref
Sun, J., Ni, X., Bi, S., Wu, W., Ye, J., Meng, J., and Windley, B.F. 2014. Synchronous turnover of flora, fauna, and climate at the Eocene–Oligocene boundary in Asia. Scientific Reports 4: 7463. Crossref
Tissier, J., Becker, D., Codrea, V.A., Costeur, L., Fărcaş, C., Solomon, A., Venczel, M., and Maridet, O. 2018. New data on Amynodontidae (Mammalia, Perissodactyla) from Eastern Europe: Phylogenetic and palaeobiogeographic implications around the Eocene–Oligocene transition. PLOS ONE 13: e0193774. Crossref
Toumoulin, A., Tardif, D., Donnadieu, Y., Licht, A., Ladant, J.-B., Kunzmann, L., and Dupont-Nivet, G. 2022. Evolution of continental temperature seasonality from the Eocene greenhouse to the Oligocene icehouse—a model-data comparison. Climate of the Past 18: 341–362. Crossref
Tramoy, R., Salpin, M., Schnyder, J., Person, A., Sebilo, M., Yans, J., Vaury, V., Fozzani, J., and Bauer, H. 2016. Stepwise palaeoclimate change across the Eocene–Oligocene transition recorded in continental NW Europe by mineralogical assemblages and δ15Norg (Rennes Basin, France). Terra Nova 28 (3): 212–220. Crossref
Trif, N. and Codrea, V.A. 2019. Batoid rays from the Oligocene of Suceag (Transylvanian Basin), Romania. In: T. Smith and A. Folie (eds.), Paleurafrica Evolution and Paleoenvironment of Early Modern Vertebrates during the Paleogene, 52. Royal Belgian Institute of natural Sciences, Brussels.
Tsubamoto, T., Takai, M., and Egi, N. 2004. Quantitative analyses of biogeography and faunal evolution of middle to late Eocene mammals in East Asia. Journal of Vertebrate Paleontology 24: 657–667. Crossref
Uhlig, U. 1999. Die Rhinocerotoidea (Mammalia) aus der unteroligozänen Spaltenfüllung Möhren 13 bei Treuchtlingen in Bayern. Abhandlungen der Bayerische Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse, Neue Folge 170: 1–254.
Ünay-Bayraktar, E. 1989. Rodents from the middle Oligocene of Turkish Thrace. Utrecht Micropaleontological Bulletins Special Publications 5: 1–119.
Valverde, J.A. 1964. Remarques sur la structure et l’évolution des communautés de vertébrés terrestres. I. Structure d’une communauté. II. Rapports entre prédateurs et proies. La Terre et la Vie 111: 121–153. Crossref
Vandenberghe, N., Hilgen, F.J., Speijer, R.P., Ogg, J.G., Gradstein, F.M., Hammer, O., Hollis, C.J., and Hooker, J.J. 2012. The Paleogene period. In: F.M. Gradstein, J.G. Ogg, M.D. Schmitz, and G.M. Ogg (eds.), The Geologic Time Scale, 855–921. Elsevier, Amsterdam. Crossref
Venczel, M. 2023. Updating the fossil record of the alligatoroid crocodilian Diplocynodon from the late Eocene of Transylvanian Basin. Frontiers Amphibian Reptile Science 1: 1217025. Crossref
Venczel, M., Codrea V.A., and Fărcaș C. 2013. A new palaeobatrachid frog from the early Oligocene of Suceag, Romania. Journal of Systematic Palaeontology 11: 179–189. Crossref
Venczel, M. and Codrea, V.A. 2018. A new proteid salamander from the early Oligocene of Romania with notes on the paleobiogeography of Eurasian proteids. Journal of Vertebrate Paleontology 38 (5): e1508027. Crossref
Vianey-Liaud, M. 1972. Contribution à l’étude des cricetidés oligocènes d’Europe occidentale. Palaeovertebrata 5: 1–44. Crossref
Vianey-Liaud, M. 1979. Evolution des rongeurs à l’Oligocène en Europe Occidentale. Palaeontographica Beiträge zur Naturgeschichte der Vorzeit 166: 136–236.
Vianey-Liaud, M. and Marivaux, L. 2016. Autopsie d’une radiation adaptative: Phylogénie des Theridomorpha, rongeurs endémiques du Paléogène d’Europe – histoire, dynamique évolutive et intérêt biochronologique. Palaeovertebrata 40: e1. Crossref
Vianey-Liaud, M. and Schmid, B. 2009. Diversité, datation et paléoenvironnement de la faune de mammifères oligocène de Cavalé (Quercy, SO France): contribution de l’analyse morphométrique des Theridomyinae (Mammalia, Rodentia). Geodiversitas 31: 909–941. Crossref
Wade, B.S. and Pearson, P.N. 2008. Planktonic foraminiferal turnover, diversity fluctuations and geochemical signals across the Eocene/Oligocene boundary in Tanzania. Marine Micropaleontology 3–4: 244–255. Crossref
Wang, B. 2007. Late Eocene cricetids (Rodentia, Mammalia) from Nei Mongol, China. Vertebrata Palasiatica 45: 195–212.
Wang, B. and Dawson, M.R. 1994. A primitive cricetid (Mammalia: Rodentia) from the middle Eocene of Jiangsu province, China. Annals of the Carnegie Museum 63: 239–256. Crossref
Wang, B. and Meng, J. 1986. Eucricetodon (Rodentia, Mammalia) from the lower Oligocene of Qujing, Yunnan, China. Vertebrata Palasiatica 24 (2): 110–122.
van de Weerd, A.A., de Bruijn, H., Marković, Z., and Wessels, W. 2018. Paracricetodontinae (Mammalia, Rodentia) from the late Eocene and early Oligocene of south-east Serbia. Palaeobiodiversity and Palaeoenvironments 98: 489–508. Crossref
van de Weerd, A.A., de Bruijn, H., and Wessels, W. 2023. A small assemblage of early Oligocene rodents and insectivores from the Sivas basin, Turkey. Palaeobiodiversity and Palaeoenvironments 103: 609–632. Crossref
Weppe, R., Condamine, F.L., Guinot, G., Maugoust, J., and Orliac, M.J. 2023. Drivers of the artiodactyl turnover in insular western Europe at the Eocene–Oligocene Transition. Proceedings of the National Academy of Sciences of the United States of America 120: e2309945120. Crossref
Weppe, R., Blondel, C., Rémy, J. A., Antoine, P. O., Pelissié, T., Vautrin, Q., and Lihoreau, F. 2024. Valbro: un nouveau site à vertébrés de l’Oligocène inférieur (MP22) de France (Quercy). V–Euongulés. Annales de Paléontologie 110 : 102678. Crossref
Woodburne, M.O., Goin, F. J., Bond, M., Carlini, A.A., Gelfo, J. N., López, G. M., Iglesias, A., and Zimicz, A. N. 2014. Paleogene land mammal faunas of South America; a response to global climatic changes and indigenous floral diversity. Journal of Mammalian Evolution 21: 1–73. Crossref
Zachos, J.C., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate, 65 Ma to Present. Science 292: 686–693. Crossref
Ziegler, R. and Storch, G. 2008. Mammals from the Cyrena Beds of Offenbach (Hesse)—biostratigraphic correlation. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 248: 267–278.
Acta Palaeontol. Pol. 70 (2): 291–327, 2025
https://doi.org/10.4202/app.01234.2024