


New light on the trophic ecology of Carcharodon hastalis from teeth embedded in Miocene cetacean vertebrae from Calvert Cliffs in Maryland, USA
STEPHEN J. GODFREY, VICTOR J. PEREZ, MARCUS JONES, PHILLIP F. CHAPMAN, NATHAN SPENCER, and JASON E. OSBORNE
Godfrey, S.J., Perez, V.J., Jones, M., Chapman, P.F., Spencer, N., and Osborne, J.E. 2025. New light on the trophic ecology of Carcharodon hastalis from teeth embedded in Miocene cetacean vertebrae from Calvert Cliffs in Maryland, USA. Acta Palaeontologica Polonica 70 (2): 329–337.
Recent isotopic analyses of the teeth of the extinct lamnid Carcharodon hastalis showed that it fed at a comparable trophic level as was the fossil and modern great white shark, Carcharodon carcharias. Although there are many examples of shark bite marks on marine mammal bones, there have not been any publications documenting the presence of C. hastalis teeth embedded in the bones of marine mammals. Here we report on the first C. hastalis teeth found embedded in vertebrae of two Miocene cetaceans. These teeth represent unequivocal evidence of trophic interactions between this shark and cetaceans. It is not known if these interactions were the result of active predation or scavenging. These embedded C. hastalis teeth offer supporting evidence to the aforementioned isotopic findings. The finding of C. hastalis teeth embedded in cetacean vertebrae demonstrate that in the Carcharodon lineage, serrated teeth were not a prerequisite to feeding on marine mammals. Carcharodon hastalis may have fed on marine mammals for millions of years prior to the evolution of lightly serrated teeth in its chronospecific descendent, Carcharodon hubbelli. The behavioral adaptation to mammalophagy in the Carcharodon lineage, regardless as to how inefficient it might have been without serrated teeth, appears to have occurred for millions of years prior to the evolution of fully serrated teeth in Carcharodon carcharias. That feeding behavior may well have given natural selection sufficient time to develop and hone the serrated teeth now seen in extant great white sharks (C. carcharias). Given that competition for high trophic resources between the Carcharodon and Otodus lineages seemingly existed for millions of years prior to the extinction of Otodus megalodon, it seems that competition alone is likely not the only explanation for why O. megalodon went extinct.
Key words: Elasmobranchii, Carcharodon hastalis, Cetacea, cetacean vertebrae, trophic interaction, Miocene, Maryland.
Stephen J. Godfrey [Stephen.Godfrey@calvertcountymd.gov; ORCID: https://orcid.org/0000-0002-7916-8791 ], Maryland Paleontology Collection and Research Center, Calvert Marine Museum, P.O. Box 97, Solomons, Maryland, 20688 USA. Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, DC, 20013-7012 USA; Vertebrate Paleontology, Natural History Museum of Los Angeles, 900 Exposition Boulevard, Los Angeles, California, 90007 USA.
Victor J. Perez [vjperez@smcm.edu; ORCID: https://orcid.org/0000-0002-0041-7151 ], St. Mary’s College of Maryland, St. Mary’s City, Maryland, 20686 USA; Maryland Paleontology Collection and Research Center, Calvert Marine Museum, P.O. Box 97, Solomons, Maryland, 20688 USA.
Marcus Jones [paleomarcjones@gmail.com; ORCID: https://orcid.org/0009-0003-9012-1438 ], Maryland Paleontology Collection and Research Center, Calvert Marine Museum, P.O. Box 97, Solomons, Maryland, 20688 USA.
Phillip F. Chapman [phillip.f.chapman@dartmouth.edu; ORCID: https://orcid.org/0009-0001-9810-1736 ], Materials Science and Engineering, Johns Hopkins University, Baltimore, Maryland. Thayer School of Engineering, Dartmouth College, 15 Thayer Drive, Hanover, New Hampshire, 03755 USA (current address).
Nathan Spencer [Nathan@syGlass.io; ORCID: https://orcid.org/0009-0001-9917-4670 ] and Jason E. Osborne [Jason@ syGlass.io; ORCID: https://orcid.org/0009-0008-4748-4352 ], syGlass, 1405 Earl Core Road PMB 1070, Morgantown, West Virginia, 26506 USA.
Received 23 January 2025, accepted 22 April 2025, published online 16 June 2025.
Copyright © 2025 S.J. Godfrey et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Recent analyses of zinc and nitrogen isotopes in fossil and extant sharks have revealed some interesting trophic patterns, especially among apex predators in the Carcharodon and Otodus lineages (Kast et al. 2022; McCormack et al. 2022). Zinc isotopes showed overlapping trophic positions for Carcharodon hastalis Agassiz, 1843, and Carcharodon carcharias (Linnaeus, 1758), indicating that both species were feeding on high-trophic level prey (e.g., cetaceans), despite differences in their dental morphology (McCormack et al. 2022). Similarly, nitrogen isotopes showed that, during the Miocene, C. hastalis was feeding at a comparable trophic level to Pliocene and modern C. carcharias, however, in the early Pliocene, C. hastalis had a lower trophic position indicative of greater emphasis on piscivory (Kast et al. 2022). These discoveries offer insight into the evolution of the Carcharodon lineage, as well as the extinction of C. hastalis and Otodus megalodon (Agassiz, 1835). Many researchers have proposed that competition between C. carcharias and O. megalodon was likely a contributing factor to the extinction of O. megalodon (e.g., Pimiento et al. 2016; Boessenecker et al. 2019; Shimada et al. 2025); however, the fossil record and isotopic data indicate that competition between these lineages predates the evolution of C. carcharias.
Previous studies have offered some evidence for the dietary preferences of C. hastalis, indicating both piscivory and mammalophagy (e.g., Takakuwa 2014; Collareta et al. 2017). The dietary differences observed in C. hastalis may reflect an ontogenetic shift in diet, geographic variability in diet, and/or temporal variability in diet. Collareta et al. (2017) described compelling evidence that, as juveniles, C. hastalis were at a minimum feeding on fish based on gut contents preserved in a partial skeleton of C. hastalis from Peru. That juvenile C. hastalis were piscivorous comes as no surprise, given that they share similar tooth-shape and dietary ontogenetic shifts (e.g., changes in trophic level from mesopredator to apex predator) as in extant C. carcharias (Estrada et al. 2006; Kim et al. 2012).
For adult C. hastalis to have zinc and nitrogen isotopic values within the range of those of fossil and extant C. carcharias, it would have had to consume organisms high on the trophic scale like marine mammals, either by active predation or, at a minimum, by regular scavenging. Although there are publications that implicate, by the presence of associated teeth and bite marks, the consumption of marine mammals by members of Carcharodon (Cigala Fulgosi 1990; Bianucci et al. 2000, 2010; Aguilera et al. 2008; Ehret et al. 2009, 2012; Govender and Chinsamy 2013), there is only one study in which a large number of C. hastalis teeth were found associated with a balaenopterid whale skeleton from the Upper Miocene Pisco Formation of Peru, strongly suggesting that it was at a minimum scavenging the whale (Takakuwa 2014).
Hitherto, there are no publications in which teeth of C. hastalis have been found embedded in the fossilized remains of a marine mammal, offering undeniable evidence of mammalophagy. Here we report on the first C. hastalis teeth found embedded in two vertebrae from Miocene cetaceans found along Calvert Cliffs, Maryland, USA (Fig. 1). These embedded C. hastalis teeth represent unequivocal evidence of trophic interactions between this Miocene shark and cetaceans. However, it is not known if the interactions represent active predation or scavenging. Either way, these specimens show that serrated teeth were not a prerequisite for feeding on marine mammals, and that competition between C. hastalis and O. megalodon quite likely existed well before the evolution of C. carcharias.
Institutional abbreviations.—CMM-V-, Vertebrate Paleontology Collection at the Maryland State Paleontology Collection and Research Center, Calvert Marine Museum, Solomons, Maryland, USA.
Other abbreviations.—Ma, mega annum; SZ, Shattuck-Zones (the informal lithologic zones of Shattuck 1904).
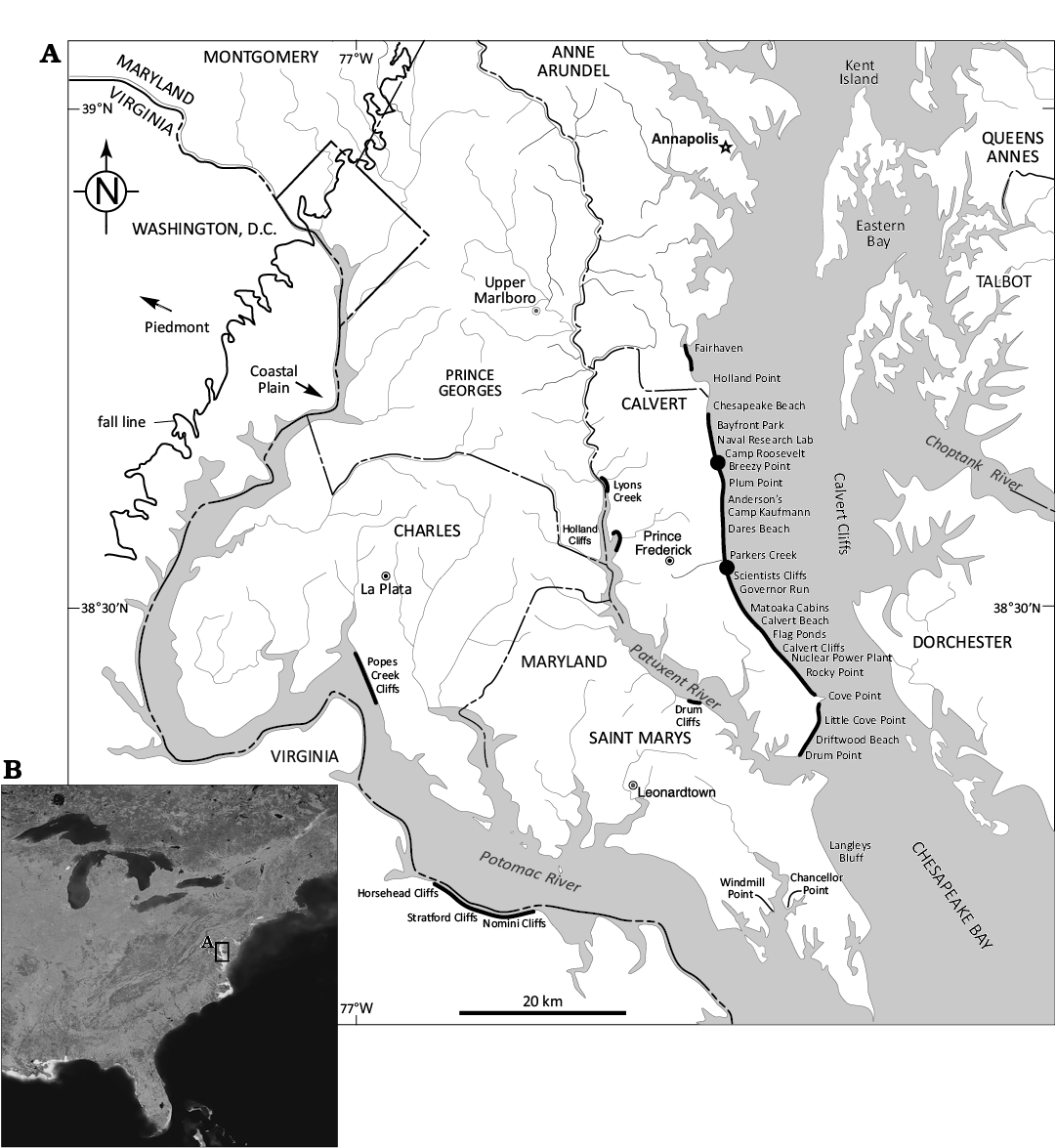
Fig. 1. A. Map of southern Maryland showing locations along Calvert Cliffs (black dots) where the two Carcharodon hastalis tooth-embedded cetacean vertebrae were found. Modified from Vogt et al. 2018. B. Satellite image of the south-eastern quadrant of North America indicating the location of southern Maryland (USA) and Calvert Cliffs along the western shore of Chesapeake Bay.
Material and methods
Micro-Computed Tomography (μ-CT) on CMM-V-11947 was performed at the Johns Hopkins University Materials Characterization and Processing facility. The 2D projection images or radiographs were acquired with a RX Solutions Easy Tom 150/160 μ-CT, using a fixed X-ray system and a rotating sample stage. The X-ray tube voltage was 50KV, and 1440 projections were collected during 360° of rotation. Each projection was produced by averaging 4 frames to reduce random noise. Voxel size was 30.38 µm.
The renderings of the μ-CT data for CMM-V-11947 in Fig. 2B were generated by importing individual image planes from the scan into syGlass virtual reality (VR) software (www.syGlass.io). These 2D image planes were automatically stacked to form a 3D image volume and were rendered in VR using the syGlass direct volume rendering engine. This technique samples the image volume along rays originating from each pixel in the resulting image, applying a transfer function to determine how each sample should affect the result. Windowing and contrast adjustments were made to more clearly represent the boundaries between the embedded tooth structure and the penetrated vertebra, allowing length measurements to be acquired along the tooth. VR controllers were used to place an oblique clipping plane passing through the center of the tooth structure where these measurements were acquired. The 3D stereoscopic rendering allowed for rapid exploration and deeper understanding of both the internal composition of the vertebra and its relationship with the penetrating tooth.
To highlight detail and improve contrast, CMM-V-11947 was whitened (i.e., very lightly dusted) with sublimed ammonium chloride (a whitening technique described by Cooper 1935 and Feldman 1989). After the specimen was photographed with a Nikon CoolPix P510 camera under fluorescent light, the ammonium chloride was removed by holding CMM-V-11947 under running water (Shelburne and Thompson 2016). A freshwater rince is essential to ensure that hydrochloric acid does not form from any residual ammonium chloride and damage the fossil. The images were edited in Adobe Photoshop ® and the figures were compiled in Adobe Illustrator ®.
Results
CMM-V-11947 (Fig. 2) was collected, as float on the beach south of Camp Roosevelt (Fig. 1), Calvert County, Maryland, USA. The specimen was found at approximately N 38º37’ 55.51”, W 76º31’01.81”. Although the specimen was not found in situ in the cliffs immediately adjacent to the beach, there is no reason to think that CMM-V-11947 did not originate from within the vicinal Miocene-age sediments. At that point along Calvert Cliffs, the only Miocene sediments are those from the Plum Point Member of the Calvert Formation (Kidwell et al. 2015; Vogt et al. 2018: fig. 1.12). The sediments comprising this section of the cliffs range in age from approximately 18–14 Ma (Perez et al. 2019: fig. 1). The very top of the cliff includes “upland deposits” or “upland gravels” present throughout southern Maryland (Hack 1955; Schlee 1957) of uncertain (possibly Pleistocene) age, from which no fossil has ever been confirmed to have been found in situ. CMM-V-11947 is consistent with its derivation from the Calvert Formation. Carcharodon hastalis is known to occur throughout the three Miocene formations that comprise Calvert Cliffs (Calvert, Choptank, and St. Marys formations in ascending order) (Kent 2018).
It is not known how long the vertebra was on the beach, from the time it eroded from the adjacent cliff until it was found. Consequently, we do not know to what extent its present state of preservation reflects postmortem taphonomic processes vs. time spent on the beach, potentially being tumbled about in the surf of the modern Chesapeake Bay.
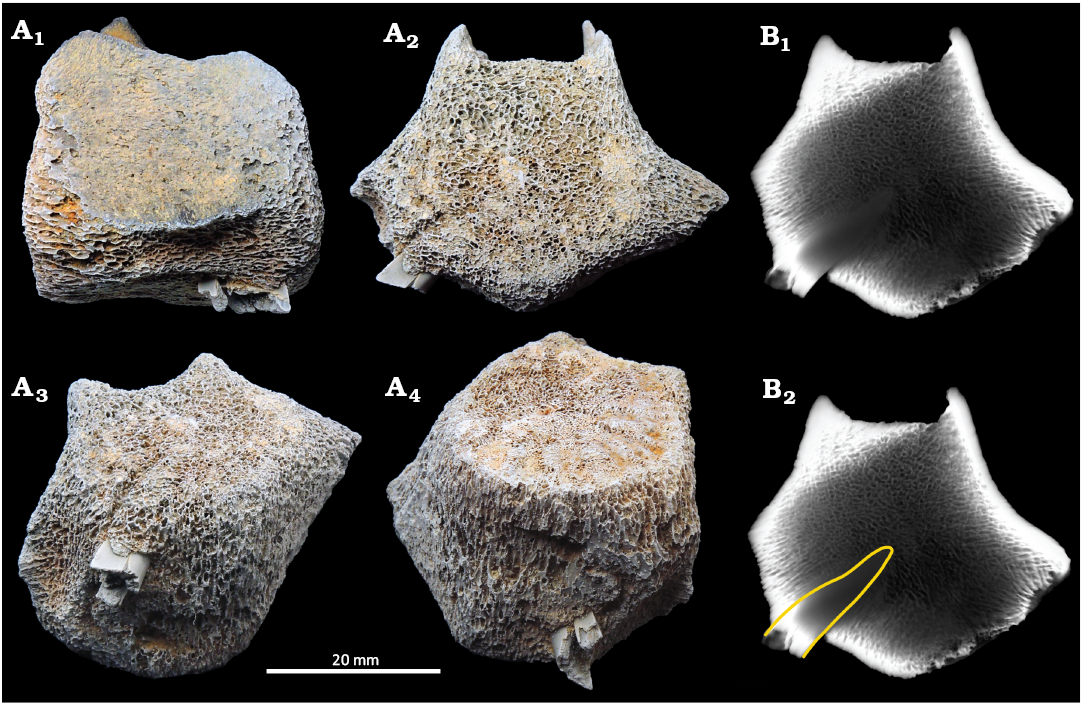
Fig. 2. CMM-V-11947, a small odontocete mammal vertebra in which is embedded a lower anterior tooth of the Miocene lamnid shark, Carcharodon hastalis Agassiz, 1843, Calvert Cliffs, USA, Miocene Calvert Formation. The neural arch, transverse processes, and both epiphyses were not preserved. The embedded C. hastalis tooth is seen protruding from the lower quadrant of the centrum. Vertebra in a left lateral (A1), posterior (A2), and posteroventral (A3) views, oblique anteroventral view from the left (A4). B. μ-CT-scan x-ray image through the vertebra at the level of the embedded C. hastalis tooth (B1). Image with the C. hastalis tooth outlined in yellow (B2). The specimen was very lightly dusted with sublimed ammonium chloride to improve contrast and highlight detail.
The small odontocete vertebra (CMM-V-11947) is incomplete (Fig. 2). Most of the centrum is preserved, less the epiphyses, however, the neural arch and the transverse processes are for the most part also missing. The centrum is 37 mm long and 30.5 mm high. The anterior and posterior articular faces of the vertebra preserve radially oriented ridges of bone indicative that the epiphyses were, at the time of death, not fused to the centrum and not preserved. The neural canal is approximately 15 mm wide at its base. The dorsoventrally thickened bases of the transverse processes originate from the lower half of the centrum. These bases extend for nearly the full length of the centrum. The ventral-most surface of the centrum does not preserve the thin outer layer of cortical compact bone.
The C. hastalis partial tooth is preserved in the lower posterior quadrant of the centrum (Fig. 2). The exposed and broken proximal end of the tooth is conspicuously fractured and ragged. The exposed sharp cutting edges of the tooth are devoid of serrations. The μ-CT images (Fig. 2B) show that the pointed crown of the tooth penetrated the centrum to a depth of 13.4 mm.
CMM-V-4573 (Fig. 3) consists of an isolated cetacean vertebra that was recovered in situ from SZ 15 (Plum Point Member of the Calvert Formation) in the second cliff south of Parkers Creek (Warriors’ Rest, Calvert Cliffs, Calvert County, Maryland, USA, Fig. 1). Approximate GPS coordinates are: N38° 31’ 59.91”, W76° 31’ 01.34”. Along Calvert Cliffs, SZ 15 is approximately 14 Ma (Perez et al. 2019: fig. 1).
The vertebra is mostly complete, lacking only the top of the neural spine and small portions of the distal-most ends of the transverse processes. Both epiphyses are complete and the epiphyseal sutures are obliterated, indicating that this was a mature individual. The centrum is 146 mm long and the vertebra has a maximum width across the transverse processes of 387 mm. The maximum horizontal diameter of the anterior epiphysis is 119 mm.
The embedded C. hastalis partial tooth is located on the right side of the vertebra where the upper surface of the transverse process meets the centrum (Fig. 3). From that portion of the tooth that is protruding from the vertebra, the sharp cutting edge of the tooth is seen to be without serrations (Fig. 3B1). The cross-sectional shape of the tooth is lenticular (Fig. 3B2). The size, shape, and lack of serrations on the embedded tooth matches that of lower anterior or lateral teeth in C. hastalis (Kent 2018: fig. 2.11).
Discussion
Identity of the cetacean.—Vertebra CMM-V-11947 (Fig. 2), belongs to a small odontocete based on the following combination of features; its small size, it is amphiplatyan, there is only a very thin outer layer of cortical bone (much of which was not preserved), and the trabecular bone comprising most of the centrum consists of uniformly low-density bone. The vertebra is thought to be from the lumbar region because in anterior or posterior view (Fig. 2A2), the outline of the centrum approximates a pentagon (Godfrey and Lambert 2023: fig. 2.39F). The top side of the pentagon is formed by the horizontal surface between the broken base of the neural spine. The other four sides of the pentagon angle ventrolaterally or dorsolaterally (two upper and two lower respectively) forming the dorsal and ventral surfaces of the incompletely preserved transverse processes. That the bases of the transverse processes are low on the centrum is also characteristic of vertebrae in the lumbar series. There are no articular facets suggesting that this vertebra held haemal arches. However, the centrum is still too poorly preserved to attempt a more specific identification.
From its size and shape, CMM-V-4573 (Fig. 3) is probably mysticete in origin; being too large to have come from one of the Calvert odontocetes (Godfrey and Lambert 2023), with the exception of Squalodon whitmorei (Dooley 2005: table 4), although the vertebrae of the physeteroid Orycterocetus crocodilinus Cope, 1867, are not yet known (Kellogg 1965). Of the mysticetes known from the Calvert Formation for which lumbar vertebrae are also known (these include Parietobalaena palmeri Kellogg, 1924, Pelocetus calvertensis Kellogg, 1965, Diorocetus hiatus Kellogg, 1968, and Atlantocetus patulus (Kellogg, 1968), CMM-V-4573 is most like those of Atlantocetus patulus (Kellogg 1968b: pl. 63: 2, 3, 5, 6), in that their transverse processes slant ventrally. However, since most Miocene mysticete lumbar vertebrae are seemingly so conservative morphologically, we do not make this taxonomic assignment with great confidence. A single vertebra is not sufficient basis, in this situation, on which to make a definitive taxonomic assignment beyond cetacean.
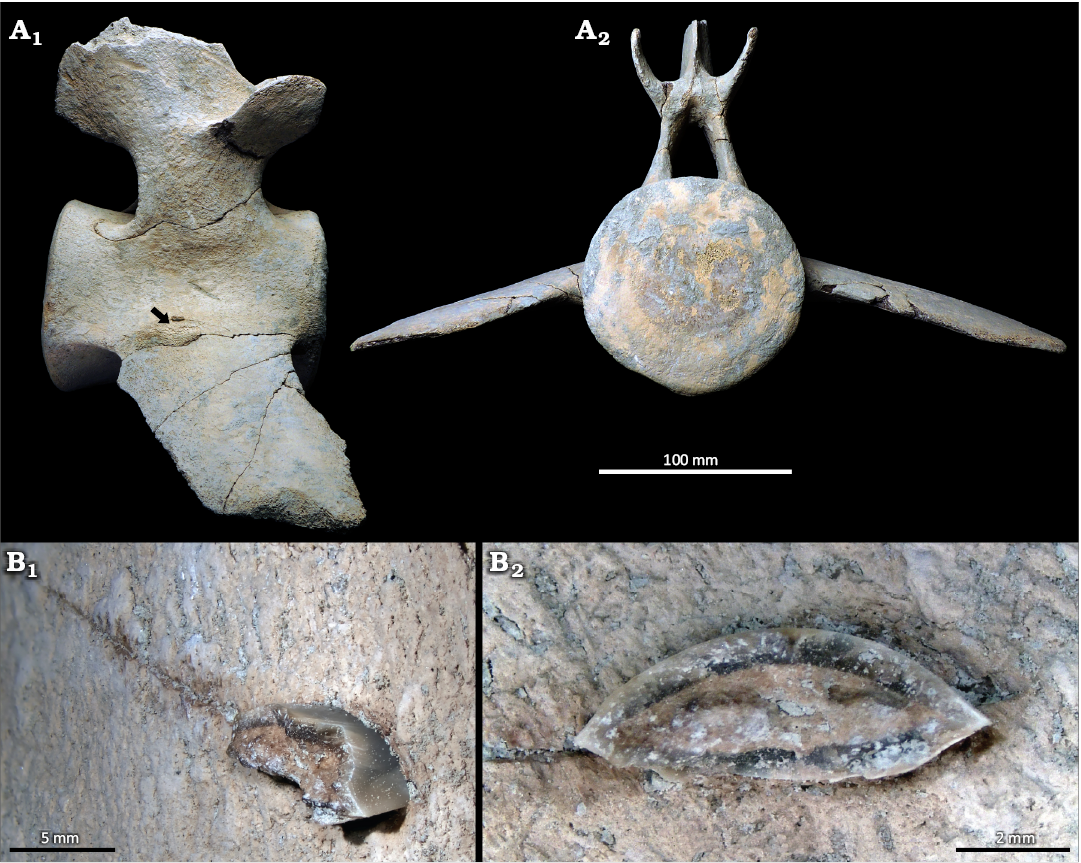
Fig. 3. A. CMM-V-4573, most of a cetacean lumbar vertebra from which the top of the neural spine is missing, from Calvert Cliffs, USA, Miocene Calvert Formation. A1, vertebra in right lateral view, the tip of the black arrow is pointing to the location of the embedded apex of a lamnid shark Carcharodon hastalis Agassiz, 1843, tooth. A2, anterior view. B. Broken end of the C. hastalis tooth showing its smooth cutting edge. B1, an oblique anteromedial view. B2, magnified view of the embedded tooth showing its biconvex cross-sectional shape.
Identity of the shark teeth.—The embedded teeth preserve several features that confirm their identity as representative of the Neogene lamnid shark, Carcharodon hastalis, including their overall size and symmetry, cutting edge lacking serrations, biconvex cross-section, and osteodont histotype (Kent 2018). The elongate narrow crown of CMM-V-11947 (Fig. 2) is undoubtedly a lower anterior tooth, based on comparisons with associated dentitions of C. hastalis (Kent 2018; Perez et al. 2021: fig. 5A). The tooth position of CMM-V-4573 within its originating jaw is less certain, given that a smaller portion of the tooth is visible; however, it seems likely that it also represents a lower tooth based on the cross-sectional shape of the crown and its position within the cetacean vertebra.
It is worth noting that there are two other known Neogene lamnid species that occur in these deposits, with somewhat similar, non-serrated dental morphologies: Isurus oxyrinchus Rafinesque, 1810, and Isurus retroflexus Agassiz, 1843, (Kent 2018). However, in comparison to C. hastalis, the crowns of Isurus oxyrinchus teeth are generally less robust and narrower, exhibiting greater asymmetry and labio-lingual recurvature. Likewise, in comparison to C. hastalis, the crowns of Isurus retroflexus teeth are more labio-lingually compressed. Other morphological differences are present between these species, but they are irrelevant to this study due to the fragmentary nature of the embedded teeth.
The size and cross-sectional shape of the embedded teeth also differs sufficiently from those of Parotodus benedeni Le Hon, 1871, to preclude their origin from that rare otodontid. While the embedded teeth are fragmentary and significant portions are obscured within the vertebrae, the portions preserved correspond best with C. hastalis based on our qualitative observations.
Ecological and macroevolutionary insights.—Within chondrichthyans, historically, distinct tooth morphologies have been used to infer prey preferences. In general, sharks that engage predominantly in piscivory have teeth that are slender and elongated, with smooth cutting edges (grasping-type teeth), whereas most generalist and macropredatory sharks have teeth that are broadly triangular and flat, with complete cutting edges that are often serrated (Moss 1977; Frazzetta 1988; Kent 1994; Whitenack and Motta 2010; Whitenack et al. 2011; Cappetta 2012; Corn et al. 2016; Huber et al. 2019; Perez 2022). Carcharodon hastalis somewhat blurs the distinction by possessing a dignathic heterodont dentition, with slender and elongate lower teeth (grasping-type teeth) and broadly triangular, flat upper teeth with complete, unserrated cutting edges (cutting-type teeth). Perez (2022) attributed this combination of dental morphologies to the grasping-cutting type dentition, with a corresponding generalist dietary behavior “capable of active predation and scavenging on a wide variety of prey”.
Despite this, due to the lack of serrated teeth, it has generally been assumed that marine mammals were not the primary prey items for C. hastalis. In fact, Ehret et al. (2012: 1150) described C. hastalis as piscivorous. Collareta et al. (2017) offered additional support for this describing the articulated partial remains of a juvenile extinct lamnid shark Carcharodon (their Cosmopolitodus) hastalis. Remarkably, remains of fish (a pilchard identified as Sardinops sp. cf. S. sagax Jenyns, 1842) were found in the abdominal region of the shark. Based on the location, individual proximity, and orientation of preservation of the fish remains, Collareta et al. (2017: fig. 2) interpreted them as the fossilized stomach contents of the shark. Their conclusions were compelling and piscivory was demonstrated for the first time in the fossil record in a juvenile C. hastalis. Additional support for their conclusions comes from the preference for piscivory in immature individuals of extant lamniform sharks (like Carcharodon carcharias and Isurus oxyrinchus). Therefore, at least as juveniles, C. hastalis were evidently and not surprisingly feeding on fish, just like their presumed descendant, C. carcharias. The default expectation would be that, at least as juveniles, C. hastalis were piscivorous; however, the question remains whether this dietary preference was maintained into adulthood, or if C. hastalis shared a similar ontogenetic shift in dietary preference to the inclusion of marine mammals as in the extant C. carcharias and Isurus oxyrinchus (Castro 2011).
Multiple cases of fossil cetacean bones with tooth marks, presumably made by C. hastalis, are now known, adding to the growing body of taphonomic insights suggesting widespread mammalophagy in C. hastalis (Bianucci et al. 2010, 2018; Takakuwa 2014; Bosio et al. 2021; Godfrey and Lowry 2021) prior to the evolution of serrated teeth in C. carcharias. To which are added the nitrogen and zinc isotopic values in C. hastalis indicating their relatively high trophic feeding level (Kast et al. 2022; McCormack et al. 2022), and now the presence of C. hastalis teeth embedded in cetacean vertebrae. Consequently, this offers relatively strong support for an ontogenetic dietary shift in C. hastalis from possibly exclusive piscivory, to piscivory and at least some measure of marine mammalophagy. Although most researchers would likely agree that C. hastalis was capable of feeding on marine mammals, there is likely no consensus as to the degree to which mammals contributed to their regular diet. Not even C. carcharias is exclusively mammalophagous. It remains a generalist predator also feeding on, for example, cephalopods, elasmobranchs, and other fishes (Pethybridge et al. 2014). However, the nitrogen and zinc isotopic analyses show that, during the Miocene, C. hastalis was high on the trophic ladder, with values comparable to that of both fossil and extant C. carcharias (Kast et al. 2022; McCormack et al. 2022). Thus, C. hastalis and C. carcharias likely shared similar dietary compositions, despite the acquisition of serrated teeth in C. carcharias. The fact that C. hastalis nitrogen isotopic values decreased during the Pliocene may indicate that competition with C. carcharias forced C. hastalis to rely more on piscivory (Kast et al. 2022), eventually leading to their extinction as the higher ecological niche was replaced and more firmly occupied by C. carcharias.
That Miocene C. hastalis were feeding on marine mammals indicates that the behavior to consume marine mammals long preceded the acquisition of serrated teeth. No matter how inefficient it might have been for C. hastalis to feed on cetaceans without serrated teeth, by so doing for millions of years, arguably enabled natural selection to shape and hone, at first the lightly serrated teeth of Carcharodon hubbelli Ehret, MacFadden, Jones, Devries, Foster, & Salas-Gismondi, 2012, and then transforming those into the fully serrated teeth of C. carcharias. The behavioral adaptation of mammalophagy preceded the morphological tooth adaptation/transformation, but the behavioral component of this long-term evolutionary adaptation allowed for directional selection of presumably an ever more efficient lightly serrated (i.e., C. hubbelli), then boldly serrated tooth (i.e., C. carcharias) in this macropredatory lineage.
It is well known that the diet of C. carcharias changes as it matures (Estrada et al. 2006; Kim et al. 2012; Le Croizier 2024). Young great whites feed predominantly on fish. However, as they mature, they variably but increasingly hunt and/or scavenge a greater diversity of prey including marine mammals (seals, sea lions, whales, and dolphins). The bitten cetacean vertebrae described herein now indicate that the diet of C. hastalis, at least occasionally, also changed or diversified during ontogeny to include cetaceans, all in the absence of serrated teeth. Likewise, a partial tooth of C. hubbelli was found embedded in a mysticete whale mandible from the Upper Miocene of Peru, thus providing direct evidence that they too as adults were taking marine mammals as prey as early as c. 6.5 Ma (Ehret et al. 2009, 2012). Therefore, the ontogenetic dietary shift from piscivory to marine mammalophagy within the Carcharodon lineage has been going on for at least 14 million years and perhaps longer (based on the age of the two Miocene vertebrae described herein).
The dietary habits of the extant shortfin mako, Isurus oxyrinchus, offer additional evidence that serrations are not a prerequisite for feeding on marine mammals. While the dental morphology of I. oxyrinchus teeth is suggestive of piscivory, previous studies of stomach contents have documented a wide range of prey items, including crustaceans, cephalopods, teleost fish, other elasmobranchs, sea turtles, sea birds, and cetaceans (e.g., Castro 2011; Calle-Morán et al. 2023; Velasco-Tarelo et al. 2024). For most individuals, squid and fish comprise most of their diet, but variation exists geographically, across sexes, and through ontogeny. Feeding on marine mammals seems to be restricted to the larger individuals or as rare instances of opportunistic scavenging by smaller individuals. Furthermore, C. hastalis was larger in body size than contemporary mako sharks (I. oxyrinchus and I. retroflexus), which might have facilitated pre-serration mammalophagy.
It has been suggested that the evolution of C. carcharias contributed to the demise of O. megalodon during the Pliocene (~3.6 Ma) because of increased competition for the limited resource of marine mammals (Boessenecker et al. 2019; Shimada et al. 2025). However, based on CMM-V-11947 (Fig. 2) and CMM-V-4573 (Fig. 3), competition between the Carcharodon and Otodus lineages could have existed well before the Pliocene. Pimiento et al. (2016) proposed that O. megalodon’s path to extinction began in the Miocene based on an apparent decline in the total abundance of O. megalodon teeth found globally. Further, while the nitrogen isotopic values of C. hastalis decreased during the Pliocene, these values actually increased slightly from the Miocene to the Pliocene in O. megalodon (Kast et al. 2022). This indicates that O. megalodon maintained its uniquely high trophic position, despite potential competition with C. carcharias. Thus, while competition with the Carcharodon lineage still may have been a contributing factor to the extinction of O. megalodon, perhaps the abiotic and other biotic factors such as range fragmentation, a cooling global climate, or a decline in cetacean diversity, played a larger (or synergistic) role in the ultimate demise of O. megalodon.
Concluding remarks
This is the first report of teeth from the Miocene lamnid shark, Carcharodon hastalis, embedded in the bones of marine mammals, specifically cetaceans. It confirms that C. hastalis was, at least as adults, feeding on marine mammals. This direct fossil evidence corroborates recent isotopic findings that C. hastalis was feeding relatively high on the trophic scale, comparable to fossil and modern C. carcharias. Furthermore, the ontogenetic dietary shift from piscivory to marine mammalophagy within the Carcharodon lineage has been going on for at least 14 million years and perhaps longer. Thus, mammalophagy in the Carcharodon lineage predates the evolution of serrated teeth. The behavioral adaptation to mammalophagy in this shark lineage gave natural selection millions of years in which to develop and hone the serrated teeth now seen in extant great white sharks. This also implies that competition between the Carcharodon and Otodus lineages likely occurred for millions of years before the eventual extinction of C. hastalis and the Otodus lineage, so perhaps competition between these lineages was not the primary driving factor in the ultimate demise of O. megalodon.
Editor: Eli Amson.
Acknowledgements
CMM-V-4573 and CMM-V-11947 were found and donated to CMM by Mike Ellwood (Port Republic, USA) and Marcus Jones (Prince Frederick, USA), respectively. CMM Research Associate Stephen Groff is gratefully thanked for generating the stacked image in Fig. 3B1. We also thank CMM Paleontology Collections Manager John R. Nance for encouraging access to specimens in his care. We greatly appreciate the valuable input and positive feedback provided by reviewers Robert (“Bobby”) W. Boessenecker (University of California Museum of Paleontology, Berkeley, USA) and Alberto Collareta (Università di Pisa, Italy), and the journal editorial team for moving this manuscript through the publication process. This research was funded in part by the citizens of Calvert County Maryland, the County Board of Calvert County Commissioners, the Clarissa and Lincoln Dryden Endowment and the Duvel-Tuve-Burris Endowment for Paleontology at the Calvert Marine Museum for helping fund a summer internship for MJ.
References
Aguilera, O.A, García, L., and Cozzuol, M.A. 2008. Giant-toothed white sharks and cetacean trophic interaction from the Pliocene Caribbean Paraguaná Formation. Paläontologische Zeitschrift 82: 204–208. Crossref
Bianucci, G., Bisconti, M., Landini, W., Storai, T., Zuffa, M., Giuliani, S., and Mojetta, A. 2000. Trophic interactions between white sharks (Carcharodon carcharias) and cetaceans: a comparison between Pliocene and recent data. In: M. Vacchi, G. La Mesa, F. Serena, and B. Sèret (eds.), Proceedings 4th Meeting of the European Elasmobranc Association, Livorno (Italy). 27–30 September 2000, 33–48. Imprimerie F. Paillart, Abbeville.
Bianucci, G., Collareta, A., Bosio, G., Landini, W., Gariboldi, K., Gioncada, A., Lambert, O., Malinverno, E., de Muizon, C., Varas-Malca, R., Villa, I.M., Coletti, G., Urbina, M., and Di Celma, C. 2018. Taphonomy and palaeoecology of the lower Miocene marine vertebrate assemblage of Ullujaya (Chilcatay Formation, East Pisco Basin, southern Peru). Palaeogeography, Palaeoclimatology, Palaeoecology 511: 256–279. Crossref
Bianucci, G., Sorce, B., Storai, T., and Landini, W. 2010, Killing in the Pliocene: shark attack on a dolphin from Italy. Palaeontology 53: 457–470. Crossref
Bosio, G., Collareta, A., Di Celma, C., Lambert, O., Marx, F.G., de Muizon, C., Gioncada, A., Gariboldi, K., Malinverno, E., Malca, R.V., Urbina, M., and Bianucci, G. 2021. Taphonomy of marine vertebrates of the Pisco Formation (Miocene, Peru): Insights into the origin of an outstanding Fossil-Lagerstätte. PLoS ONE 16 (7): e0254395. Crossref
Boessenecker, R.W., Ehret, D.J., Long, D.J., Churchill, M., Martin, E., and Boessenecker, S.J. 2019. The Early Pliocene extinction of the mega-toothed shark Otodus megalodon: a view from the eastern North Pacific. PeerJ 7: e6088. Crossref
Calle-Morán, M.D., Erazo-Garcés, H.M., Hernández-Téllez, A.R., Galván-Magaña, F. and Estupiñán-Montaño, C. 2023. Feeding ecology of the shortfin mako shark, Isurus oxyrinchus, in the Ecuadorian Pacific Ocean. Journal of the Marine Biological Association of the United Kingdom 103: e96. Crossref
Cappetta, H. 2012. Handbook of Paleoichthyology, Volume 3E: Chondrichthyes · Mesozoic and Cenozoic Elasmobranchii: Teeth. 512 pp. Verlag Dr. Friedrich Pfeil, Munich.
Castro, J.I. 2011. The Sharks of North America. 612 pp. Oxford University Press, New York.
Cigala Fulgosi, F. 1990 Predation (or possible scavenging) by a great white shark on an extinct species of bottlenosed dolphin in the Italian Pliocene. Tertiary Research 12: 17–36.
Collareta, A., Lambert, O., Landini, W., Di Celma, C., Malinverno, E., Varas-Malca, R., Urbina, M., and Bianucci, G. 2017. Did the giant extinct shark Carcharocles megalodon target small prey? Bite marks on marine mammal remains from the late Miocene of Peru. Palaeogeography, Palaeoclimatology, Palaeoecology 469: 84–91. Crossref
Cooper, C.L. 1935. Ammonium chloride sublimate apparatus. Journal of Paleontology 9: 357–359.
Corn, K.A., Farina, S C., Brash, J., and A.P. Summers. 2016. Modelling tooth-prey interactions in sharks: the importance of dynamic testing. Royal Society Open Science 3 (8): 160141. Crossref
Dooley, A.C. 2005. A new species of Squalodon (Mammalia, Cetacea) from the Middle Miocene of Virginia. Virginia Museum of Natural History Memoir 8: 1–43.
Ehret, D.J., MacFadden, B.J., Jones, D.S., DeVries, T.J., and Salas-Gismondi, R. 2009. Caught in the act: trophic interactions between a 4-million-year-old white shark (Carcharodon) and mysticete whale from Peru. Palaios 24: 329–333. Crossref
Ehret, D J., MacFadden, B.J., Jones, D.S., Devries, T.J., Foster, D.A., and Salas-Gismondi, R. 2012. Origin of the White Shark Carcharodon (Lamniformes: Lamnidae) based on recalibration of the Upper Neogene Pisco Formation of Peru. Palaeontology 55: 1139–1153. Crossref
Estrada, J.A., Rice, A.N., Natanson, L.J., and Skomal, G.B. 2006. Use of isotopic analysis of vertebrae in reconstructing ontogenetic feeding ecology in white sharks. Ecology 87: 829–834. Crossref
Feldman, R.M. 1989. Whitening fossils for photographic purposes. In: R.M. Feldman (ed.), Paleotechniques. The Paleontological Society Special Publication 4: 342–346. Crossref
Frazzetta, T.H. 1988. The mechanics of cutting and the form of shark teeth (Chondrichthyes, Elasmobranchii). Zoomorphology 108: 93–107. Crossref
Godfrey, S.J. and Lowry, A.J. 2021. The ichnospecies Linichnus bromleyi on a Miocene baleen whale radius preserving multiple shark bite-shake traces suggests scavenging. Carnets de Geologie 21 (17): 391–398. Crossref
Godfrey, S.J. and Lambert, O. 2023. Miocene toothed whales (Odontoceti) from Calvert Cliffs, Atlantic Coastal Plain, USA. Smithsonian Contributions to Paleobiology, Washington 107: 49–186.
Govender, R. and Chinsamy, A. 2013. Early Pliocene (5 Ma) shark-cetacean trophic interaction from Langebaanweg, Western Coast of South Africa. Palaios 28: 270–277. Crossref
Hack, J.T. 1955, Geology of the Brandywine area and origin of the upland of southern Maryland. U.S. Geological Survey Professional Paper 267-A: 1–43. Crossref
Huber, D.R., Wilga, C.D. Dean, M.N., Ferry, L.A., Gardiner, J.M., Habegger, M.L., Papastamatiou, Y.P., Ramsay, J., and Whitenack, L. 2019. Feeding in cartilaginous fishes: an interdisciplinary synthesis. In: V. Bels and I. Whishaw (eds.), Feeding in Vertebrates. Fascinating Life Sciences, 231–295. Springer, Cham. Crossref
Kast, E.R., Griffiths, M.L., Kim, S.L., Rao, Z.C., Shimada, K., Becker, M.A., Maisch, H.M., Eagle, R.A., Clarke, C.A., Neumann, A.N., Karnes, M.E., Lüdecke, T., Leichliter, J.N., Martínez-García, A., Akhtar, A.A., Wang, X.T., Haug, G.H., and Sigman, D.M. 2022. Cenozoic megatooth sharks occupied extremely high trophic positions. Science Advances 8 (25): eabl6529. Crossref
Kellogg, A.R. 1965. A new whalebone whale from the Miocene Calvert Formation. United States National Museum Bulletin 247: 1–45.
Kellogg, A.R. 1968. Fossil marine mammals from the Miocene Calvert Formation of Maryland and Virginia. United States National Museum Bulletin 247: 103–197.
Kent, B.W. 1994. Fossil Sharks of the Chesapeake Bay Region. 146 pp. Egan Rees & Boyer, Columbia, MD.
Kent, B.W. 2018. The cartilaginous fishes (chimaeras, sharks, and rays) of Calvert Cliffs, Maryland, USA. Smithsonian Contributions to Paleobiology 100: 45–157.
Kidwell, S.M., Powars, D.S., Edwards, L.E., and Vogt, P.R. 2015. Miocene stratigraphy and paleoenvironments of the Calvert Cliffs, Maryland. The Geological Society of America, Field Guide 40: 231–279. Crossref
Kim S.L., Tinker M.T., Estes J.A., and Koch P.L. 2012. Ontogenetic and among-individual variation in foraging strategies of northeast Pacific white sharks based on stable isotope analysis. PloS One 7: e45068. Crossref
Le Croizier, G., Hoyos-Padilla, M., Amezcua-Martínez, F., Aquino-Baleytó, M., Besnard, L., Le Grand, F., Le Loc’h, F., Mathieu-Resuge, M., Munaron, J.M., Ory, A., Sardenne, F., Schaal, G., and Lorrain, A. 2024. Can biochemical tracers reveal ontogenetic trophic shift and individual prey selection in white sharks from Guadalupe Island, Northeast Pacific? Environmental Research 262: 119507. Crossref
Moss, M.L. 1977. Skeletal tissues in sharks. American Zoologist 17: 335–342. Crossref
McCormack, J.M. Griffiths, M.L., Kim, S.L., Shimada, K., Karnes, M., Maisch, H., Pederzani, S., Bourgon N., Jaouen, K., Becker, M.A., Jöns, N., Sisma-Ventura, G., Straube, N., Pollerspöck, J., Hublin, J.-J., Eagle, R.A., and Tütken, T. 2022. Trophic position of Otodus megalodon and great white sharks through time revealed by zinc isotopes. Nature Communications 13: 2980. Crossref
Perez, V.J. 2022. The chondrichthyan fossil record of the Florida Platform (Eocene–Pleistocene). Paleobiology 48: 622–654. Crossref
Perez, V.J., Godfrey, S.J., Kent, B.W., Weems, R.E., and Nance, J.R. 2019. The transition between Carcharocles chubutensis and Carcharocles megalodon (Otodontidae, Chondrichthyes): lateral cusplet loss through time. Journal of Vertebrate Paleontology 38 (6): e1546732. Crossref
Perez, V.J., Leder, R.M., and Badaut, T. 2021. Body length estimation of Neogene macrophagous lamniform sharks (Carcharodon and Otodus) derived from associated fossil dentitions. Palaeontologia Electronica 24 (1): a10. Crossref
Pethybridge, H.R., Parrish, C.C., Bruce, B.D., Young, J.W., and Nichols, P.D. 2014. Lipid, fatty acid and energy density profiles of white sharks: Insights into the feeding ecology and ecophysiology of a complex top predator. PLoS ONE 9 (5): e97877. Crossref
Pimiento, C., MacFadden, B.J., Clements, C.F., Varela, S., Jaramillo, C., Velez‐Juarbe, J., and Silliman, B.R. 2016. Geographical distribution patterns of Carcharocles megalodon over time reveal clues about extinction mechanisms. Journal of Biogeography 43: 1645–1655. Crossref
Schlee, J.S. 1957. Upland gravels of Southern Maryland: Geological Society of America Bulletin 68: 1371–1409. Crossref
Shattuck, G.B. 1904. Geological and paleontological relations, with a review of earlier investigations. In: W.B. Clark, G.B. Shattuck, and W.H. Dall (eds.), The Miocene Deposits of Maryland. Maryland Geological Survey Systematic Report, Miocene, V. 1, xxxiii–cxxxvii. The Johns Hopkins Press, Baltimore.
Shelburne, E.C.H. and Thompson, A.C. 2016. Specimen whitening: An assessment of methods of ammonium chloride smoke removal. Collection Forum 30 (1): 63–72. Crossref
Shimada, K., Motani, R., Wood, J.J., Sternes, P.C., Tomita, T., Bazzi, M., Collareta, A., Gayford, J.H., Türtscher, J., Jambura, P.L., Kriwet, J., Vullo, R., Long, D.J., Summers, A.P., Maisey, J.G., Underwood, C., Ward, D.J., Maisch, H.M. IV, Perez, V.J., Feichtinger, I., Naylor, G.J.P., Moyer, J.K., Higham, T.E., da Silva, J.P.C.B., Bornatowski, H., González-Barba, G., Griffiths, M.L., Becker, M.A., and Siversson, M. 2025. Reassessment of the possible size, form, weight, cruising speed, and growth parameters of the extinct megatooth shark, Otodus megalodon (Lamniformes: Otodontidae), and new evolutionary insights into its gigantism, life history strategies, ecology, and extinction. Palaeontologia Electronica 28 (1): a12. Crossref
Takakuwa, Y. 2014. A dense occurrence of teeth of fossil “mako” shark (“Isurus” hastalis: Chondrichthyes, Lamniformes), associated with a balaenopterid-whale skeleton of the Late Miocene Pisco Formation, Peru, South America. Bulletin of the Gunma Museum of Natural History 18: 77–86.
Velasco-Tarelo, P.M., Galván-Magaña, F., and Estupiñán-Montaño, C. 2024. Feeding ontogeny and trophic ecology of the shortfin mako shark, Isurus oxyrinchus, on the west coast of Baja California Sur, Mexico. Regional Studies in Marine Science 77 (1): 103631. Crossref
Vogt, P.R., Eshelman, R.E., and Godfrey, S.J. 2018. Calvert Cliffs: eroding mural escarpment, fossil dispensary, and paleoenvironmental archive in space and time. Smithsonian Contributions to Paleobiology 100: 3–44.
Whitenack, L.B. and Motta, P.J. 2010. Performance of shark teeth during puncture and draw: implications for the mechanics of cutting. Biological Journal of the Linnean Society 100: 271–286. Crossref
Whitenack, L.B., Simkins Jr., D.C., and Motta, P.J. 2011. Biology meets engineering: the structural mechanics of fossil and extant shark teeth. Journal of Morphology 272: 169–179. Crossref
Acta Palaeontol. Pol. 70 (2): 329–337, 2025
https://doi.org/10.4202/app.01241.2025