


Fossilized gill soft tissues in Mesozoic freshwater unionoid bivalves: reinvestigation and new evidence of the evolution of adaptation to the freshwater environment
ALEKSANDRA SKAWINA and RENATO PIRANI GHILARDI
Skawina, A. and Ghilardi, R.P. 2025. Fossilized gill soft tissues in Mesozoic freshwater unionoid bivalves: reinvestigation and new evidence of the evolution of adaptation to the freshwater environment. Acta Palaeontologica Polonica 70 (2): 375–384.
Large freshwater unionoid bivalves today are a globally distributed and diverse group, with a history dating back to the Triassic. Their evolutionary success largely depends on their reproductive behavior, today, they all take maternal care via brooding their embryos, which is only possible in the modified (advanced) eulamellibranch gills, facilitating survival in freshwaters. It can be expected that such an essential trait could have originated in the last common ancestor of advanced Unionida lineages sometime after Carnian, Late Triassic, where unionoids with filibranch gills were known, and before the Middle Jurassic, when advanced unionoids already existed. The gill anatomy of unionoids, because of their calcified gill filaments, enhances exceptional preservation of associated soft gill tissues. New Late Triassic unionoid materials from Poland with gill tissues preserved, Silesunio parvus, Tihkia silesiaca, Tihkia? sp., together with other Mesozoic materials from archival collections from Cretaceous of United Kingdom (“Unio porrectus”) and Brazil (Anodontites freitasi) provide new insights into gill evolution in Unionida. The traits observed during surface investigation supported a filibranch trait in the gill anatomy of S. parvus and Tihkia? sp. indicating the origin of evolving advanced gills took place after the Triassic. Yet, the eulamellibrach anatomy was confirmed only in Late Cretaceous A. freitasi. The preservation of the gills in the remaining specimens studied does not provide new data on the origins of advanced gills in unionoids and its more detailed timing.
Key words: Unionida, brooding, soft tissues, gill anatomy, freshwater, Triassic, Cretaceous, Mesozoic.
Aleksandra Skawina [a.skawina@uw.edu.pl; ORCID: https://orcid.org/0000-0002-8287-1568 ], University of Warsaw, Faculty of Biology, Institute of Evolutionary Biology, ul. Żwirki i Wigury 101, 02089 Warsaw, Poland.
Renato Pirani Ghilardi [renato.ghilardi@unesp.br; ORCID: https://orcid.org/0000-0003-0410-8011 ], São Paulo State University (UNESP), School of Sciences, Lapalma, Av. Eng. Luís Edmundo Carrijo Coube, 2085, CEP 17033-360 Bauru-SP, Brazil.
Received 20 February 2025, accepted 5 June 2025, published online 30 June 2025.
Copyright © 2025 A. Skawina and R.P. Ghilardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Unionoid bivalves (order Unionida) are now an important and highly diverse member of freshwater ecosystems, with almost 1000 living species, from which up to 40% are threatened with extinction (Lopes-Lima et al. 2018; Graf and Cummings 2021). They also have a long evolutionary history dating back to the Triassic (Skawina and Dzik 2011). Their evolutionary success and risk of future extinction might at least partially relate to the fact that larvae of all living species are today obligate parasites of fish (e.g., Bauer and Wachtler 2001), with a few exceptions including no-host development or an amphibian as a host (e.g., Watters and O’Dee 1998). Unionoids take maternal care of their offspring by brooding in the cavities of modified eulamellibranch gills, a character which is independently shared with at least some members of other freshwater bivalve families, e.g., Sphaeridae, Cyrenidae, and some Dreissenidae (Morton and Puljas 2013; Graf 2013; Mansur et al. 2019). Both larval parasitism, and maternal brooding, are widely accepted as adaptations for dispersion and survival in freshwaters. Unfortunately, although unionoid fossils are known from the freshwater deposits of all regions of Pangea since the Triassic prior to the onset of the continental breakup (ca. 200 million years ago; Seton et al. 2012), the questions of how and when these crucial adaptations evolved and how they impacted the evolution of this bivalve group remain to be fully understood.
The parasitic character of the larvae and their brooding in eulamellibranch gills are independent features yet both crucial for the unionoid life cycle (and today present in all the Recent representatives). One may thus hypothesize that these traits existed in the Middle Jurassic last common ancestor of modern lineages of Unionida (Boucot and Poinar 2010; Skawina and Dzik 2011; Graf et al. 2015; Bolotov et al. 2016, 2017; Huang et al. 2018; Skawina 2021). Unfortunately, early evolution of larva in Unionida may remain undetermined, likely because of low fossilization potential of the larva (the oldest fossil unionoid glochidia were discovered in the Miocene by Gross and Piller 2018). The gill anatomy of adult Unionida, on the other hand, has a better preservation potential than their larvae, because of the presence of chitinous gill supports with calcium concretions (“calcified rods” formed from calcium phosphate), a feature present also in the gills of marine trigoniids, closest living relatives and possible ancestors of Unionida (Ridewood 1903; Silverman et al. 1989; Whyte 1991; Richard et al. 1991; Wilby and Whyte 1995; Torrens et al. 2000; Hinzmann et al. 2015). These concretions are known to serve as a source of calcium for mineralization of unionoid offspring shell in calcium-poor freshwater habitats (Silverman et al. 1985, 1987, 1989). Both Trigoniida and Unionida share similarities in general anatomy, shell microstructure, and gill physiology, e.g., ciliary patterns (Tevesz 1975; Morton 1987; Graf and Cummings 2006). Filibranch gill anatomy is present in both fossil and living trigoniids, while only the oldest unionoids share this character with their trigoniid ancestors. All the living members of the Unionida today share the eulamellibranch anatomy of their gills, additionally modified for brooding (Klug et al. 2005; Graf and Cummings 2006; Skawina and Dzik 2011; Skawina 2021).
Eulamellibranch gills have transverse interfilamentar tissue junctions, which connect gill filaments (of which gill supports are their skeleton) side by side within the demibranchs (see for definition in Carter et al. 2012; Knight et al. 2014). Such arrangement allows for a “basket” shape anatomy of the demibranchs to form. In contrast, in filibranch gills, the gill filaments are connected by the ciliary connections only, the so-called “ciliated discs” of interlocking cilia, which facilitate the mechanical disconnection and reconnection of single filaments, and the shuffled arrangement of filaments; see SOM 1: A–G, Supplementary Online Material available at http://app.pan.pl/SOM/app70-Skawina_Ghilardi_SOM.pdf; see Ridewood 1903; Knight et al. 2014; Freitas et al. 2022). The filibranch anatomy of the gills does not exclude larval brooding behavior, but the incubation of embryos is in the mantle (infrabranchial) cavity, not within the gills (Morton 1978; Mackie 1984).
Although the eulamellibranchy provides the crucial ability for unionoids to live in freshwaters to brood the embryos inside their gills, and all Recent members of Unionida share this trait, the oldest putative eulamellibranch gills in this group are currently known from the Senonian, Upper Cretaceous of Brazil (single specimen in Simone and Mezzalira 1993). The Jurassic age of other examples of soft tissue gill preservation in unnamed fossils and unnumbered unionoids was once mentioned by Richard et al. (1991: 1753): “Fossil records contain phosphatized impressions in unionid gills from sample dating back to the Jurassic period. Apparently these fossil bivalves contained calcium concretions and calcified chitinous rods (B. Runyger, British Museum of Natural History, personal communication).” Finally, the phosphatized gills in unionoids were more recently described in two specimens of Silesunio parvus from the Upper Triassic of Poland (Skawina 2010; Skawina and Dzik 2011), but these gills share their filibranch gill anatomy with trigoniid ancestors, and no transverse tissue connections were observed.
In this paper, a new Late Triassic material is described for the first time. This includes new findings of: Carnian Silesunio parvus, Norian Tihkia? sp. and Norian–Rhaetian Tihkia silesiaca specimens. These specimens join the two previously described specimens of S. parvus (ZPAL Ab. III/2168 and ZPAL Ab. III/2208) and are the only known specimens with the potential to provide data about gill microstructure. This material provides an exceptional opportunity to constrain the early evolution of eulamellibranchy and reproductive behavior in Unionida in the Triassic, at the time when crucial adaptations to the successful dispersal in freshwaters worldwide probably emerged or were already established. This material also allows to observe the anatomy of these crucial soft tissue organs in a sequence of a dozen million-year time intervals. The paper also contains a reinvestigation of younger Mesozoic (Cretaceous) specimens bearing fossilized gills, unnamed specimen(s) from the Lower Cretaceous of the United Kingdom and Anodontites freitasi from Upper Cretaceous of Brazil, which provide additional information about the evolution of eulamellibranchy in Unionida during the Mesozoic.
Institutional abbreviations.—IG, Geological Institute of the State of São Paulo, São Paulo, Brazil; NHMUK, Natural History Museum, London, United Kingdom; ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
Material and methods
Locality descriptions.—Three localities, Krasiejów, Poręba, and Lipie Śląskie-Lisowice, with freshwater Keuper (Upper Triassic) strata in Silesia and the Kraków-Częstochowa Upland of SW Poland are known for spectacular vertebrate fossils like dipnoan and ganoid fishes, large temnospondyl amphibians, and aquatic and land reptiles of different lineages, including phytosaurs, dicynodonts, archosauromorphs, carnivorous archosaurs and turtles (e.g., Dzik et al. 2000, 2008; Dzik and Sulej 2007; Sulej et al. 2012).
Locality Krasiejów, near Opole, SW Poland preserves fossils of middle–late Carnian age. Silesunio parvus occurring in lacustrine deposits therein, preserves juvenile sculpture on the post-larval shell, calcified shells or internal molds with muscle attachment scars, and those with phosphatized gills (e.g., Skawina and Dzik 2011; Skawina 2013). The features mentioned above are variably preserved in the fossiliferous interval studied (about 1 m thick), however, the specimens with phosphatized gills were discovered only within a thin “yellow basal mudstone layer” on the very bottom of the profile (Skawina 2013: fig. 4).
Locality Poręba, near Zawiercie, Kraków-Częstochowa Upland, SW Poland, preserves fossils of the Norian age. This locality is famous for the occurrence of the Proterochersis porebensis turtle (Szczygielski and Sulej 2016). The fluvial strata at Poręba are about 6 m thick, comprising yellowish conglomerates with bones, and grey mud- and claystones interbedded with sandstones (Sulej et al. 2012; Szczygielski and Sulej 2016). Bivalves are occasionally found as shell imprints, calcified shells, and internal molds with preserved muscle scars. Specimens with preserved gills were only discovered as internal molds. These molds were concentrated due to Recent weathering and rainwater washing across the exposed strata.
Locality Lipie Śląskie-Lisowice near Lubliniec, SW Poland preserves fossils of the late Norian–Rhaetian age. The locality is chiefly known for its large dicynodonts and the large predatory archosaur Smok wawelski (Dzik et al. 2008; Niedźwiedzki et al. 2012; Kowal-Linka et al. 2019). The locality is currently flooded clay-pit but at the time of collection it was approximately 12 m thick section exposing fluvial deposits consisting mostly of grey silt- and mudstones interbedded with sandstones. Unionoid bivalves Tihkia silesiaca are preserved as internal molds with preserved muscle attachment scars, and poorly preserved shell imprints (Skawina and Dzik 2011). The phosphatized gills were only found on two specimens, partial internal molds pressed with the rock with a poorly preserved shell palimpsest on it.
The provenance and age of Mesozoic unionoid bivalves from the UK, mentioned by Richard et al. (1991) in the collection hosted by NHMUK is unclear. Re-examination of this collection led to the re-discovery of a drawer of selected internal molds of Mesozoic trigoniids (a dozen of specimens) and unionoid (single specimen) with preserved gill tissues (thank to analyses of Noel J. Morris, NHMUK and Martin A. Whyte, Sheffield University; AS personal communication with Katie Collins, 2024). Some trigoniids in the material in question, like Laevitrigonia gibbosa, NHMUK PI MB 2424, came from the Upper Jurassic limestones, in the Isle of Portland, Dorset, UK (see Wilby and Whyte 1995; Torrens et al. 2000; see SOM 2). However, the single internal mold originally labeled as “Unio porrectus”, NHMUK PI MB 2396, had limited information regarding the locality of origin. The specimen’s collection number and accompanying notes (including names of Martin A. Whyte, who also mentioned an unnamed single fossil Early Cretaceous unionoid in Whyte 1991), and original taxonomical assignment indicate its origin from the lowermost Lower Cretaceous of Wealden Group, comprising non-marine mudstones and sandstones (Newton 1910; Radley 2006; Radley and Allen 2012).
The Late Cretaceous (Senonian) Anodontites freitasi, IG 1033-I comes from the Adamantina Formation, Bauru Group, containing terrestrial vertebrates like titanosaur Gondwanatitan; Kellner and Azevedo 1999; Bandeira et al. 2016; Aureliano et al. 2021). This specimen has been discovered in a brown sandstone cropping out at the railway line, 16 km from Adamantina toward Flórida Paulista city (Simone and Mezzalira 1993). It has been described and illustrated previously by Simone and Mezzalira (1993). The age of these strata is unclear (e.g., Simone 1994; Nava and Martinelli 2011; Ghilardi et al. 2011; Silva Junior et al. 2022), but is most likely Senonian. IG 1033-I is preserved as an internal mold.
Methods.—The surface of specimens were examined under optical magnifiers and Keyence digital microscope, with acquired digital images analysed further on the screen). The lack of tissue connections between gill supports, thus filibranchy, was inferred when no visible interlamellar tissue junctions were observed. Such junctions are assumed to have been visible if present according to experimental taphonomy and comparison with other fossil material (Skawina 2010; Knight et al. 2014; SOM 1). Likewise, the presence of shuffled gill supports possible due to the disconnection of interlocking cilia, could also be visually detected if present (e.g., Klug et al 2005). The presence of the tissue connections between gill supports, thus eulamellibranchy, was inferred by observing the interfilamentar tissue junctions themselves and the parallel arrangement of gill supports as a result of these tissue junctions.
Results
Carnian (Triassic).—Two specimens (ZPAL Ab. III/2168 and 2208) were already described in Skawina (2010) and Skawina and Dzik (2011). Five new specimens (ZPAL Ab. III/2209, 2214–2216, 4409), all preserved as partial molds with shell imprints (probably a kind of shell “palimpsest”) have been discovered since then. Only ZPAL Ab. III/2209 (Fig. 1A) has both valves nearly shut close, while remaining has either a single valve, or two valves preserved in a butterfly position (Fig. 1B–E) (Skawina and Dzik 2011: fig. 1; SOM 1: G–J herein). None of them form a complete internal mold. The gill supports of demibranchs are preserved as brownish linear structures three-dimensionally preserved or flat, partially as color imprints (see Fig. 1A2, B, SOM 1: G–J). The demibranchs are recognizable as more (Fig. 1A2, B–D) or less (Fig. 1A1, A3, E) complete fragments. They are located in the center and dorsal part of the shell area, expanding to the posterior part of the bivalve, which corresponds to their expected anatomical position (see SOM 1: A). In ZPAL Ab. III/2214–2216 parts of both the inner and outer demibranch are visible. Gill supports are preserved as most commonly brownish, paired linear structures with a mid-deposit color line that likely corresponds to decay-driven collapsed gill supports, which are torn in the mid-line (compare with SOM 1: D, F). Only ZPAL Ab. III/4409 bears brownish flat linear structures with no change in color in the middle (Fig. 1E). No signs of connections between gill supports are recognizable in all these specimens, while some fragments of filaments are shuffled or tangled (e.g., Fig. 1A1, A3, C2).

Fig. 1. Late Triassic Unionida Silesunio parvus Skawina & Dzik, 2011, with preserved gills, from Carnian, Krasiejów, Poland. A. ZPAL Ab. III/2209. A1, preserved larger fragments of demibranchs visible in dorsal-middle areas both valves of the bivalve; A2, right-valve gills with 22 parallel gill supports, likely collapsed in the middle, preserved as paired dark-brown linear structures continuing into deposit-color lines; A3, left valve gills with brownish demibranchs fragmented into sections, gill supports are shuffled and tangled, transported within the valve and thus coming from outer or inner demibranchs, or from both. B. ZPAL Ab. III/2214. B1, probably a complete inner and outer demibranchs in three-dimensional preservation; gill supports of demibranchs start near the area of umbo and continue up to near the posterior end of the bivalve.; one (outer?) demibranch is translocated toward the ventral part; the gills in the center of the shell are preserved as brownish linear structures, otherwise in the color of the deposits. B2, brownish (collapsed in the middle?) gill supports visible as fine paired linear structures, shuffled and tangled within the demibranch; underneath the layer, the gill supports likely belonging to the inner demibranchs visible. C. ZPAL Ab. III/2216. C1, interpreted parts of inner and outer demibranch, arranged in opposite directions while the lines of attachment of gill supports of each demibranch are slightly shifted from each other. Some of the gill supports are shifted according to their neighbors ones (black arrows). C2, indicated with white arrows examples of collapsed middle part of each gill support visible as deposit color-line surrounded by brownish parallel lines. D. ZPAL Ab. III/2215. D1, two partially preserved demibranchs, and one (outer?) translocated toward the ventral part of a bivalve, gill supports brownish, collapsed, in majority parallel to each other. D2, detail showing translocated demibranchs; no tissue junctions between adjacent filaments are visible. E. ZPAL Ab. III/4409. E1, brownish, mostly parallel lines of believed gill supports covering the middle part of the left valve of the bivalve. E2, brownish linear structures devoid of the collapsed central part of each line, thus different from the other examples.
Norian (Triassic).—All the specimens of Tihkia? sp. are preserved as internal molds. Those are: the best preserved ZPAL V. 39/496 (Fig. 2A1–A3), and ZPAL V. 39/473 and ZPAL V. 39/474. Both latter specimens show whitish repeated parallel lines typical for the demibranch position (Fig. 2B, C). ZPAL V. 39/496 preserves a few fragments of a demibranch comprising of dark-brown paired, parallel linear structures, in the middle and dorsal area. These structures are similar in shape to equivalent structures in S. parvus and of Recent decaying material (SOM 1: D, F). No morphological observation of tissue connections is visible in this material.
Norian–Rhaetian (Triassic).—The specimens of Tihkia silesiaca with fossilized gills are poorly preserved. Two specimens, ZPAL V. 33/298 and ZPAL V. 33/305, have brownish areas in the middle or dorsal-middle of the shells. ZPAL V. 33/298 is a left valve. It has visible darker and lighter brown linear structures interpreted as gill supports, which are corresponding to the expected position of demibranchs (Fig. 2D). The lighter brown part expands toward the posterior part of the specimen; here, some filaments are translocated/shuffled (Fig. 2D2). The second specimen is a right valve ZPAL V. 33/305. It preserves an agglomerate of brown linear structures that is difficult to interpret, however, single structures are distinguishable (Fig. 2E). The poor preservation of these specimens does not allow for inquiries about the possible presence or absence of interfilamentar tissue junctions of filaments.

Fig. 2. Late Triassic Unionida Tihkia? sp. from Norian, Poręba, Poland (A–C) and Tihkia silesiaca Skawina & Dzik, 2011, from Norian–Rhaetian, Lipie Śląskie-Lisowice, Poland (D, E). A. ZPAL V. 39/496, internal mold. A1, right lateral view; A2, left lateral view, with brownish three-dimensionally preserved linear structures, likely a few fragments of torn demibranchs; A3, A4, details with paired brownish linear structures interpreted as gill supports; the gill supports are here collapsed in the middle. B. ZPAL V. 39/473, internal mold. B1, left lateral view with multiple parallel linear structures; B2, detail of parallel linear structures. Linear structures interpreted as gill supports are preserved brighter than the sediment. C. ZPAL V. 39/474, partially preserved, damaged internal mold. C1, whitish linear structures interpreted as gill supports forming what resembles a demibranch; C2, a fragment of which looks partially rotated from the natural position, from the surface into the depth of the specimen (arrowed). D. ZPAL V. 33/298, left valve imprint with darker and lighter brownish linear structures corresponding to gill supports; these form a poorly preserved larger fragment of a demibranch in middle-dorsal area of the specimen (D1); D2, single gill supports are interpretable, collapsed in the middle, note the tangled arrangement. E. ZPAL V. 33/305, poorly preserved left valve imprint with a dark brownish area of poorly preserved, phosphatized gill supports (E1); E2, detail with single gill supports recognizable (arrows).
Early Cretaceous.—NHMUK PI MB 2396 is an internal mold, originally labeled as “Unio porrectus”. It displays an agglomerate-like area in the postero-middle part of the left side (Fig. 3A–D). The area, ca. 20 mm long and 10 mm wide, consists of delicate whitish linear structures similar to gill supports and situated in the area of the expected demibranch position. The preservation state of this structure prevents more detailed morphological analysis or discussion of the presence/absence of interfilamentar tissue junctions of filaments. The specimen has its muscle attachment scars visible. The presence of a few minute attachment scars inside the umbonal cavity, and absence of a strong, commonly single pedal elevator in the apex of each umbonal cavity which is typical for Trigoniida (Newell and Boyd 1975; Graf and Cummings 2006; Skawina and Dzik 2011) suggests unionoid affinities of this specimen (Fig. 3A5, A6).
Late Cretaceous (Senonian).—The specimen of Anodontites freitasi, IG 1033-I, is an internal mold. The fossilized fragment of a demibranch is preserved on the middle-dorsal area of the left side of the specimen. This structure is about 10 mm long and 5 mm wide. It is preserved as a whitish net-like of vertical and horizontal linear structures. The horizontal structures may correspond to gill supports, while the vertical structures indicate the presence of the interfilamentar tissue junctions preserved in this specimen.
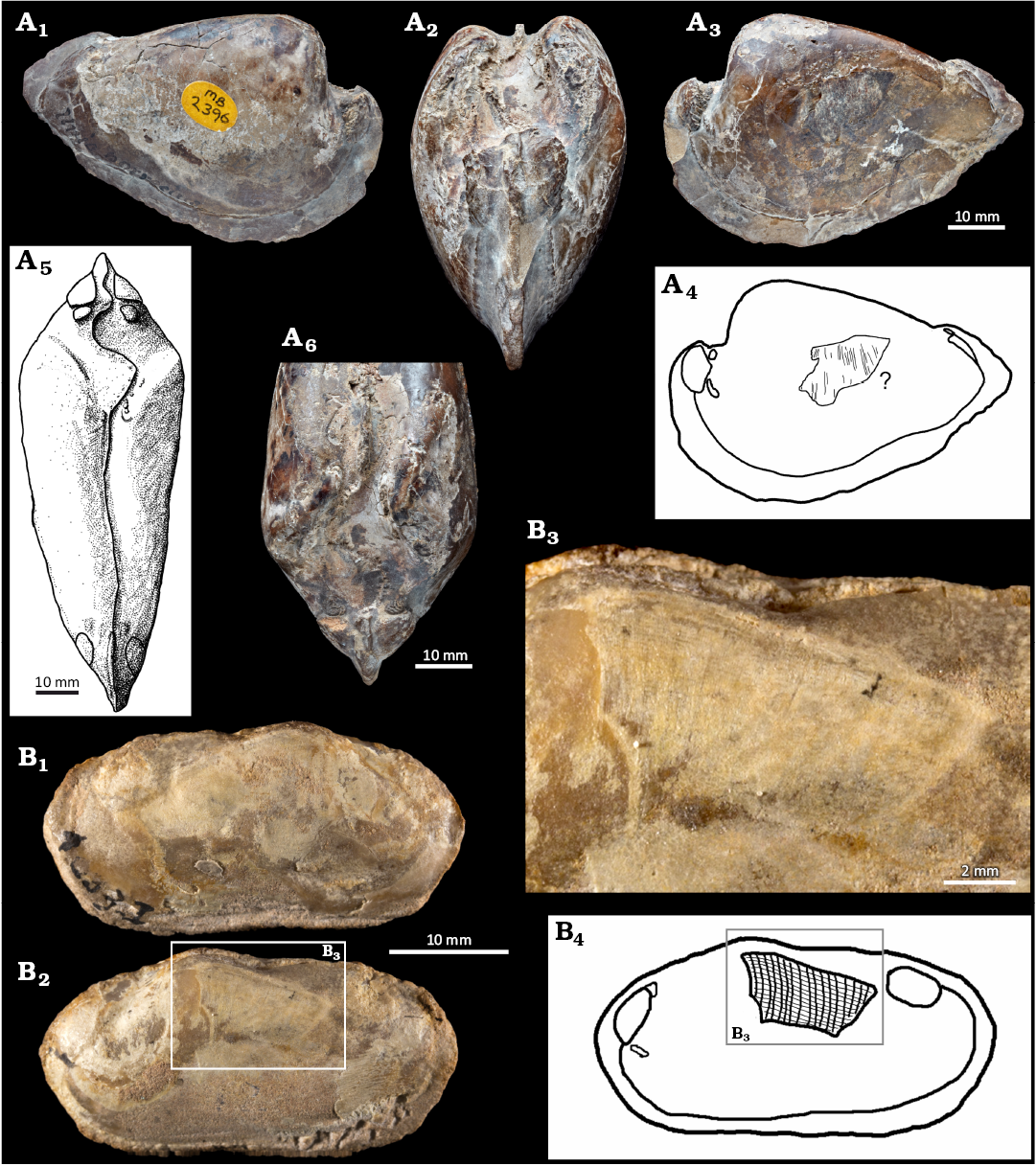
Fig. 3. Cretaceous Unionida with partial gill preservation. A. “Unio porrectus” J. De C. Sowerby, 1828, NHMUK PI MB 2396, Lower Cretaceous, Wealden Group, Isle of Portland, UK. A1, internal mold in right lateral view; A2, anterior view with hinge line and anterior set of muscle scars visible; A3, left lateral view, on this side an about 20 mm long and 10 mm wide area linear structures resembling gill supports of a demibranch visible; A4, drawing of supposed part of demibranch; drawing (A5) and photograph (A6) of dorsal view. The presence of a few minute muscle attachment scars in the area of the umbo suggests a relation with Unionida. B. Anodontites freitasi Mezzalira, 1974, IG 1033-I, Upper Cretaceous of Adamantina Formation, Bauru Group, Senonian, Brazil. B1, internal mold in right lateral view; photograph (B2) and drawing (B4) of left lateral view in the dorsalo-middle part of this side about 10 mm length and 5 mm wide, a brighter structure is visible; B3, a detail with area interpreted as preserved gills.The hypothesized mineralized gill supports form a net-like structure (lines are transversely connected), indicating eulamellibranch anatomy.
Discussion
Phosphatized soft tissues were previously occasionally reported from mollusks (e.g., mantle, muscles in cephalopods; Allison 1988; Wilby and Briggs 1997; Wilby et al. 2004), while only a few such structures were discovered in fossil bivalves (gills, muscles, sometimes labial palps; e.g., Harper and Todd 1995; Klug et al. 2005; Knight et al. 2014; Klug et al. 2022). Preservation of gills has been reported more frequently from the fossil group traditionally called the palaeoheterodonts thus Unionida + Trigoniida (e.g., Graf and Cummings 2006; Carter et al. 2011) than from other bivalve taxa. The accepted explanation is that this group have chitinous gills supports calcified, that can serve as nucleation center and source of mineral precursors which facilitate early mineralization and protect gill tissue against post-mortem decomposition (Ridewood 1903; Silverman et al. 1985; Silverman et al. 1989; Whyte 1991; Skawina 2010). Unfortunately, gill preservation in freshwater bivalves occurs rarely (Whyte 1991; Simone and Mezzalira 1993; Skawina and Dzik 2011) in comparison to trigoniids (e.g., Klug et al 2005). Also, all or most of the trigoniids with fossilized soft tissues are preserved as internal molds. Wilby and Whyte (1995) proposed that the enclosure of decaying soft tissue within the tightly closed shell was a necessary prerequisite to establish an environment enabling soft tissue mineralization. This proposition is not supported fully by the fossil record of freshwater bivalves. Although a single Norian specimen and both Cretaceous specimens studied herein are internal molds, the well-preserved gills of Carnian S. parvus and poorly preserved tissues of Rhaetian T. silesiaca occur in the specimens with open shells (or not fully opened like in ZPAL Ab. III/2209). It is possible that osmotic freshwater conditions could influence the decay and impact on early mineralization processes, or some other yet unknown traits of these particular “taphonomic window” localities were involved.
Most of the fossilized demibranchs in the specimens from the Polish Upper Triassic preserved two brownish linear structures with a break in-between. This character as interpreted by comparison with modern decaying material, could have appeared within 150 hours postmortem, providing no subsequent disturbance occurred (Skawina 2010: fig. 1; SOM 1: D–F). After this time, ciliated tissue decays and interfilamentar junctions should be visible at least in some areas of the preserved demibranchs, proving that interfilamentar junctions were initially present. Such preservation of interfilamentar junctions connecting gill demibranchs was indeed observed in the specimen of Late Cretaceous A. freitasi, as well as in unrelated Cretaceous inoceramid (Knight et al. 2014). However, no similar structures were observed in Carnian and Norian bivalves, and their preservation of Rhaetian and Early Cretaceous specimens did not allow for their clear interpretation. The materials studied herein, including new specimens with the oldest preserved unionoid gills, confirm the filibranch anatomy of the Carnian, Late Triassic Unionida. The same can be assumed for Norian unionoids based on morphological analyses (no interfilamentar junctions observed in preserved gills). There is thus a clear similarity between the gill anatomy of Triassic Unionida and the gill anatomy of their closest marine relatives, Trigoniida. Although filibranchy does not fully exclude it, it represents an incomplete state of maternal care in the evolution of Unionida in freshwaters. Future research, including more fieldwork and careful screening of museum collections, is needed to provide more evidence on evolution of the reproductive behavior and maternal care in Unionida.
Conclusions
A new Late Triassic fossil material of Unionida with extraordinarily preserved gill tissues is described from Poland (Silesunio parvus, Tihkia silesiaca, Tihkia? sp.), together with a reinvestigation of Cretaceous fossils from archival museum collections from United Kingdom (“Unio porrectus”) and Brazil (Anodontites freitasi).
The preserved anatomy of gills may provide paleobiological information about the possibility of fossil behavior, brooding embryos in maternal gills (if anatomy is advanced, eulamellibranch), which is accepted as an adaptation to freshwater habitats and may support the evolutionary success of this group of bivalves.
The visible features of the gills of Late Triassic unionoids (S. parvus and Tihkia? sp.) do not support the advanced anatomy, while eulamellibranchy was confirmed in the specimen of Late Cretaceous A. freitasi. The preservation of gills in remaining specimens is not allowing for considerations about their anatomy, thus inferring about the detailed timing of origins of the crucial anatomical adaptations to life in freshwaters.
Author’s contributions
AS: conceptualization, methodology, investigation, visualization; AS and RPG: resources, writing—original draft, writing—review and editing, funding acquisition.
Acknowledgments
The authors are grateful to a wide group of supportive persons, our gratitude is given to: Kenneth De Baets (Faculty of Biology, University of Warsaw, Poland), Débora Eliza Baumann (UNESP-São Paulo State University, Bauru, Brazil), Graciela Delvene (Instituto Geológico y Minero de España, Madrid, Spain), Marian Dziewiński (ZPAL Image Services), Jerzy Dzik (ZPAL), Peter Grugeon (NHMUK Image Services; pictures of NHMUK PI MB 2396 “Unio porrectus” on Fig. 3A1, A2, A3, A6), Bernardo Peixoto (UNESP Image Services), Luiz Ricardo L. Simone (Museum of Zoology of the University of São Paulo, São Paulo, Brazil), Tomasz Sulej (ZPAL), for their presence, discussions, and kind help during working and analyzing of the presented material. Special thanks are dedicated to Katie Collins (NHMUK) for her support with the collection. We are grateful to the journal referees Arthur Bogan (North Carolina State Museum of Natural Sciences, Raleigh, USA) and Daniel L. Graf (University of Wisconsin, Stevens Point, USA) for their helpful suggestions that improved the manuscript. The authors are thankful to Holly Anderson (Faculty of Biology, University of Warsaw, Poland) for her English support. This research was funded by the grant of the National Science Centre, Poland, No. 2023/07/X/NZ8/01313 (MINIATURA 7) to AS, and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2023/01470-2) to RPG. Part of the research (AS) was funded by I.3.4 Action of the Excellence Initiative-Research University Programme at the University of Warsaw (Project: PARADIVE). Fieldworks (AS) were supported by the National Science Centre, Poland grant No. 7986/B/PO1/2011/40.
Editor: Krzysztof Hryniewicz.
References
Allison, P.A. 1988. Phosphatized soft‐bodied squids from the Jurassic Oxford Clay. Lethaia 21: 403–410. Crossref
Aureliano, T., Ghilardi, A.M., Navarro, B.A., Fernandes, M.A., Ricardi-Branco, F., and Wedel, M.J. 2021. Exquisite air sac histological traces in a hyperpneumatized nanoid sauropod dinosaur from South America. Scientific Reports 11 (1): 24207. Crossref
Bandeira, K.L., Medeiros Simbras, F., Batista Machado, E., de Almeida Campos, D., Oliveira, G.R., and Kellner, A.W. 2016. A new giant Titanosauria (Dinosauria: Sauropoda) from the Late Cretaceous Bauru Group, Brazil. PLoS One 11: e0163373. Crossref
Bauer, G. and Wachtler, K. 2001. Ecology and Evolution of the Freshwater Mussels Unionoida. 396 pp. Springer-Verlag, Berlin. Crossref
Bolotov, I.N., Vikhrev, I.V., Bespalaya, Y.V., Gofarov, M.Y., Kondakov, A.V., Konopleva, E.S., Bolotov, N.N., and Lyubas, A.A. 2016. Multi-locus fossil-calibrated phylogeny, biogeography and a subgeneric revision of the Margaritiferidae (Mollusca: Bivalvia: Unionoida). Molecular Phylogenetics and Evolution 103: 104–121. Crossref
Bolotov, I.N., Vikhrev, I.V., Kondakov, A.V., Konopleva, E.S., Gofarov, M.Y., Aksenova, O.V., and Tumpeesuwan, S. 2017. New taxa of freshwater mussels (Unionidae) from a species-rich but overlooked evolutionary hotspot in Southeast Asia. Scientific Reports 7: 11573. Crossref
Boucot, A.J. and Poinar, G.O.J. 2010. Fossil Behavior Compendium. 391 pp. CRC Press, Boca Raton. Crossref
Carter, J.G., Altaba, C.R., Anderson, L.C., Araujo, R., Biakov, A.S., Bogan, A.E., Campbell, D.C., Campbell, M., Jin-hua, C., Cope, J.C.W., Delvene, G., Dijkstra, H.H., Zong-jie, F., Gardner, R.N., Gavrilova, V.A., Goncharova, I.A., Harries, P.J., Hartman, J.H., Hautmann, M., Hoeh, W.R., Hylleberg, J., Bao-yu, J., Johnston, P., Kirkendale, L., Kleemann, K., Koppka, J., Kříž, J., Machado, D., Malchus, N., Márquez-Aliaga, A., Masse, J.-P., McRoberts, C.A., Middelfart, P.U., Mitchell, S., Nevesskaja, L.A., Özer, S., Pojeta, J., Polubotko, I.V., Pons, J.M., Popov, S., Sánchez, T., Sartori, A.F., Scott, R.W., Sey, I.I., Signorelli, J.H., Silantiev, V.V., Skelton, P.W., Steuber, T., Waterhouse, J.B., Wingard, G.L., and Yancey, T. 2011. A synoptical classification of the Bivalvia (Mollusca). Paleontological Contributions 4: 1–47.
Carter, J.G., Harries, P., Malchus, N., Sartori, A., Anderson, L., Bieler, R., Bogan, A., Coan, E., Cope, J., Cragg, S., Garcia-March, J., Hylleberg, J., Kelley, P., Kleemann, K., Kriz, J., McRoberts, C., Mikkelsen, P., Pojeta, J.J., Skelton, P.W., Temkin, I., Yancey, T., and Zieritz, A. 2012. Treatise Online no. 48: Part N, Revised, Volume 1, Chapter 31: Illustrated glossary of the Bivalvia. Treatise Online [available online, https://doi.org/10.17161/to.v0i0.4322] Crossref
Dzik, J. and Sulej, T. 2007. A review of the early Late Triassic Krasiejów biota from Silesia, Poland. Palaeontologia Polonica 64: 3–27.
Dzik, J., Sulej, T., and Niedźwiedzki, G. 2008. A dicynodont-theropod association in the latest Triassic of Poland. Acta Palaeontologica Polonica 53: 733–738. Crossref
Dzik, J., Sulej, T., Kaim, A., and Niedźwiedzki, R. 2000. Późnotriasowe cmentarzysko kręgowców lądowych w Krasiejowie na Śląsku Opolskim. Przegląd Geologiczny 48: 226–235.
Freitas, E.T.F., Moreira, A.M.S., de Paula, R.S., Andrade, G.R., de Carvalho, M.D., Assis, P.S., Jorge, E.C., and Cardoso, A.V. 2022. Ultrastructure of the gill ciliary epithelium of Limnoperna fortunei (Dunker 1857), the invasive golden mussel. BMC Zoology 7: 6. Crossref
Ghilardi, R.P., Carbonaro, F.A., and Simone, L.R.L. 2011. Physa mezzalirai, a new Cretaceous basommatophoran from Adamantina formation, Brazil. Strombus 18: 7–9.
Graf, D.L. 2013. Patterns of freshwater bivalve global diversity and the state of phylogenetic studies on the Unionoida, Sphaeriidae, and Cyrenidae. American Malacological Bulletin 31: 135–153. Crossref
Graf, D.L. and Cummings, K.S. 2006. Palaeoheterodont diversity (Mollusca: Trigonioida + Unionoida): what we know and what we wish we knew about freshwater mussel evolution. Zoological Journal of the Linnean Society 148: 343–394. Crossref
Graf, D.L. and Cummings, K.S. 2021. A ‘big data’ approach to global freshwater mussel diversity (Bivalvia: Unionoida), with an updated checklist of genera and species. Journal of Molluscan Studies 87: eyaa034. Crossref
Graf, D.L., Jones, H., Geneva, A.J., Pfeiffer, J.M., 3rd, and Klunzinger, M.W. 2015. Molecular phylogenetic analysis supports a Gondwanan origin of the Hyriidae (Mollusca: Bivalvia: Unionida) and the paraphyly of Australasian taxa. Molecular Phylogenetics and Evolution 85: 1–9. Crossref
Gross, M. and Piller, W.E. 2018. Fossil glochidia (Bivalvia: Unionida: Hyriidae) from the middle Miocene of western Amazonia (Peru). Journal of Systematic Palaeontology 17: 1117–1128. Crossref
Harper, E.M. and Todd, J.A. 1995. Preservation of the adductor muscle of an Upper Jurassic oyster. Paläontologische Zeitschrift 69: 55–59. Crossref
Hinzmann, M.F., Lopes-Lima, M., Bobos, I., Ferreira, J., Domingues, B., and Machado, J. 2015. Morphological and chemical characterization of mineral concretions in the freshwater bivalve Anodonta cygnea (Unionidae). Journal of Morphology 276: 65–76. Crossref
Huang, X.C., Wu, R.W., An, C.T., Xie, G.L., Su, J.H., Ouyang, S., Zhou, C.H., and Wu, X.P. 2018. Reclassification of Lamprotula rochechouartii as Margaritifera rochechouartii comb. nov. (Bivalvia: Margaritiferidae) revealed by time-calibrated multi-locus phylogenetic analyses and mitochondrial phylogenomics of Unionoida. Molecular Phylogenetics and Evolution 120: 297–306. Crossref
Kellner, A.W. and Azevedo, S.D. 1999. A new sauropod dinosaur (Titanosauria) from the Late Cretaceous of Brazil. National Science Museum Monographs 15: 111–142.
Klug, C., Hagdorn, H., and Montenari, M. 2005. Phosphatized soft-tissue in Triassic bivalves. Palaeontology 48: 833–852. Crossref
Klug, C., Hüne, L., Roth, R., and Hautmann, M. 2022. Phosphatized adductor muscle remains in a Cenomanian limid bivalve from Villers-sur-Mer (France). Swiss Journal of Palaeontology 141: 10. Crossref
Knight, R.I., Morris, N.J., Todd, J.A., Howard, L.E., Ball, A.D., and Jagt, J. 2014. Exceptional preservation of a novel gill grade in large Cretaceous inoceramids: systematic and palaeobiological implications. Palaeontology 57: 37–54. Crossref
Kowal-Linka, M., Krzemińska, E., and Czupyt, Z. 2019. The youngest detrital zircons from the Upper Triassic Lipie Śląskie (Lisowice) continental deposits (Poland): Implications for the maximum depositional age of the Lisowice bone-bearing horizon. Palaeogeography, Palaeoclimatology, Palaeoecology 514: 487–501. Crossref
Lopes-Lima, M., Burlakova, L.E., Karatayev, A.Y., Mehler, K., Seddon, M., and Sousa, R. 2018. Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia 810: 1–14. Crossref
Mackie, G.L. 1984. Reproduction in Bivalvia. In: A.S. Tompa, N.H. Verdonk, and J.A.M. van de Biggelaar (eds.), The Mollusca. Reproduction, 351–418. Academic Press, New York.
Mansur, M.C.D., Pereira, D., Bergonci, P.E.A., Pimpão, D.M., Barradas, J.R.d.S., and Sabaj, M.H. 2019. Morphological assessment of Rheodreissena (Bivalvia: Veneroida: Dreissenidae) with an updated diagnosis of the genus, descriptions of two new species, redescription of R. lopesi, and the first account of larval brooding in New World dreissenids. Proceedings of the Academy of Natural Sciences of Philadelphia 166: 1–45. Crossref
Morton, B. 1978. The biology and functional morphology of Philobrya munita (Bivalvia: Philobryidae). Journal of Zoology 185: 173–196. Crossref
Morton, B. 1987. The functional morphology of Neotrigonia margaritacea (Bivalvia: Trigoniacea), with a discussion of phylogenetic affinities. Records of the Australian Museum 39: 339–354. Crossref
Morton, B. and Puljas, S. 2013. Life‐history strategy, with ctenidial and pallial larval brooding, of the troglodytic ‘living fossil’ Congeria kusceri (Bivalvia: Dreissenidae) from the subterranean Dinaric Alpine karst of Croatia. Biological Journal of the Linnean Society 108: 294–314.
Nava, W.R. and Martinelli, A.G. 2011. A new squamate lizard from the Upper Cretaceous Adamantina Formation (Bauru Group), São Paulo State, Brazil. Anais da Academia Brasileira de Ciências 83: 291–299. Crossref
Newell, N.D. and Boyd, D.W. 1975. Parallel evolution in early trigoniacean bivalves. Bulletin of the American Museum of Natural History 154: 53–162.
Newton, R.B. 1910. On an undescribed Anodonta from the English Wealden Formation, with remarks on the other Unionidae of the same period. Proceedings of the Malacological Society 9: 114–117.
Niedźwiedzki, G., Sulej, T., and Dzik, J. 2012. A large predatory archosaur from the Late Triassic of Poland. Acta Palaeontologica Polonica 57: 267–276. Crossref
Radley, J.D. 2006. A Wealden guide I: the Weald Sub-basin. Geology Today 22: 109–118. Crossref
Radley, J.D. and Allen, P. 2012. The Wealden (non-marine Lower Cretaceous) of the Wessex Sub-basin, southern England. Proceedings of the Geologists’ Association 123: 319–373. Crossref
Richard, P.E., Dietz, T.H., and Silverman, H. 1991. Structure of the gill during reproduction in the unionids Anodonta grandis, Ligumia subrostrata, and Carunculina parva taxasensis. Canadian Journal of Zoology 69: 1744–1754. Crossref
Ridewood, W.G. 1903. On the structure of the gills of the lamellibranchia. Philosophical Transactions of the Royal Society of London. Series B, Containing Papers of a Biological Character 195: 147–284. Crossref
Seton, M., Müller, R.D., Zahirovic, S., Gaina, C., Torsvik, T., Shephard, G., Talsma, A., Gurnis, M., Turner, M., Maus, S., and Chandler, M. 2012. Global continental and ocean basin reconstructions since 200 Ma. Earth-Science Reviews 113: 212–270. Crossref
Silva Junior, J.C.G., Martinelli, A.G., Iori, F.V., Marinho, T.S., Hechenleitner, E.M., and Langer, M.C. 2022. Reassessment of Aeolosaurus maximus, a titanosaur dinosaur from the Late Cretaceous of Southeastern Brazil. Historical Biology 34: 403–411. Crossref
Silverman, H., Richard, P.E., Goddard, R.H., and Dietz, T.H. 1989. Intracellular formation of calcium concretions by phagocytic cells in freshwater mussels. Canadian Journal of Zoology 67: 198–207. Crossref
Silverman, H., Steffens, W.L., and Dietz, T.H. 1985. Calcium from extracellular concretions in the gills of freshwater unionid mussels is mobilized during reproduction. Journal of Experimental Zoology 236: 137–147. Crossref
Silverman, H., Todd Kays, W., and Dietz Thomas, H. 1987. Maternal calcium contribution to glochidial shells in freshwater mussels (Eulamellibranchia: Unionidae). Journal of Experimental Zoology 242: 137–146. Crossref
Simone, L. 1994. Fossil molluscs of Brazil. Boletim do Instituto Geológico 11: 1–202.
Simone, L. and Mezzalira, S. 1993. Vestígios de partes moles em um Bivalve fóssil (Unionoida, Mycetopodidae) do Grupo Bauru (Cretáceo Superioi), São Paulo, Brasil. Anais da Academia Brasileira de Ciências 65: 155–159.
Skawina, A. 2010. Experimental decay of gills in freshwater bivalves as a key to understand their preservation in Upper Triassic lacustrine deposits. Palaios 25: 215–220. Crossref
Skawina, A. 2013. Population dynamics and taphonomy of the Late Triassic (Carnian) freshwater bivalves from Krasiejów (Poland). Palaeogeography, Palaeoclimatology, Palaeoecology 379–380: 68–80. Crossref
Skawina, A. 2021. Evolutionary history of bivalves as parasites. In: K. De Baets and J.W. Huntley (eds.), The Evolution and Fossil Record of Parasitism: Identification and Macroevolution of Parasites, 153–207. Springer International Publishing, Cham. Crossref
Skawina, A. and Dzik, J. 2011. Umbonal musculature and relationships of the Late Triassic filibranch unionoid bivalves. Zoological Journal of the Linnean Society 163: 863–883. Crossref
Sulej, T., Niedźwiedzki, G., and Bronowicz, R. 2012. A new Late Triassic vertebrate fauna from Poland with turtles, aetosaurs, and coelophysoid dinosaurs. Journal of Vertebrate Paleontology, 32: 1033–1041. Crossref
Szczygielski, T. and Sulej, T. 2016. Revision of the Triassic European turtles Proterochersis and Murrhardtia (Reptilia, Testudinata, Proterochersidae), with the description of new taxa from Poland and Germany. Zoological Journal of the Linnean Society 177: 395–427. Crossref
Tevesz, M.J.S. 1975. Structure and habits of the ‘living fossil’ pelecypod Neotrigonia. Lethaia 8: 321–327. Crossref
Torrens, H., Benamy, E., Daeschler, E., Spamer, E., and Bogan, A. 2000. Etheldred Benett of Wiltshire, England, the first lady geologist—her fossil collection in the Academy of Natural Sciences of Philadelphia, and the rediscovery of “lost” specimens of Jurassic Trigoniidae (Mollusca: Bivalvia) with their soft anatomy preserved. Proceedings of the Academy of Natural Sciences of Philadelphia 150: 59–123.
Watters, G.T. and O’Dee, S.H. 1998. Metamorphosis of freshwater mussel glochidia (Bivalvia: Unionidae) on amphibians and exotic fishes. The American Midland Naturalist 139: 49–57. Crossref
Whyte, M.A. 1991. Phosphate gill supports in living and fossil bivalves. In: S. Suga and H. Nakahara (eds.), Mechanisms and Phylogeny of Mineralization in Biological Systems, 427–431. Springer Japan, Tokyo. Crossref
Wilby, P.R. and Briggs, D.E.G. 1997. Taxonomic trends in the resolution of detail preserved in fossil phosphatized soft tissues. Geobios 30: 493–502. Crossref
Wilby, P.R. and Whyte, M.A. 1995. Phosphatized soft tissues in bivalves from the Portland Roach of Dorset (Upper Jurassic). Geological Magazine 132: 117–120. Crossref
Wilby, P.R., Hudson, J.D., Clements, R.G., and Hollingworth, N.T.J. 2004. Taphonomy and origin of an accumulate of soft-bodied cephalopods in the Oxford Clay Formation (Jurassic, England). Palaeontology 47: 1159–1180. Crossref
Acta Palaeontol. Pol. 70 (2): 375–384, 2025
https://doi.org/10.4202/app.01251.2025